Ammonium Hydroxide's Role in Ammonia Stripping Processes
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonia Stripping Background and Objectives
Ammonia stripping is a crucial process in various industrial applications, particularly in wastewater treatment and environmental remediation. The technique has gained significant attention over the past few decades due to its effectiveness in removing ammonia from liquid streams. The evolution of this technology can be traced back to the early 20th century when the basic principles of gas stripping were first applied to ammonia removal.
The primary objective of ammonia stripping is to transfer ammonia from the liquid phase to the gas phase, facilitating its removal from the system. This process is particularly important in addressing environmental concerns related to excessive ammonia levels in water bodies, which can lead to eutrophication and harm aquatic ecosystems. As environmental regulations have become more stringent, the demand for efficient ammonia removal technologies has increased, driving further research and development in this field.
Ammonium hydroxide plays a pivotal role in the ammonia stripping process. It serves as a key intermediate compound, existing in equilibrium with ammonia in aqueous solutions. The relationship between ammonium hydroxide and free ammonia is governed by pH and temperature, which are critical parameters in optimizing the stripping process. Understanding this equilibrium is essential for designing effective ammonia stripping systems and predicting process efficiency.
The technology has evolved from simple air stripping towers to more sophisticated designs incorporating advanced packing materials, improved mass transfer devices, and optimized operating conditions. Recent developments have focused on enhancing energy efficiency, reducing environmental impact, and improving overall process economics. These advancements have expanded the applicability of ammonia stripping to a wider range of industries, including fertilizer production, petrochemical processing, and food and beverage manufacturing.
As we look towards the future, the objectives for ammonia stripping technology continue to evolve. Key goals include developing more sustainable processes with lower energy consumption, minimizing chemical usage, and exploring novel hybrid technologies that combine stripping with other treatment methods. There is also a growing interest in recovering ammonia as a valuable resource, aligning with circular economy principles and promoting resource efficiency.
The role of ammonium hydroxide in these processes remains central, with ongoing research aimed at better understanding and controlling its behavior under various operating conditions. This includes investigating the impact of different chemical additives on the ammonium hydroxide-ammonia equilibrium and exploring innovative ways to shift this equilibrium in favor of ammonia removal. As we progress, the integration of advanced monitoring and control systems is expected to play a crucial role in optimizing the performance of ammonia stripping processes, further enhancing their efficiency and reliability.
The primary objective of ammonia stripping is to transfer ammonia from the liquid phase to the gas phase, facilitating its removal from the system. This process is particularly important in addressing environmental concerns related to excessive ammonia levels in water bodies, which can lead to eutrophication and harm aquatic ecosystems. As environmental regulations have become more stringent, the demand for efficient ammonia removal technologies has increased, driving further research and development in this field.
Ammonium hydroxide plays a pivotal role in the ammonia stripping process. It serves as a key intermediate compound, existing in equilibrium with ammonia in aqueous solutions. The relationship between ammonium hydroxide and free ammonia is governed by pH and temperature, which are critical parameters in optimizing the stripping process. Understanding this equilibrium is essential for designing effective ammonia stripping systems and predicting process efficiency.
The technology has evolved from simple air stripping towers to more sophisticated designs incorporating advanced packing materials, improved mass transfer devices, and optimized operating conditions. Recent developments have focused on enhancing energy efficiency, reducing environmental impact, and improving overall process economics. These advancements have expanded the applicability of ammonia stripping to a wider range of industries, including fertilizer production, petrochemical processing, and food and beverage manufacturing.
As we look towards the future, the objectives for ammonia stripping technology continue to evolve. Key goals include developing more sustainable processes with lower energy consumption, minimizing chemical usage, and exploring novel hybrid technologies that combine stripping with other treatment methods. There is also a growing interest in recovering ammonia as a valuable resource, aligning with circular economy principles and promoting resource efficiency.
The role of ammonium hydroxide in these processes remains central, with ongoing research aimed at better understanding and controlling its behavior under various operating conditions. This includes investigating the impact of different chemical additives on the ammonium hydroxide-ammonia equilibrium and exploring innovative ways to shift this equilibrium in favor of ammonia removal. As we progress, the integration of advanced monitoring and control systems is expected to play a crucial role in optimizing the performance of ammonia stripping processes, further enhancing their efficiency and reliability.
Market Analysis for Ammonia Recovery
The market for ammonia recovery is experiencing significant growth, driven by increasing environmental regulations and the rising demand for sustainable agricultural practices. The global ammonia recovery market is expected to expand at a steady rate over the next decade, with a particular focus on wastewater treatment and fertilizer production sectors.
In the wastewater treatment industry, ammonia recovery technologies are gaining traction due to stricter environmental regulations on nitrogen discharge. Municipal wastewater treatment plants are increasingly adopting ammonia recovery systems to comply with these regulations and to generate additional revenue streams from recovered ammonia. The industrial wastewater sector, particularly from food processing and chemical manufacturing, also presents a substantial market opportunity for ammonia recovery solutions.
