Ammonium Hydroxide's Role in the Formation of Nanostructures
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonium Hydroxide Nanostructure Formation Background
Ammonium hydroxide has played a pivotal role in the formation of nanostructures, marking a significant milestone in the field of nanotechnology. This compound, with its unique chemical properties, has become an indispensable tool for researchers and engineers in the synthesis and manipulation of nanoscale materials.
The journey of ammonium hydroxide in nanostructure formation began in the late 20th century when scientists started exploring its potential in controlling the growth and morphology of various nanomaterials. Its ability to act as both a pH regulator and a complexing agent made it particularly attractive for use in wet chemical synthesis methods.
One of the earliest applications of ammonium hydroxide in nanostructure formation was in the synthesis of metal oxide nanoparticles. Researchers discovered that by carefully controlling the concentration of ammonium hydroxide in the reaction medium, they could influence the size, shape, and crystallinity of the resulting nanoparticles. This breakthrough opened up new avenues for tailoring the properties of nanomaterials for specific applications.
As the field of nanotechnology progressed, the role of ammonium hydroxide expanded beyond simple nanoparticle synthesis. It became a crucial component in the fabrication of more complex nanostructures, such as nanowires, nanotubes, and hierarchical nanoarchitectures. The compound's ability to modulate surface tension and interfacial energies proved instrumental in directing the self-assembly of these intricate structures.
In the realm of template-assisted synthesis, ammonium hydroxide emerged as a versatile etching agent. Its controlled use allowed for the selective removal of sacrificial templates, resulting in the creation of hollow and porous nanostructures with high surface areas and unique optical and catalytic properties.
The advent of green chemistry principles in the early 21st century further highlighted the importance of ammonium hydroxide in nanostructure formation. As a relatively benign and easily recyclable reagent, it aligned well with the growing emphasis on environmentally friendly synthesis methods. This led to increased research into ammonium hydroxide-based processes as alternatives to more hazardous or energy-intensive techniques.
Recent years have seen a surge in the application of ammonium hydroxide in the synthesis of two-dimensional nanomaterials, such as graphene and transition metal dichalcogenides. Its role in exfoliation processes and as a intercalating agent has been crucial in developing scalable production methods for these materials, which hold immense promise for next-generation electronics and energy storage devices.
As we look towards the future, the role of ammonium hydroxide in nanostructure formation continues to evolve. Ongoing research is exploring its potential in biomimetic synthesis, where it could help replicate complex natural nanostructures. Additionally, its integration with advanced characterization techniques is providing deeper insights into the mechanisms of nanostructure growth and assembly, paving the way for even more precise control over nanomaterial properties.
The journey of ammonium hydroxide in nanostructure formation began in the late 20th century when scientists started exploring its potential in controlling the growth and morphology of various nanomaterials. Its ability to act as both a pH regulator and a complexing agent made it particularly attractive for use in wet chemical synthesis methods.
One of the earliest applications of ammonium hydroxide in nanostructure formation was in the synthesis of metal oxide nanoparticles. Researchers discovered that by carefully controlling the concentration of ammonium hydroxide in the reaction medium, they could influence the size, shape, and crystallinity of the resulting nanoparticles. This breakthrough opened up new avenues for tailoring the properties of nanomaterials for specific applications.
As the field of nanotechnology progressed, the role of ammonium hydroxide expanded beyond simple nanoparticle synthesis. It became a crucial component in the fabrication of more complex nanostructures, such as nanowires, nanotubes, and hierarchical nanoarchitectures. The compound's ability to modulate surface tension and interfacial energies proved instrumental in directing the self-assembly of these intricate structures.
In the realm of template-assisted synthesis, ammonium hydroxide emerged as a versatile etching agent. Its controlled use allowed for the selective removal of sacrificial templates, resulting in the creation of hollow and porous nanostructures with high surface areas and unique optical and catalytic properties.
