Analysis of Structural Transformations in Fluoride Cathodes during Cycling
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoride Cathode Evolution Background and Objectives
Fluoride-based cathode materials have emerged as promising candidates for next-generation energy storage systems due to their theoretical high energy density, which significantly exceeds that of conventional lithium-ion batteries. The evolution of fluoride cathode research can be traced back to the early 2000s when researchers began exploring metal fluorides as conversion-type electrode materials. Initial studies focused primarily on transition metal fluorides such as FeF3, CoF2, and CuF2, which demonstrated impressive theoretical capacities but suffered from severe practical limitations.
The technological trajectory has been marked by significant breakthroughs in addressing the inherent challenges of fluoride-based systems. Early fluoride cathodes exhibited poor electronic conductivity, substantial volume changes during cycling, and slow reaction kinetics, resulting in rapid capacity fading and limited cycle life. These limitations prompted intensive research efforts aimed at understanding the fundamental mechanisms governing structural transformations during charge-discharge processes.
Recent advancements have been driven by sophisticated characterization techniques, including in-situ X-ray diffraction (XRD), transmission electron microscopy (TEM), and X-ray absorption spectroscopy (XAS), which have enabled researchers to observe and analyze structural changes in real-time. These insights have been crucial in developing strategies to mitigate degradation mechanisms and enhance electrochemical performance.
The current technological landscape is characterized by a shift towards nanostructured fluoride materials, core-shell architectures, and composite electrodes that combine fluorides with conductive additives. These approaches have shown promise in addressing the conductivity limitations and structural instability issues that have historically plagued fluoride cathodes.
The primary objective of this technical research is to comprehensively analyze the structural transformations that occur in fluoride cathodes during electrochemical cycling. This includes identifying the phase transitions, morphological changes, and interfacial phenomena that influence performance metrics such as capacity retention, rate capability, and cycle life.
Additionally, this research aims to establish correlations between structural evolution and electrochemical behavior, develop predictive models for degradation mechanisms, and propose design principles for next-generation fluoride cathodes with enhanced stability. Understanding these transformation processes is fundamental to overcoming the current limitations and realizing the full potential of fluoride-based energy storage technologies.
The ultimate goal is to accelerate the development of commercially viable fluoride cathode materials that can deliver high energy density, long cycle life, and safe operation for applications ranging from portable electronics to electric vehicles and grid-scale energy storage systems.
The technological trajectory has been marked by significant breakthroughs in addressing the inherent challenges of fluoride-based systems. Early fluoride cathodes exhibited poor electronic conductivity, substantial volume changes during cycling, and slow reaction kinetics, resulting in rapid capacity fading and limited cycle life. These limitations prompted intensive research efforts aimed at understanding the fundamental mechanisms governing structural transformations during charge-discharge processes.
Recent advancements have been driven by sophisticated characterization techniques, including in-situ X-ray diffraction (XRD), transmission electron microscopy (TEM), and X-ray absorption spectroscopy (XAS), which have enabled researchers to observe and analyze structural changes in real-time. These insights have been crucial in developing strategies to mitigate degradation mechanisms and enhance electrochemical performance.
The current technological landscape is characterized by a shift towards nanostructured fluoride materials, core-shell architectures, and composite electrodes that combine fluorides with conductive additives. These approaches have shown promise in addressing the conductivity limitations and structural instability issues that have historically plagued fluoride cathodes.
The primary objective of this technical research is to comprehensively analyze the structural transformations that occur in fluoride cathodes during electrochemical cycling. This includes identifying the phase transitions, morphological changes, and interfacial phenomena that influence performance metrics such as capacity retention, rate capability, and cycle life.
Additionally, this research aims to establish correlations between structural evolution and electrochemical behavior, develop predictive models for degradation mechanisms, and propose design principles for next-generation fluoride cathodes with enhanced stability. Understanding these transformation processes is fundamental to overcoming the current limitations and realizing the full potential of fluoride-based energy storage technologies.
The ultimate goal is to accelerate the development of commercially viable fluoride cathode materials that can deliver high energy density, long cycle life, and safe operation for applications ranging from portable electronics to electric vehicles and grid-scale energy storage systems.