The fertilizer industry represents another major market segment for ammonia recovery. As the global population continues to grow, the demand for fertilizers is increasing, driving the need for more efficient and sustainable ammonia production methods. Recovered ammonia can be used directly as a fertilizer or as a raw material for other nitrogen-based fertilizers, offering a circular economy approach to fertilizer production.
Geographically, North America and Europe are currently leading the ammonia recovery market, owing to stringent environmental regulations and well-established wastewater treatment infrastructure. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, urbanization, and increasing awareness of environmental issues.
The market is also seeing a shift towards more advanced and efficient ammonia recovery technologies. Membrane-based systems and biological treatment processes are gaining popularity due to their lower energy requirements and higher recovery rates compared to traditional stripping methods. This trend is likely to continue as companies invest in research and development to improve process efficiency and reduce operational costs.
Key market drivers include the increasing focus on resource recovery from waste streams, the rising cost of synthetic ammonia production, and growing concerns about the environmental impact of nitrogen pollution. Additionally, government initiatives promoting circular economy principles and sustainable waste management practices are expected to further boost the ammonia recovery market.
However, the market also faces challenges, such as high initial investment costs for ammonia recovery systems and the need for skilled operators. Overcoming these barriers will be crucial for wider market adoption, particularly in developing regions where cost sensitivity is higher.
In the wastewater treatment industry, ammonia recovery technologies are gaining traction due to stricter environmental regulations on nitrogen discharge. Municipal wastewater treatment plants are increasingly adopting ammonia recovery systems to comply with these regulations and to generate additional revenue streams from recovered ammonia. The industrial wastewater sector, particularly from food processing and chemical manufacturing, also presents a substantial market opportunity for ammonia recovery solutions.
The fertilizer industry represents another major market segment for ammonia recovery. As the global population continues to grow, the demand for fertilizers is increasing, driving the need for more efficient and sustainable ammonia production methods. Recovered ammonia can be used directly as a fertilizer or as a raw material for other nitrogen-based fertilizers, offering a circular economy approach to fertilizer production.
Geographically, North America and Europe are currently leading the ammonia recovery market, owing to stringent environmental regulations and well-established wastewater treatment infrastructure. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, urbanization, and increasing awareness of environmental issues.
The market is also seeing a shift towards more advanced and efficient ammonia recovery technologies. Membrane-based systems and biological treatment processes are gaining popularity due to their lower energy requirements and higher recovery rates compared to traditional stripping methods. This trend is likely to continue as companies invest in research and development to improve process efficiency and reduce operational costs.
Key market drivers include the increasing focus on resource recovery from waste streams, the rising cost of synthetic ammonia production, and growing concerns about the environmental impact of nitrogen pollution. Additionally, government initiatives promoting circular economy principles and sustainable waste management practices are expected to further boost the ammonia recovery market.
However, the market also faces challenges, such as high initial investment costs for ammonia recovery systems and the need for skilled operators. Overcoming these barriers will be crucial for wider market adoption, particularly in developing regions where cost sensitivity is higher.
Current Challenges in Ammonia Stripping
Ammonia stripping processes face several significant challenges that hinder their efficiency and widespread adoption. One of the primary issues is the high energy consumption associated with the process. The stripping of ammonia from wastewater typically requires elevated temperatures, often ranging from 60°C to 80°C, which necessitates substantial energy input. This energy demand not only increases operational costs but also raises environmental concerns due to the associated carbon footprint.
Another challenge lies in the pH control of the stripping process. Effective ammonia removal requires maintaining an alkaline pH, typically above 10.5, to shift the ammonia-ammonium equilibrium towards free ammonia. However, precise pH control can be difficult to achieve and maintain, especially in large-scale operations or when dealing with variable wastewater compositions. Fluctuations in pH can significantly impact the stripping efficiency and lead to inconsistent treatment results.
The presence of interfering compounds in wastewater streams poses additional complications. Organic matter, suspended solids, and other contaminants can interfere with the stripping process, reducing its effectiveness and potentially causing fouling or scaling of equipment. This issue is particularly pronounced in industrial wastewaters with complex compositions, necessitating additional pretreatment steps that further increase process complexity and costs.
Ammonia recovery and utilization present another set of challenges. While stripped ammonia can be potentially recovered as a valuable resource, the concentration and purity of the recovered ammonia often fall short of industrial standards. This limitation hampers the economic viability of ammonia recovery and reuse schemes, despite their potential environmental benefits.
The design and optimization of stripping columns also remain challenging. Factors such as gas-liquid contact time, mass transfer efficiency, and column height must be carefully balanced to achieve optimal performance. However, scaling up laboratory results to industrial-scale operations often reveals unforeseen issues, leading to suboptimal performance in real-world applications.