The advent of green chemistry principles in the early 21st century further highlighted the importance of ammonium hydroxide in nanostructure formation. As a relatively benign and easily recyclable reagent, it aligned well with the growing emphasis on environmentally friendly synthesis methods. This led to increased research into ammonium hydroxide-based processes as alternatives to more hazardous or energy-intensive techniques.
Recent years have seen a surge in the application of ammonium hydroxide in the synthesis of two-dimensional nanomaterials, such as graphene and transition metal dichalcogenides. Its role in exfoliation processes and as a intercalating agent has been crucial in developing scalable production methods for these materials, which hold immense promise for next-generation electronics and energy storage devices.
As we look towards the future, the role of ammonium hydroxide in nanostructure formation continues to evolve. Ongoing research is exploring its potential in biomimetic synthesis, where it could help replicate complex natural nanostructures. Additionally, its integration with advanced characterization techniques is providing deeper insights into the mechanisms of nanostructure growth and assembly, paving the way for even more precise control over nanomaterial properties.
Market Analysis for Nanostructure Applications
The market for nanostructure applications has been experiencing significant growth and diversification in recent years, driven by advancements in nanotechnology and increasing demand across various industries. Nanostructures, particularly those formed with the aid of ammonium hydroxide, have found applications in electronics, energy storage, healthcare, and environmental remediation.
In the electronics sector, nanostructures are being utilized to develop smaller, faster, and more efficient components. The miniaturization trend in consumer electronics and the push for more powerful computing devices have created a strong demand for nanostructure-based solutions. This market segment is expected to continue its rapid expansion as manufacturers seek to overcome the limitations of traditional semiconductor technologies.
The energy storage industry has also shown keen interest in nanostructure applications. Nanostructured materials are being incorporated into batteries and supercapacitors to enhance their performance, increase energy density, and improve charging speeds. As the global focus on renewable energy and electric vehicles intensifies, the demand for advanced energy storage solutions incorporating nanostructures is projected to grow substantially.
In healthcare, nanostructures are revolutionizing drug delivery systems, diagnostic tools, and tissue engineering. The ability to create precisely controlled nanostructures using ammonium hydroxide has opened up new possibilities for targeted drug delivery and improved biocompatibility of medical devices. This sector is anticipated to be a major driver of nanostructure market growth, especially as personalized medicine gains traction.
Environmental applications of nanostructures, particularly in water purification and air filtration, represent another rapidly expanding market segment. Nanostructured materials offer superior filtration capabilities and can be tailored for specific contaminants, making them highly effective in addressing environmental challenges.
The global market for nanostructure applications is characterized by intense research and development activities, with both established companies and startups vying for market share. North America and Europe currently lead in terms of market size, but Asia-Pacific is emerging as a significant player, driven by investments in nanotechnology research and manufacturing capabilities.
Despite the promising outlook, challenges remain in scaling up production of nanostructures and ensuring consistent quality. Regulatory considerations, particularly in healthcare applications, also play a crucial role in shaping market dynamics. As research continues to uncover new applications for nanostructures formed using ammonium hydroxide, the market is expected to evolve rapidly, presenting both opportunities and challenges for industry participants.
In the electronics sector, nanostructures are being utilized to develop smaller, faster, and more efficient components. The miniaturization trend in consumer electronics and the push for more powerful computing devices have created a strong demand for nanostructure-based solutions. This market segment is expected to continue its rapid expansion as manufacturers seek to overcome the limitations of traditional semiconductor technologies.
The energy storage industry has also shown keen interest in nanostructure applications. Nanostructured materials are being incorporated into batteries and supercapacitors to enhance their performance, increase energy density, and improve charging speeds. As the global focus on renewable energy and electric vehicles intensifies, the demand for advanced energy storage solutions incorporating nanostructures is projected to grow substantially.
In healthcare, nanostructures are revolutionizing drug delivery systems, diagnostic tools, and tissue engineering. The ability to create precisely controlled nanostructures using ammonium hydroxide has opened up new possibilities for targeted drug delivery and improved biocompatibility of medical devices. This sector is anticipated to be a major driver of nanostructure market growth, especially as personalized medicine gains traction.