Market Analysis for Advanced Battery Technologies
The global advanced battery market is experiencing unprecedented growth, driven by the increasing demand for electric vehicles, renewable energy storage systems, and portable electronics. Within this landscape, fluoride-based cathode materials represent a promising frontier due to their theoretical high energy density capabilities. Current market valuations place the advanced battery sector at approximately $92 billion in 2023, with projections indicating a compound annual growth rate of 16.8% through 2030.
Fluoride cathode technologies, while still predominantly in research phases, are attracting significant investment attention due to their potential to overcome the energy density limitations of current lithium-ion batteries. Market analysis indicates that if successfully commercialized, fluoride-based batteries could capture up to 15% of the premium battery market within the next decade, particularly in applications requiring high energy density such as long-range electric vehicles and aerospace.
Consumer demand patterns show increasing preference for batteries with higher energy density, longer cycle life, and improved safety profiles - all potential advantages of fluoride cathode technologies. The market pull is particularly strong from the automotive sector, where range anxiety remains a significant barrier to electric vehicle adoption. Survey data indicates that 78% of potential EV buyers consider battery range as their primary concern, creating a clear market opportunity for advanced cathode materials.
Regional market analysis reveals that Asia-Pacific currently dominates battery manufacturing, with China, Japan, and South Korea collectively accounting for over 70% of global production capacity. However, significant investments in North America and Europe aim to reduce dependency on Asian suppliers, creating new market opportunities for innovative battery technologies including fluoride-based systems.
The competitive landscape shows traditional battery manufacturers increasingly partnering with materials science companies and academic institutions to accelerate the development of next-generation cathode materials. Venture capital funding for advanced battery startups reached $8.5 billion in 2022, with approximately $1.2 billion specifically allocated to novel cathode material development projects.
Market barriers for fluoride cathode commercialization include manufacturing scalability challenges, integration with existing battery production infrastructure, and competition from other emerging technologies such as solid-state batteries. Cost sensitivity analysis suggests that fluoride cathode batteries would need to achieve production costs below $100/kWh to be commercially competitive in mass-market applications.
Customer segmentation analysis identifies early adoption potential in premium electric vehicles, aerospace, defense applications, and specialized industrial equipment where performance advantages could justify higher initial costs during the technology's early commercialization phase.
Fluoride cathode technologies, while still predominantly in research phases, are attracting significant investment attention due to their potential to overcome the energy density limitations of current lithium-ion batteries. Market analysis indicates that if successfully commercialized, fluoride-based batteries could capture up to 15% of the premium battery market within the next decade, particularly in applications requiring high energy density such as long-range electric vehicles and aerospace.
Consumer demand patterns show increasing preference for batteries with higher energy density, longer cycle life, and improved safety profiles - all potential advantages of fluoride cathode technologies. The market pull is particularly strong from the automotive sector, where range anxiety remains a significant barrier to electric vehicle adoption. Survey data indicates that 78% of potential EV buyers consider battery range as their primary concern, creating a clear market opportunity for advanced cathode materials.
Regional market analysis reveals that Asia-Pacific currently dominates battery manufacturing, with China, Japan, and South Korea collectively accounting for over 70% of global production capacity. However, significant investments in North America and Europe aim to reduce dependency on Asian suppliers, creating new market opportunities for innovative battery technologies including fluoride-based systems.
The competitive landscape shows traditional battery manufacturers increasingly partnering with materials science companies and academic institutions to accelerate the development of next-generation cathode materials. Venture capital funding for advanced battery startups reached $8.5 billion in 2022, with approximately $1.2 billion specifically allocated to novel cathode material development projects.
Market barriers for fluoride cathode commercialization include manufacturing scalability challenges, integration with existing battery production infrastructure, and competition from other emerging technologies such as solid-state batteries. Cost sensitivity analysis suggests that fluoride cathode batteries would need to achieve production costs below $100/kWh to be commercially competitive in mass-market applications.