Lastly, the management of off-gases from the stripping process presents environmental and safety concerns. The ammonia-rich gas stream produced during stripping must be properly treated to prevent air pollution and ensure worker safety. This often requires additional treatment steps, such as acid scrubbing or thermal oxidation, which add to the overall process complexity and cost.
Another challenge lies in the pH control of the stripping process. Effective ammonia removal requires maintaining an alkaline pH, typically above 10.5, to shift the ammonia-ammonium equilibrium towards free ammonia. However, precise pH control can be difficult to achieve and maintain, especially in large-scale operations or when dealing with variable wastewater compositions. Fluctuations in pH can significantly impact the stripping efficiency and lead to inconsistent treatment results.
The presence of interfering compounds in wastewater streams poses additional complications. Organic matter, suspended solids, and other contaminants can interfere with the stripping process, reducing its effectiveness and potentially causing fouling or scaling of equipment. This issue is particularly pronounced in industrial wastewaters with complex compositions, necessitating additional pretreatment steps that further increase process complexity and costs.
Ammonia recovery and utilization present another set of challenges. While stripped ammonia can be potentially recovered as a valuable resource, the concentration and purity of the recovered ammonia often fall short of industrial standards. This limitation hampers the economic viability of ammonia recovery and reuse schemes, despite their potential environmental benefits.
The design and optimization of stripping columns also remain challenging. Factors such as gas-liquid contact time, mass transfer efficiency, and column height must be carefully balanced to achieve optimal performance. However, scaling up laboratory results to industrial-scale operations often reveals unforeseen issues, leading to suboptimal performance in real-world applications.
Lastly, the management of off-gases from the stripping process presents environmental and safety concerns. The ammonia-rich gas stream produced during stripping must be properly treated to prevent air pollution and ensure worker safety. This often requires additional treatment steps, such as acid scrubbing or thermal oxidation, which add to the overall process complexity and cost.
Existing Ammonia Stripping Solutions
01 Use in chemical processes
Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it useful for neutralizing acids and controlling pH levels in different applications.- Use in chemical processes: Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the production of different compounds and materials, including polymers, pharmaceuticals, and industrial chemicals. Its alkaline properties make it useful for neutralization reactions and as a cleaning agent in industrial applications.
- Application in wastewater treatment: Ammonium hydroxide is utilized in wastewater treatment processes for pH adjustment and nitrogen removal. It can help neutralize acidic effluents and promote the growth of beneficial microorganisms in biological treatment systems. Additionally, it may be used in the precipitation of heavy metals from industrial wastewater.
- Role in agricultural and fertilizer production: Ammonium hydroxide serves as a key component in the manufacturing of fertilizers and agricultural chemicals. It provides a source of nitrogen for plant growth and can be used to adjust soil pH. In some formulations, it may be combined with other nutrients to create balanced fertilizer blends for various crops.
- Use in cleaning and surface treatment: Ammonium hydroxide is employed in cleaning formulations for household and industrial purposes. Its alkaline nature makes it effective in removing grease, oils, and other organic contaminants. It is also used in surface treatment processes, such as etching and texturing of materials in the semiconductor and electronics industries.
- Application in textile and dyeing processes: Ammonium hydroxide finds applications in the textile industry, particularly in dyeing and finishing processes. It can be used as a pH regulator in dye baths, helping to improve color fastness and uniformity. Additionally, it may be employed in the treatment of natural fibers to enhance their properties or in the production of synthetic fibers.
02 Application in cleaning and surface treatment
Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. Its ability to dissolve certain metals and oxides makes it valuable in metal surface treatment and electroplating applications.Expand Specific Solutions03 Role in textile and leather processing
In the textile and leather industries, ammonium hydroxide serves multiple purposes. It is used in dyeing processes to adjust pH levels and improve color fastness. In leather tanning, it helps in dehairing and preparing hides for further treatment. Its alkaline nature aids in breaking down proteins and fats in these materials, enhancing their quality and appearance.Expand Specific Solutions04 Environmental and agricultural applications
Ammonium hydroxide finds applications in environmental protection and agriculture. It is used in flue gas treatment to reduce nitrogen oxide emissions from power plants. In agriculture, it serves as a source of nitrogen for fertilizers and can be directly applied to soil to increase nitrogen content. Its ability to neutralize acidic soils makes it valuable in soil treatment and crop management.Expand Specific Solutions05 Use in pharmaceutical and personal care products
Ammonium hydroxide is utilized in the pharmaceutical and personal care industries. It serves as a pH adjuster in various formulations, including medications, shampoos, and cosmetics. Its ability to neutralize acids makes it useful in antacid preparations. In hair care products, it can help in adjusting the pH of hair dyes and perming solutions to achieve desired results.Expand Specific Solutions
Key Industry Players in Ammonia Recovery
The ammonia stripping process utilizing ammonium hydroxide is in a mature stage of development, with a well-established market and proven technologies. The global market for this technology is substantial, driven by increasing environmental regulations and the need for efficient wastewater treatment solutions. Key players in this field include industry giants like China Petroleum & Chemical Corp., DuPont de Nemours, Inc., and Air Liquide SA, as well as specialized firms such as Ammonia Casale SpA and Nijhuis Water Technology BV. These companies have demonstrated advanced technical capabilities and commercial success in implementing ammonia stripping processes. The technology's maturity is further evidenced by ongoing research and development efforts from academic institutions like Beijing University of Chemical Technology and the University of Kentucky Research Foundation, focusing on optimizing efficiency and exploring new applications.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an advanced ammonia stripping process using ammonium hydroxide for efficient nitrogen removal from wastewater. Their technology employs a multi-stage stripping tower with optimized pH control and temperature management. The process achieves over 95% ammonia removal efficiency [1] by maintaining the pH between 10.5-11.5 and temperatures around 60-70°C. Sinopec's system incorporates a novel gas-liquid contactor design that enhances mass transfer and reduces energy consumption. Additionally, they have implemented a closed-loop ammonia recovery system, which captures and concentrates the stripped ammonia for potential reuse in fertilizer production [3].