Environmental applications of nanostructures, particularly in water purification and air filtration, represent another rapidly expanding market segment. Nanostructured materials offer superior filtration capabilities and can be tailored for specific contaminants, making them highly effective in addressing environmental challenges.
The global market for nanostructure applications is characterized by intense research and development activities, with both established companies and startups vying for market share. North America and Europe currently lead in terms of market size, but Asia-Pacific is emerging as a significant player, driven by investments in nanotechnology research and manufacturing capabilities.
Despite the promising outlook, challenges remain in scaling up production of nanostructures and ensuring consistent quality. Regulatory considerations, particularly in healthcare applications, also play a crucial role in shaping market dynamics. As research continues to uncover new applications for nanostructures formed using ammonium hydroxide, the market is expected to evolve rapidly, presenting both opportunities and challenges for industry participants.
Current Challenges in Ammonium Hydroxide-Based Synthesis
Despite the widespread use of ammonium hydroxide in nanostructure synthesis, several challenges persist in its application. One of the primary issues is the precise control of pH during the synthesis process. Ammonium hydroxide's volatility can lead to fluctuations in pH levels, affecting the nucleation and growth of nanostructures. This variability can result in inconsistent size distributions and morphologies of the final products, compromising their quality and reproducibility.
Another significant challenge is the potential for agglomeration of nanoparticles during synthesis. The high pH environment created by ammonium hydroxide can sometimes lead to rapid particle growth and subsequent aggregation, particularly in metal and metal oxide nanostructure synthesis. This agglomeration can negatively impact the desired properties of the nanostructures, such as their surface area and reactivity.
The scalability of ammonium hydroxide-based synthesis methods also presents a considerable hurdle. While these methods often work well at laboratory scales, translating them to industrial production levels can be problematic. Issues such as heat dissipation, mixing efficiency, and maintaining uniform reaction conditions become more pronounced at larger scales, potentially affecting the quality and consistency of the nanostructures produced.
Environmental and safety concerns associated with ammonium hydroxide usage pose additional challenges. The compound's strong odor and potential for ammonia gas release necessitate stringent safety measures and proper ventilation systems. Moreover, the disposal of ammonium hydroxide-containing waste requires careful consideration to minimize environmental impact, adding complexity to the overall synthesis process.
The formation of unwanted byproducts during ammonium hydroxide-based synthesis is another area of concern. These byproducts can interfere with the desired nanostructure formation, potentially altering their properties or requiring additional purification steps. This not only complicates the synthesis process but can also increase production costs and time.
Lastly, the long-term stability of nanostructures synthesized using ammonium hydroxide remains a challenge. Some nanostructures may undergo gradual changes in morphology or composition over time, particularly when exposed to different environmental conditions. This instability can limit the shelf life and applicability of the synthesized nanostructures in various applications, necessitating the development of stabilization strategies or alternative synthesis methods.
Another significant challenge is the potential for agglomeration of nanoparticles during synthesis. The high pH environment created by ammonium hydroxide can sometimes lead to rapid particle growth and subsequent aggregation, particularly in metal and metal oxide nanostructure synthesis. This agglomeration can negatively impact the desired properties of the nanostructures, such as their surface area and reactivity.
The scalability of ammonium hydroxide-based synthesis methods also presents a considerable hurdle. While these methods often work well at laboratory scales, translating them to industrial production levels can be problematic. Issues such as heat dissipation, mixing efficiency, and maintaining uniform reaction conditions become more pronounced at larger scales, potentially affecting the quality and consistency of the nanostructures produced.
Environmental and safety concerns associated with ammonium hydroxide usage pose additional challenges. The compound's strong odor and potential for ammonia gas release necessitate stringent safety measures and proper ventilation systems. Moreover, the disposal of ammonium hydroxide-containing waste requires careful consideration to minimize environmental impact, adding complexity to the overall synthesis process.