Customer segmentation analysis identifies early adoption potential in premium electric vehicles, aerospace, defense applications, and specialized industrial equipment where performance advantages could justify higher initial costs during the technology's early commercialization phase.
Current Challenges in Fluoride Cathode Stability
Fluoride cathodes represent a promising frontier in battery technology due to their high theoretical energy density, which significantly exceeds that of conventional lithium-ion batteries. However, these materials face substantial stability challenges during cycling that currently limit their practical implementation. The primary issue lies in the structural degradation that occurs during the charge-discharge process, where fluoride ions are repeatedly extracted and inserted into the cathode material.
One of the most significant challenges is the volume change that occurs during cycling. As fluoride ions are removed from the cathode structure during charging, the material undergoes substantial contraction. Conversely, during discharge, the reintroduction of fluoride ions causes expansion. These repeated volume fluctuations induce mechanical stress that can lead to microcracking, particle isolation, and ultimately capacity fading over multiple cycles.
The high reactivity of fluoride ions presents another critical challenge. Unlike lithium-ion systems, fluoride ions are highly electronegative and form strong bonds with host atoms in the cathode material. This strong bonding often results in poor reversibility of the fluorination/defluorination process, leading to incomplete reactions and capacity loss. Additionally, side reactions between the electrolyte and the highly reactive fluoride species can form insulating layers on the cathode surface, increasing internal resistance and further degrading performance.
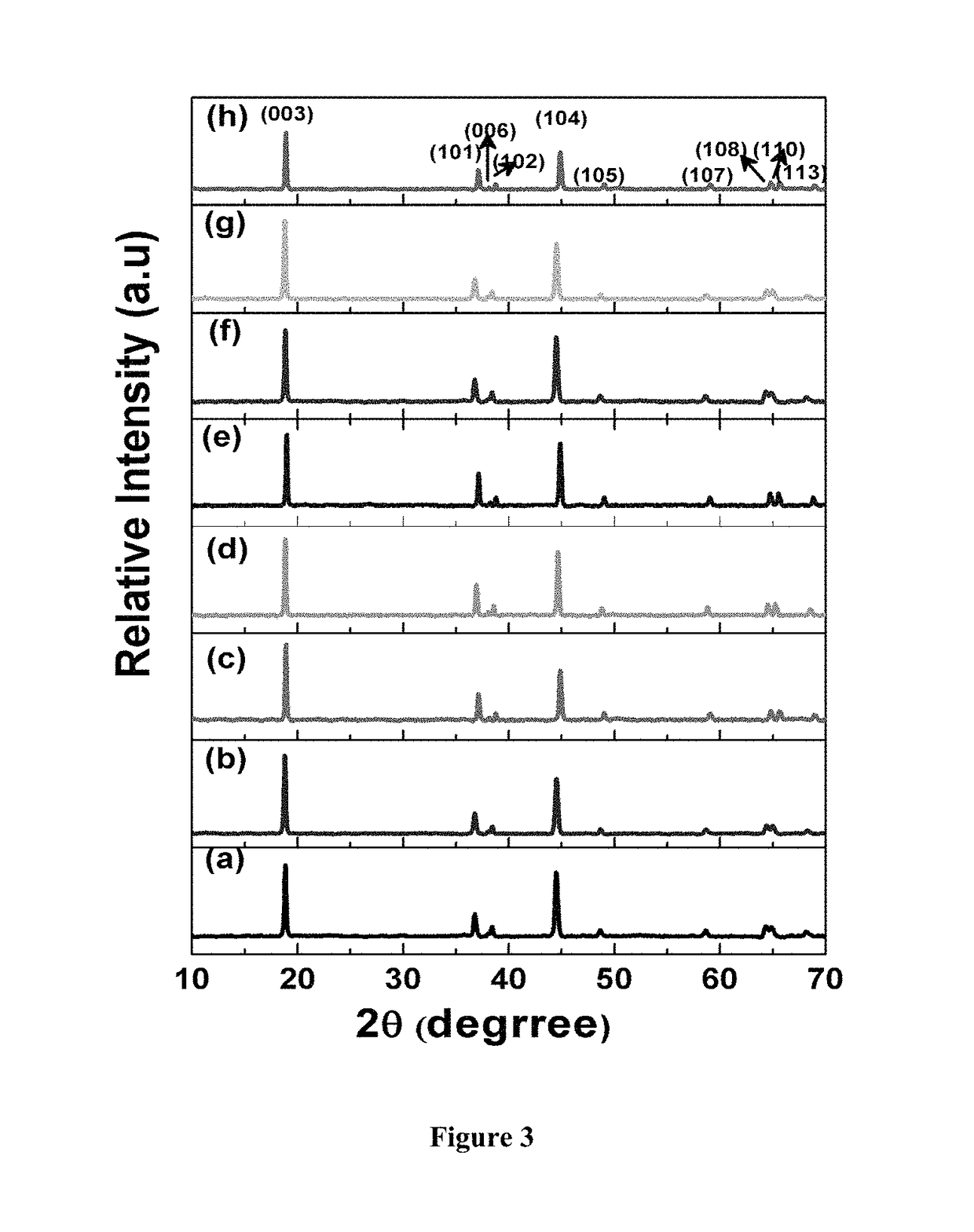
Phase transformations during cycling represent another significant obstacle. Many fluoride cathode materials undergo complex phase changes as fluoride ions are inserted or extracted. These transformations can be partially irreversible, leading to the formation of electrochemically inactive phases that contribute to capacity loss. X-ray diffraction studies have revealed that some materials transition through multiple crystallographic phases during cycling, with each transition potentially introducing structural defects.
The electronic conductivity of fluoride cathodes also presents challenges. Many fluoride compounds are inherently insulating, which limits electron transport during electrochemical reactions. This poor conductivity slows reaction kinetics and increases polarization, resulting in reduced rate capability and energy efficiency. The situation is further complicated by the formation of highly resistive fluoride-rich phases during discharge, which can effectively isolate active material from the conductive network.