Strengths: High ammonia removal efficiency, energy-efficient design, and potential for ammonia recovery and reuse. Weaknesses: May require significant initial capital investment and ongoing maintenance for the complex multi-stage system.
Ammonia Casale SpA
Technical Solution: Ammonia Casale SpA has pioneered a cutting-edge ammonia stripping technology that utilizes ammonium hydroxide in a highly efficient manner. Their process employs a proprietary structured packing material in the stripping column, which significantly increases the surface area for gas-liquid contact. This innovation has led to a 30% reduction in column height compared to conventional designs [2]. The company's technology also incorporates an advanced process control system that dynamically adjusts operating parameters based on real-time monitoring of influent characteristics. Ammonia Casale's process achieves ammonia removal rates of up to 99% while minimizing chemical consumption through precise dosing of ammonium hydroxide [4]. Furthermore, they have developed a heat integration scheme that recovers thermal energy from the stripping process, reducing overall energy requirements by up to 25% [5].
Strengths: Exceptional ammonia removal efficiency, compact design, and energy-efficient operation. Weaknesses: May require specialized maintenance and operator training due to the advanced control systems and proprietary packing material.
Innovations in Ammonium Hydroxide Use
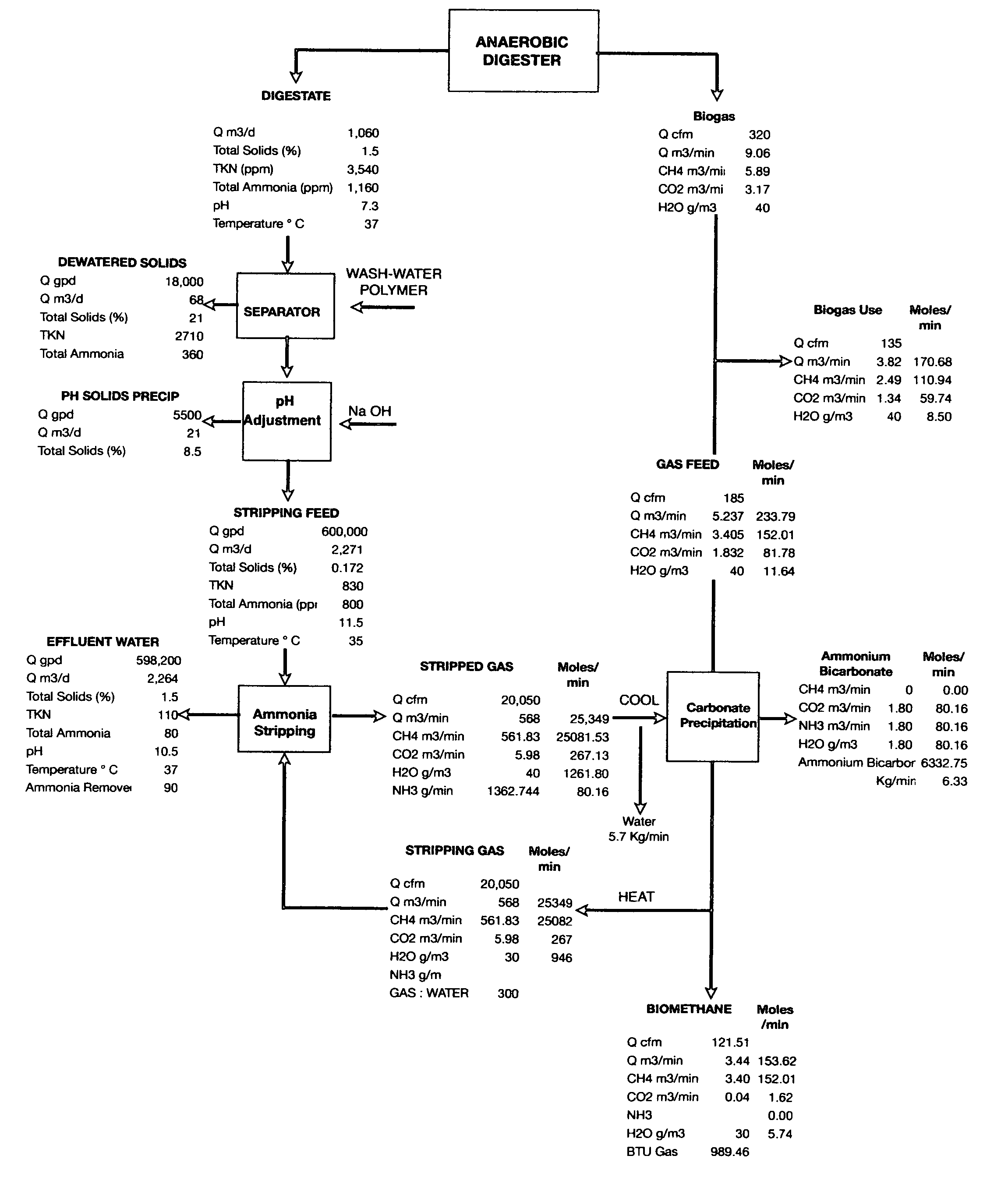