The formation of unwanted byproducts during ammonium hydroxide-based synthesis is another area of concern. These byproducts can interfere with the desired nanostructure formation, potentially altering their properties or requiring additional purification steps. This not only complicates the synthesis process but can also increase production costs and time.
Lastly, the long-term stability of nanostructures synthesized using ammonium hydroxide remains a challenge. Some nanostructures may undergo gradual changes in morphology or composition over time, particularly when exposed to different environmental conditions. This instability can limit the shelf life and applicability of the synthesized nanostructures in various applications, necessitating the development of stabilization strategies or alternative synthesis methods.
Existing Ammonium Hydroxide Synthesis Protocols
01 Synthesis of ammonium hydroxide nanostructures
Various methods are employed to synthesize ammonium hydroxide nanostructures, including chemical precipitation, sol-gel processes, and hydrothermal techniques. These methods allow for the controlled formation of nanoparticles, nanorods, or other nanostructures with specific morphologies and properties.- Synthesis of ammonium hydroxide nanostructures: Various methods are employed to synthesize ammonium hydroxide nanostructures, including chemical precipitation, sol-gel processes, and hydrothermal techniques. These methods allow for the controlled formation of nanoparticles, nanorods, or other nanostructures with specific morphologies and properties.
- Application in catalysis and chemical reactions: Ammonium hydroxide nanostructures are utilized as catalysts or catalyst supports in various chemical reactions. Their high surface area and unique properties enhance catalytic activity and selectivity, making them valuable in industrial processes and environmental applications.
- Use in material science and nanotechnology: Ammonium hydroxide nanostructures find applications in advanced materials and nanotechnology. They are used in the development of novel composites, coatings, and functional materials with enhanced properties such as improved mechanical strength, thermal stability, or electrical conductivity.
- Environmental and waste treatment applications: Ammonium hydroxide nanostructures are employed in environmental remediation and waste treatment processes. Their high adsorption capacity and reactivity make them effective in removing pollutants from water and air, as well as in the treatment of industrial effluents.
- Characterization and analysis techniques: Various analytical techniques are used to characterize ammonium hydroxide nanostructures, including electron microscopy, X-ray diffraction, and spectroscopic methods. These techniques help in determining the size, shape, composition, and properties of the nanostructures, which is crucial for optimizing their synthesis and applications.
02 Application in catalysis and chemical reactions
Ammonium hydroxide nanostructures are utilized as catalysts or catalyst supports in various chemical reactions. Their high surface area and unique properties enhance catalytic activity and selectivity, making them valuable in industrial processes and environmental applications.Expand Specific Solutions03 Use in material science and nanotechnology
Ammonium hydroxide nanostructures find applications in material science and nanotechnology. They are used in the development of advanced materials, nanocomposites, and functional coatings with improved mechanical, thermal, or electrical properties.Expand Specific Solutions04 Environmental and water treatment applications
Ammonium hydroxide nanostructures are employed in environmental remediation and water treatment processes. Their high adsorption capacity and reactivity make them effective in removing contaminants from water and air, as well as in the treatment of industrial effluents.Expand Specific Solutions05 Characterization and analysis techniques
Various analytical techniques are used to characterize ammonium hydroxide nanostructures, including electron microscopy, X-ray diffraction, and spectroscopic methods. These techniques help in understanding the morphology, composition, and properties of the nanostructures, which is crucial for optimizing their synthesis and applications.Expand Specific Solutions
Key Players in Nanostructure Manufacturing
The field of nanostructure formation using ammonium hydroxide is in a growth phase, with increasing research and commercial interest. The market size is expanding as applications in electronics, materials science, and biotechnology emerge. Technologically, it's progressing from basic research to applied development, with varying levels of maturity across different applications. Key players like BASF Corp., Massachusetts Institute of Technology, and Canon, Inc. are driving innovation, while academic institutions such as Rutgers State University and Emory University contribute fundamental research. Companies like Halliburton Energy Services and China Petroleum & Chemical Corp. are exploring industrial applications, indicating the technology's potential for diverse sectors. The involvement of both established corporations and research institutions suggests a competitive landscape with opportunities for breakthrough advancements.