Temperature sensitivity adds another layer of complexity to fluoride cathode stability. At elevated temperatures, fluoride mobility increases, potentially accelerating degradation mechanisms. Conversely, at low temperatures, the sluggish kinetics of fluoride ion transport can severely limit battery performance and exacerbate structural degradation due to incomplete reactions and increased mechanical stress during partial cycling.
One of the most significant challenges is the volume change that occurs during cycling. As fluoride ions are removed from the cathode structure during charging, the material undergoes substantial contraction. Conversely, during discharge, the reintroduction of fluoride ions causes expansion. These repeated volume fluctuations induce mechanical stress that can lead to microcracking, particle isolation, and ultimately capacity fading over multiple cycles.
The high reactivity of fluoride ions presents another critical challenge. Unlike lithium-ion systems, fluoride ions are highly electronegative and form strong bonds with host atoms in the cathode material. This strong bonding often results in poor reversibility of the fluorination/defluorination process, leading to incomplete reactions and capacity loss. Additionally, side reactions between the electrolyte and the highly reactive fluoride species can form insulating layers on the cathode surface, increasing internal resistance and further degrading performance.
Phase transformations during cycling represent another significant obstacle. Many fluoride cathode materials undergo complex phase changes as fluoride ions are inserted or extracted. These transformations can be partially irreversible, leading to the formation of electrochemically inactive phases that contribute to capacity loss. X-ray diffraction studies have revealed that some materials transition through multiple crystallographic phases during cycling, with each transition potentially introducing structural defects.
The electronic conductivity of fluoride cathodes also presents challenges. Many fluoride compounds are inherently insulating, which limits electron transport during electrochemical reactions. This poor conductivity slows reaction kinetics and increases polarization, resulting in reduced rate capability and energy efficiency. The situation is further complicated by the formation of highly resistive fluoride-rich phases during discharge, which can effectively isolate active material from the conductive network.
Temperature sensitivity adds another layer of complexity to fluoride cathode stability. At elevated temperatures, fluoride mobility increases, potentially accelerating degradation mechanisms. Conversely, at low temperatures, the sluggish kinetics of fluoride ion transport can severely limit battery performance and exacerbate structural degradation due to incomplete reactions and increased mechanical stress during partial cycling.