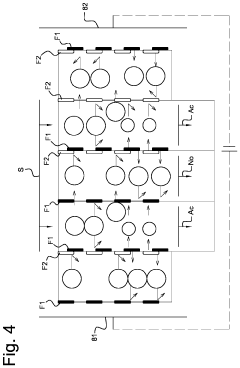
Removal of ammonia from fermentation effluent and sequestration as ammonium bicarbonate and/or carbonate
PatentInactiveUS7811455B2
Innovation
- A low-pressure, low-temperature process that strips ammonia from anaerobic digestion effluent using a gas deficient in CO2 and ammonia, followed by pH adjustment and precipitation to form ammonium bicarbonate/carbonate, which is then recovered as a solid product, improving biogas quality and reducing environmental impact.
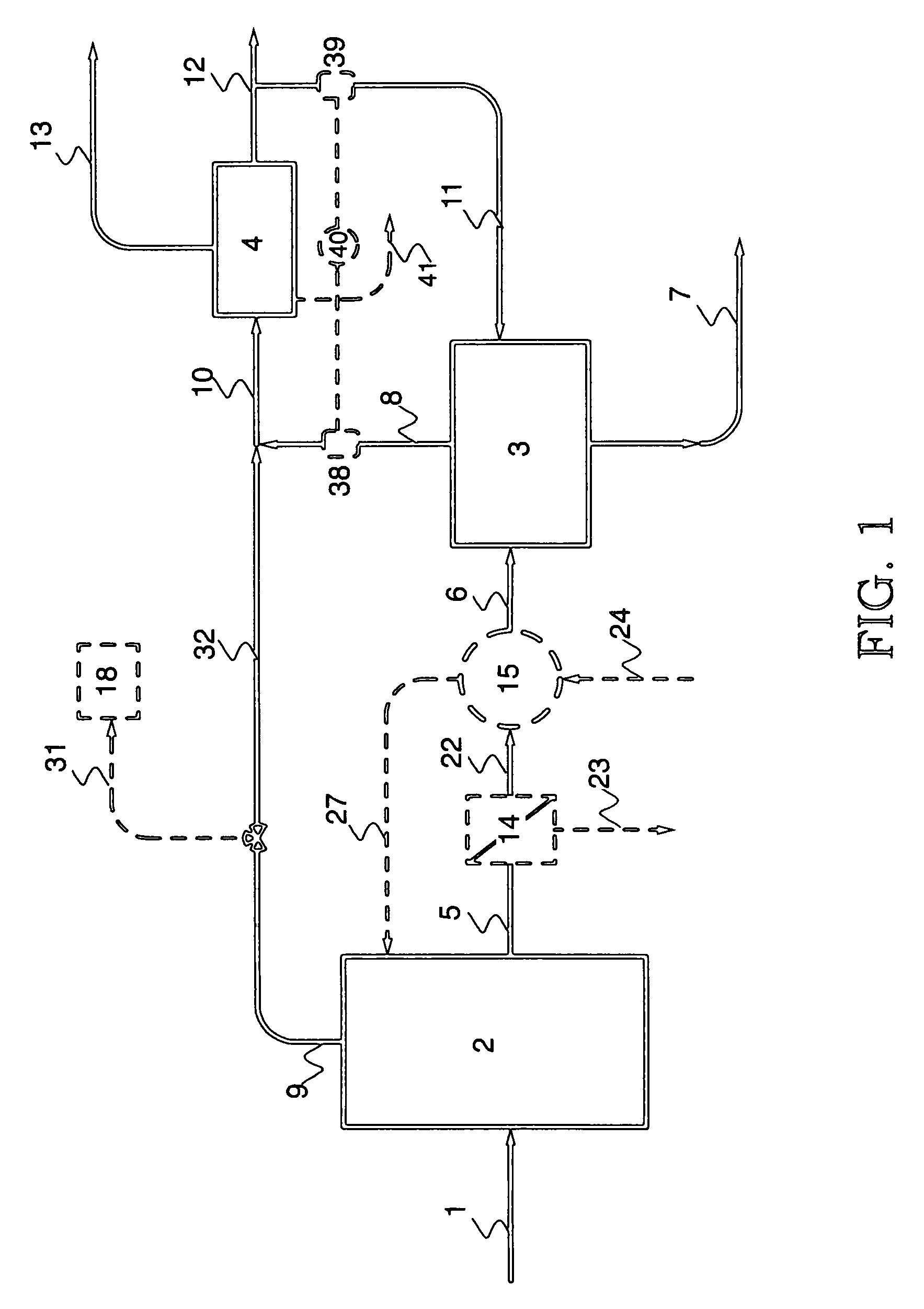
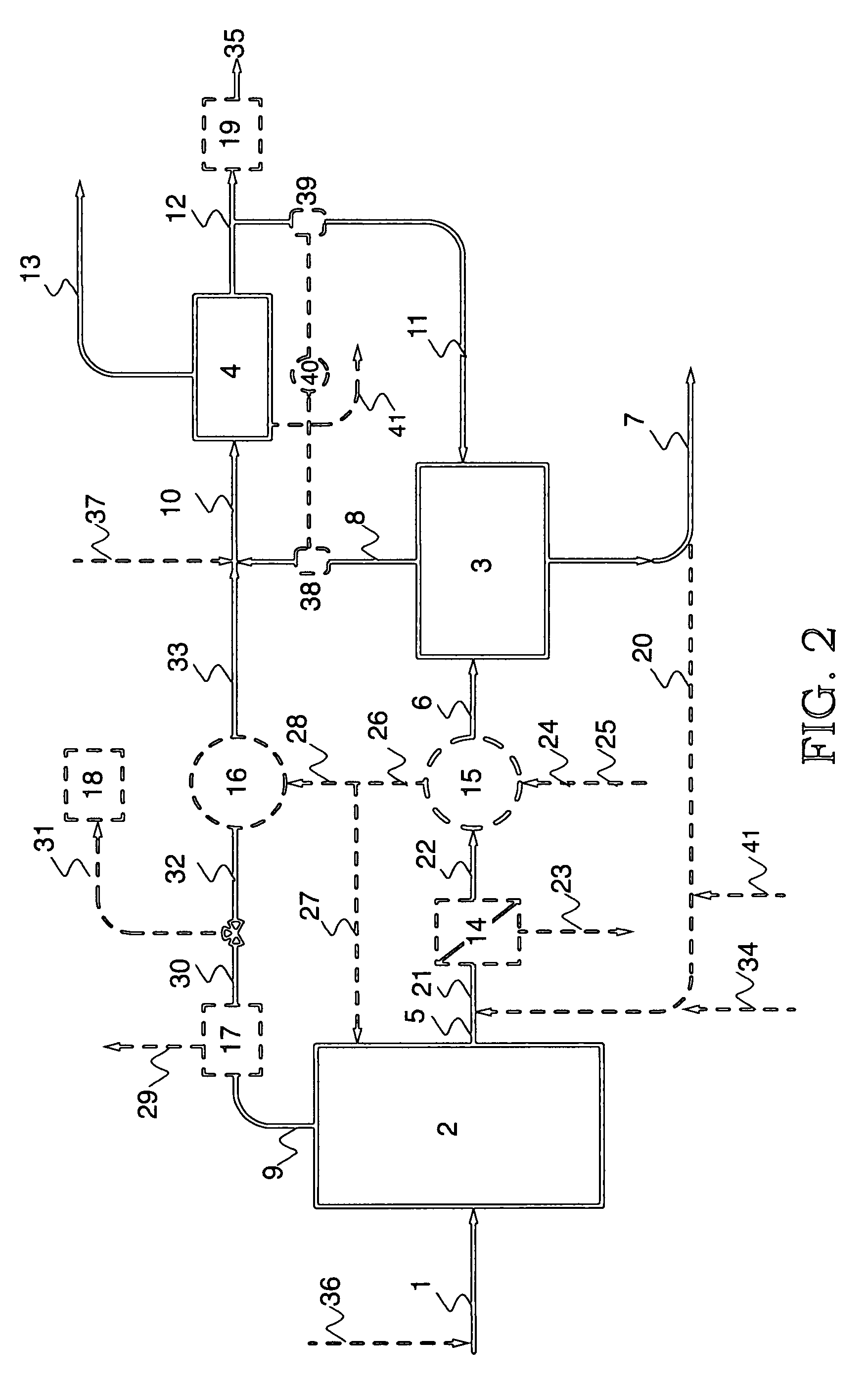
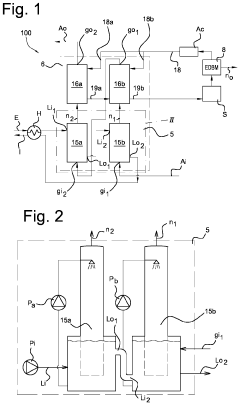
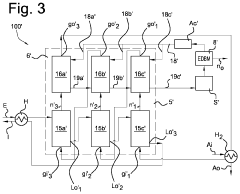
A system for removing ammonia from an ammonia-containing effluent, and method for the same.