BASF Corp.
Technical Solution: BASF Corp. has developed a novel approach for nanostructure formation using ammonium hydroxide as a key component. Their method involves a controlled precipitation process where ammonium hydroxide acts as a pH regulator and structure-directing agent[1]. By carefully adjusting the concentration of ammonium hydroxide, BASF researchers have achieved precise control over the size, shape, and composition of nanoparticles[3]. This technique has been particularly successful in synthesizing metal oxide nanostructures with high surface area and unique morphologies, such as hollow spheres and hierarchical structures[5]. The company has also integrated this approach into their scalable production processes, allowing for industrial-scale manufacture of nanostructured materials for various applications, including catalysis, energy storage, and advanced coatings[7].
Strengths: Precise control over nanostructure morphology, scalable production capabilities, and versatility in material composition. Weaknesses: Potential environmental concerns due to ammonia use and the need for careful process control to ensure consistency.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have pioneered a groundbreaking technique utilizing ammonium hydroxide for the synthesis of complex nanostructures. Their approach leverages the unique properties of ammonium hydroxide as both a base and a complexing agent to create intricate 3D nanoarchitectures[2]. By controlling the rate of ammonia release from ammonium hydroxide, they have developed a method to grow nanostructures with unprecedented levels of hierarchical organization[4]. This technique has been particularly effective in creating biomimetic nanostructures, such as those inspired by diatoms and other natural systems[6]. MIT's work has also focused on the use of ammonium hydroxide in template-free synthesis of hollow nanostructures, which have shown promise in applications ranging from drug delivery to energy storage[8]. Their research has significantly advanced the understanding of the role of ammonium hydroxide in nanostructure formation mechanisms.
Strengths: Cutting-edge research in complex nanoarchitectures, biomimetic approaches, and fundamental understanding of formation mechanisms. Weaknesses: Potential challenges in scaling up laboratory techniques to industrial production and the need for specialized equipment.
Core Innovations in Nanostructure Formation
A process for fabricating metal organic frameworks-sensitized nanostructured metal oxide-based hybrids for photoelectrochemical water splitting
PatentActiveIN202321020219A
Innovation
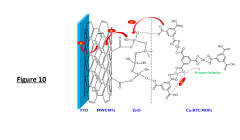
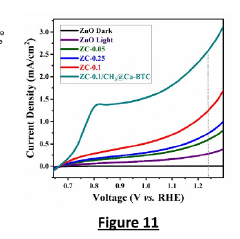
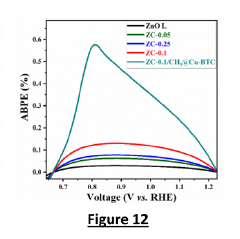
- The development of metal-organic frameworks (MOFs) sensitized nanostructured ZnO/CNs nanocomposites using a sonochemical assisted sol-gel technique and Cu-BTC MOFs, with methyl shielding to enhance stability and charge transfer, and a layer-by-layer sensitization method for improved photoanodes.
Surface treatment method for magnesium or its alloy substrate and nanostructure
PatentInactiveJPWO2011021571A1
Innovation
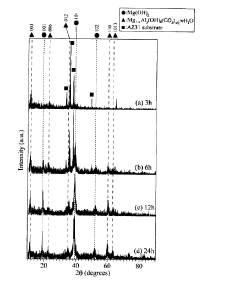
- A method involving immersion of magnesium or its alloy in pure water and controlled heating in a sealed container to grow magnesium nanostructures, without using chemicals, allowing for efficient and environmentally friendly formation of nanostructures with high adhesion and structural color variation.
Environmental Impact of Ammonium Hydroxide Use
The use of ammonium hydroxide in nanostructure formation processes raises significant environmental concerns that warrant careful consideration. As a widely used reagent in various nanomaterial synthesis methods, its environmental impact extends across multiple domains.