PatentPendingUS20240132376A1
Innovation
- A system comprising multiple stripper and scrubber units in series connection, with counter-current gas and liquid flow, and a bipolar membrane electrodialysis element for acid recycling, reduces energy consumption and chemical use by optimizing ammonia stripping and pH conditions, and reusing heat and acid.
Environmental Impact Assessment
The environmental impact assessment of ammonium hydroxide's role in ammonia stripping processes is crucial for understanding the broader implications of this technology. Ammonia stripping, while effective for removing ammonia from wastewater, can have both positive and negative environmental consequences.
One of the primary environmental benefits of ammonia stripping using ammonium hydroxide is the reduction of ammonia levels in wastewater effluents. This process helps prevent the release of excess ammonia into aquatic ecosystems, which can be harmful to fish and other aquatic life. By effectively removing ammonia, the process contributes to improved water quality in receiving water bodies and helps maintain ecological balance.
However, the use of ammonium hydroxide in ammonia stripping processes also presents potential environmental challenges. The production and transportation of ammonium hydroxide require energy and resources, contributing to carbon emissions and potential environmental risks associated with chemical handling and storage. Additionally, the ammonia removed during the stripping process needs to be properly managed to prevent its release into the atmosphere, as ammonia is a potent greenhouse gas and can contribute to air pollution.
The energy consumption of ammonia stripping processes is another important consideration in the environmental impact assessment. The process typically requires heating and aeration, which can be energy-intensive. The source of this energy (renewable vs. non-renewable) plays a significant role in determining the overall carbon footprint of the technology. Implementing energy-efficient designs and utilizing renewable energy sources can help mitigate these impacts.
Water usage is also a critical factor to consider. While ammonia stripping helps improve water quality, the process itself may require significant amounts of water, particularly in the form of makeup water to replace losses due to evaporation. In water-stressed regions, this could pose challenges and necessitate the implementation of water conservation measures or the use of alternative water sources.
The potential for air emissions during the stripping process must be carefully evaluated. Proper design and operation of air pollution control systems are essential to minimize the release of ammonia and other volatile compounds into the atmosphere. This includes the use of efficient scrubbers and proper stack design to ensure compliance with air quality regulations.
Lastly, the environmental impact assessment should consider the fate of the recovered ammonia. Ideally, the stripped ammonia should be captured and reused in beneficial applications, such as fertilizer production or industrial processes. This approach not only reduces waste but also promotes a circular economy model, turning a potential pollutant into a valuable resource.
In conclusion, while ammonium hydroxide-based ammonia stripping offers significant environmental benefits in terms of water quality improvement, a comprehensive assessment must account for energy use, air emissions, water consumption, and the lifecycle management of chemicals and byproducts to ensure a net positive environmental impact.
One of the primary environmental benefits of ammonia stripping using ammonium hydroxide is the reduction of ammonia levels in wastewater effluents. This process helps prevent the release of excess ammonia into aquatic ecosystems, which can be harmful to fish and other aquatic life. By effectively removing ammonia, the process contributes to improved water quality in receiving water bodies and helps maintain ecological balance.
However, the use of ammonium hydroxide in ammonia stripping processes also presents potential environmental challenges. The production and transportation of ammonium hydroxide require energy and resources, contributing to carbon emissions and potential environmental risks associated with chemical handling and storage. Additionally, the ammonia removed during the stripping process needs to be properly managed to prevent its release into the atmosphere, as ammonia is a potent greenhouse gas and can contribute to air pollution.
The energy consumption of ammonia stripping processes is another important consideration in the environmental impact assessment. The process typically requires heating and aeration, which can be energy-intensive. The source of this energy (renewable vs. non-renewable) plays a significant role in determining the overall carbon footprint of the technology. Implementing energy-efficient designs and utilizing renewable energy sources can help mitigate these impacts.
Water usage is also a critical factor to consider. While ammonia stripping helps improve water quality, the process itself may require significant amounts of water, particularly in the form of makeup water to replace losses due to evaporation. In water-stressed regions, this could pose challenges and necessitate the implementation of water conservation measures or the use of alternative water sources.
The potential for air emissions during the stripping process must be carefully evaluated. Proper design and operation of air pollution control systems are essential to minimize the release of ammonia and other volatile compounds into the atmosphere. This includes the use of efficient scrubbers and proper stack design to ensure compliance with air quality regulations.
Lastly, the environmental impact assessment should consider the fate of the recovered ammonia. Ideally, the stripped ammonia should be captured and reused in beneficial applications, such as fertilizer production or industrial processes. This approach not only reduces waste but also promotes a circular economy model, turning a potential pollutant into a valuable resource.
In conclusion, while ammonium hydroxide-based ammonia stripping offers significant environmental benefits in terms of water quality improvement, a comprehensive assessment must account for energy use, air emissions, water consumption, and the lifecycle management of chemicals and byproducts to ensure a net positive environmental impact.
Regulatory Framework for Ammonia Handling
The regulatory framework for ammonia handling is a critical aspect of ensuring safety and environmental protection in industries that utilize ammonia, including those employing ammonia stripping processes. These regulations are designed to address the potential hazards associated with ammonia, such as its toxicity, flammability, and corrosive properties.
At the federal level in the United States, the Occupational Safety and Health Administration (OSHA) sets standards for workplace safety related to ammonia handling. OSHA's Process Safety Management (PSM) standard (29 CFR 1910.119) applies to processes involving more than 10,000 pounds of anhydrous ammonia. This standard requires employers to develop and implement a comprehensive safety program that includes process hazard analysis, operating procedures, employee training, and incident investigation.
The Environmental Protection Agency (EPA) also plays a crucial role in regulating ammonia handling through various programs. The Risk Management Program (RMP) under the Clean Air Act requires facilities storing or handling large quantities of ammonia to develop and implement risk management plans. Additionally, the Emergency Planning and Community Right-to-Know Act (EPCRA) mandates reporting of ammonia releases and inventories to local and state emergency planning committees.
At the state level, regulations may vary but often build upon federal standards. For example, California's Division of Occupational Safety and Health (Cal/OSHA) has more stringent requirements for ammonia refrigeration systems than federal OSHA standards. Many states also have their own environmental agencies that enforce and sometimes expand upon EPA regulations.
International regulations, such as those set by the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) program, also impact global ammonia handling practices. These regulations often focus on the registration and assessment of chemical substances, including ammonia and its derivatives.
Industry-specific guidelines and best practices complement regulatory requirements. Organizations like the International Institute of Ammonia Refrigeration (IIAR) provide standards and guidelines for the safe use of ammonia in refrigeration systems, which can be applicable to ammonia stripping processes.
Compliance with these regulations typically involves implementing robust safety management systems, conducting regular risk assessments, maintaining proper documentation, and providing comprehensive employee training. Facilities must also have emergency response plans in place and coordinate with local emergency services.
As environmental concerns grow, regulations are evolving to address issues such as ammonia emissions and water pollution. This may lead to more stringent controls on ammonia stripping processes and increased emphasis on technologies that minimize environmental impact while maintaining operational efficiency.
At the federal level in the United States, the Occupational Safety and Health Administration (OSHA) sets standards for workplace safety related to ammonia handling. OSHA's Process Safety Management (PSM) standard (29 CFR 1910.119) applies to processes involving more than 10,000 pounds of anhydrous ammonia. This standard requires employers to develop and implement a comprehensive safety program that includes process hazard analysis, operating procedures, employee training, and incident investigation.
The Environmental Protection Agency (EPA) also plays a crucial role in regulating ammonia handling through various programs. The Risk Management Program (RMP) under the Clean Air Act requires facilities storing or handling large quantities of ammonia to develop and implement risk management plans. Additionally, the Emergency Planning and Community Right-to-Know Act (EPCRA) mandates reporting of ammonia releases and inventories to local and state emergency planning committees.
At the state level, regulations may vary but often build upon federal standards. For example, California's Division of Occupational Safety and Health (Cal/OSHA) has more stringent requirements for ammonia refrigeration systems than federal OSHA standards. Many states also have their own environmental agencies that enforce and sometimes expand upon EPA regulations.
International regulations, such as those set by the European Union's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) program, also impact global ammonia handling practices. These regulations often focus on the registration and assessment of chemical substances, including ammonia and its derivatives.
Industry-specific guidelines and best practices complement regulatory requirements. Organizations like the International Institute of Ammonia Refrigeration (IIAR) provide standards and guidelines for the safe use of ammonia in refrigeration systems, which can be applicable to ammonia stripping processes.
Compliance with these regulations typically involves implementing robust safety management systems, conducting regular risk assessments, maintaining proper documentation, and providing comprehensive employee training. Facilities must also have emergency response plans in place and coordinate with local emergency services.
As environmental concerns grow, regulations are evolving to address issues such as ammonia emissions and water pollution. This may lead to more stringent controls on ammonia stripping processes and increased emphasis on technologies that minimize environmental impact while maintaining operational efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!