Aquatic ecosystems are particularly vulnerable to ammonium hydroxide contamination. When released into water bodies, it can lead to increased ammonia levels, potentially causing eutrophication and disrupting aquatic life. Fish and other aquatic organisms are highly sensitive to ammonia toxicity, which can result in population declines and ecosystem imbalances.
Air quality is another area of concern. Ammonium hydroxide can volatilize, releasing ammonia gas into the atmosphere. This contributes to the formation of particulate matter and can react with other air pollutants, exacerbating smog and acid rain problems. In urban areas with high industrial activity, these emissions may significantly impact local air quality and human health.
Soil contamination is a potential risk when ammonium hydroxide is improperly disposed of or accidentally released. It can alter soil pH and nitrogen content, affecting plant growth and soil microbial communities. This may lead to long-term changes in soil fertility and ecosystem functioning.
The production and transportation of ammonium hydroxide also carry environmental risks. Industrial accidents or spills during transport can result in localized environmental damage and pose health hazards to nearby communities. Additionally, the energy-intensive production process contributes to greenhouse gas emissions and resource depletion.
Waste management is a critical aspect of mitigating environmental impacts. Proper handling, storage, and disposal of ammonium hydroxide and related waste products are essential to prevent environmental contamination. This includes implementing effective treatment processes for wastewater containing ammonium hydroxide before release into the environment.
Regulatory frameworks play a crucial role in managing these environmental risks. Many countries have established guidelines and regulations for the use, handling, and disposal of ammonium hydroxide in industrial processes, including nanostructure formation. Compliance with these regulations is essential for minimizing environmental impact.
Research into alternative, more environmentally friendly reagents for nanostructure formation is ongoing. This includes exploring green chemistry approaches and developing novel synthesis methods that reduce or eliminate the need for ammonium hydroxide. Such innovations could significantly mitigate the environmental footprint of nanomaterial production in the future.
Aquatic ecosystems are particularly vulnerable to ammonium hydroxide contamination. When released into water bodies, it can lead to increased ammonia levels, potentially causing eutrophication and disrupting aquatic life. Fish and other aquatic organisms are highly sensitive to ammonia toxicity, which can result in population declines and ecosystem imbalances.
Air quality is another area of concern. Ammonium hydroxide can volatilize, releasing ammonia gas into the atmosphere. This contributes to the formation of particulate matter and can react with other air pollutants, exacerbating smog and acid rain problems. In urban areas with high industrial activity, these emissions may significantly impact local air quality and human health.
Soil contamination is a potential risk when ammonium hydroxide is improperly disposed of or accidentally released. It can alter soil pH and nitrogen content, affecting plant growth and soil microbial communities. This may lead to long-term changes in soil fertility and ecosystem functioning.
The production and transportation of ammonium hydroxide also carry environmental risks. Industrial accidents or spills during transport can result in localized environmental damage and pose health hazards to nearby communities. Additionally, the energy-intensive production process contributes to greenhouse gas emissions and resource depletion.
Waste management is a critical aspect of mitigating environmental impacts. Proper handling, storage, and disposal of ammonium hydroxide and related waste products are essential to prevent environmental contamination. This includes implementing effective treatment processes for wastewater containing ammonium hydroxide before release into the environment.
Regulatory frameworks play a crucial role in managing these environmental risks. Many countries have established guidelines and regulations for the use, handling, and disposal of ammonium hydroxide in industrial processes, including nanostructure formation. Compliance with these regulations is essential for minimizing environmental impact.
Research into alternative, more environmentally friendly reagents for nanostructure formation is ongoing. This includes exploring green chemistry approaches and developing novel synthesis methods that reduce or eliminate the need for ammonium hydroxide. Such innovations could significantly mitigate the environmental footprint of nanomaterial production in the future.
Scale-up Strategies for Industrial Production
Scaling up the production of nanostructures using ammonium hydroxide presents both opportunities and challenges for industrial applications. The transition from laboratory-scale synthesis to large-scale manufacturing requires careful consideration of several key factors to ensure consistent quality, cost-effectiveness, and safety.
One of the primary strategies for scaling up production involves the optimization of reactor design. Continuous flow reactors have shown promise in maintaining precise control over reaction conditions, such as temperature and concentration gradients, which are critical for the formation of uniform nanostructures. These reactors allow for better heat and mass transfer, reducing the risk of agglomeration and ensuring consistent product quality across larger volumes.
Process intensification techniques can significantly enhance production efficiency. This may include the use of microfluidic devices or ultrasonic-assisted synthesis methods, which can accelerate reaction rates and improve uniformity in nanostructure formation. Such approaches can lead to reduced reaction times and increased throughput without compromising product quality.
The development of in-line monitoring and quality control systems is crucial for maintaining consistent nanostructure properties during scale-up. Real-time analysis techniques, such as dynamic light scattering or UV-Vis spectroscopy, can provide immediate feedback on particle size and distribution, allowing for rapid adjustments to process parameters as needed.
Addressing safety concerns is paramount when scaling up processes involving ammonium hydroxide. Implementation of closed-loop systems and advanced ventilation can minimize worker exposure to ammonia fumes. Additionally, the use of automated handling and dosing systems can reduce the risk of human error and improve overall safety.
Recycling and waste management strategies must be integrated into the scaled-up process to enhance sustainability and reduce environmental impact. This may involve the development of efficient ammonia recovery systems or the exploration of alternative, more environmentally friendly alkaline agents that can replicate the role of ammonium hydroxide in nanostructure formation.
Cost considerations play a significant role in scale-up strategies. Optimizing raw material usage, exploring bulk purchasing options for precursors, and improving energy efficiency in heating and mixing processes can help maintain economic viability at larger scales. The potential for continuous production methods to reduce labor costs and increase equipment utilization should also be evaluated.
Lastly, the development of modular and flexible production systems can facilitate easier scale-up and allow for rapid adaptation to changing market demands. This approach enables manufacturers to incrementally increase production capacity while minimizing initial capital investment risks.
One of the primary strategies for scaling up production involves the optimization of reactor design. Continuous flow reactors have shown promise in maintaining precise control over reaction conditions, such as temperature and concentration gradients, which are critical for the formation of uniform nanostructures. These reactors allow for better heat and mass transfer, reducing the risk of agglomeration and ensuring consistent product quality across larger volumes.
Process intensification techniques can significantly enhance production efficiency. This may include the use of microfluidic devices or ultrasonic-assisted synthesis methods, which can accelerate reaction rates and improve uniformity in nanostructure formation. Such approaches can lead to reduced reaction times and increased throughput without compromising product quality.
The development of in-line monitoring and quality control systems is crucial for maintaining consistent nanostructure properties during scale-up. Real-time analysis techniques, such as dynamic light scattering or UV-Vis spectroscopy, can provide immediate feedback on particle size and distribution, allowing for rapid adjustments to process parameters as needed.
Addressing safety concerns is paramount when scaling up processes involving ammonium hydroxide. Implementation of closed-loop systems and advanced ventilation can minimize worker exposure to ammonia fumes. Additionally, the use of automated handling and dosing systems can reduce the risk of human error and improve overall safety.
Recycling and waste management strategies must be integrated into the scaled-up process to enhance sustainability and reduce environmental impact. This may involve the development of efficient ammonia recovery systems or the exploration of alternative, more environmentally friendly alkaline agents that can replicate the role of ammonium hydroxide in nanostructure formation.
Cost considerations play a significant role in scale-up strategies. Optimizing raw material usage, exploring bulk purchasing options for precursors, and improving energy efficiency in heating and mixing processes can help maintain economic viability at larger scales. The potential for continuous production methods to reduce labor costs and increase equipment utilization should also be evaluated.
Lastly, the development of modular and flexible production systems can facilitate easier scale-up and allow for rapid adaptation to changing market demands. This approach enables manufacturers to incrementally increase production capacity while minimizing initial capital investment risks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!