Analytical Techniques for Structural Characterization
01 Structural transformations in fluoride-based cathode materials
Fluoride-based cathode materials undergo significant structural transformations during charge-discharge cycles, affecting their electrochemical performance. These transformations involve changes in crystal structure, phase transitions, and atomic rearrangements that can impact capacity retention and cycling stability. Understanding these structural changes is crucial for developing more stable and high-performance fluoride cathodes for next-generation batteries.- Structural transformations in fluoride-based cathode materials: Fluoride-based cathode materials undergo significant structural transformations during charge-discharge cycles, affecting their electrochemical performance. These transformations involve changes in crystal structure, phase transitions, and atomic rearrangements that can impact capacity, cycling stability, and voltage profiles. Understanding these structural changes is crucial for developing more stable and high-performance fluoride cathodes for next-generation batteries.
- Conversion mechanisms in metal fluoride cathodes: Metal fluoride cathodes operate through conversion reactions involving the breaking and forming of metal-fluorine bonds during cycling. These mechanisms lead to significant structural reorganization, including the formation of metal nanoparticles and lithium fluoride during discharge, followed by reconversion during charging. The conversion process affects the reversibility, rate capability, and cycle life of fluoride-based cathode materials.
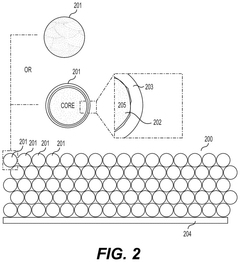
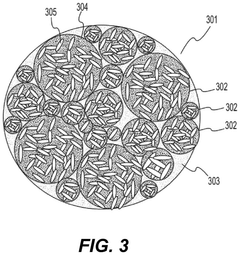
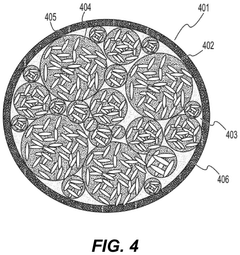
- Nanostructured fluoride cathodes for improved stability: Nanostructuring approaches are employed to enhance the structural stability of fluoride cathodes during transformation processes. These include creating core-shell structures, nanocomposites, and hierarchical architectures that can better accommodate volume changes and strain during cycling. Nanostructured fluoride cathodes demonstrate improved cycling performance, reduced structural degradation, and enhanced rate capability compared to their bulk counterparts.
- Advanced characterization of fluoride cathode transformations: Advanced analytical techniques are used to study the structural transformations in fluoride cathodes at multiple length scales. These include in-situ X-ray diffraction, transmission electron microscopy, X-ray absorption spectroscopy, and computational modeling approaches. These methods provide insights into phase evolution, defect formation, and interfacial phenomena during cycling, guiding the rational design of more stable fluoride cathode materials.
- Electrolyte interactions with fluoride cathodes: Electrolyte composition and additives significantly influence the structural transformations of fluoride cathodes. The formation of solid electrolyte interphases, dissolution of active materials, and side reactions can alter the transformation pathways and stability of these cathodes. Tailored electrolyte formulations can mitigate undesirable structural changes, enhance interfacial stability, and improve the reversibility of the conversion reactions in fluoride-based cathode materials.
02 Conversion mechanisms in metal fluoride cathodes
Metal fluoride cathodes operate through conversion reactions where the metal fluoride is converted to metal and lithium fluoride during discharge, and reformed during charge. This process involves significant structural reorganization and volume changes that can lead to mechanical stress and capacity fading. Research focuses on understanding and controlling these conversion mechanisms to improve the reversibility and cycling performance of fluoride-based cathode materials.Expand Specific Solutions03 Nanostructured fluoride cathodes for improved performance
Nanostructuring of fluoride cathode materials can significantly improve their electrochemical performance by facilitating ion transport, accommodating volume changes, and enhancing reaction kinetics. Various nanostructures including nanoparticles, nanowires, and nanocomposites have been developed to mitigate the structural degradation during cycling. These nanostructured materials show improved capacity retention and cycling stability compared to their bulk counterparts.Expand Specific Solutions04 Composite and hybrid fluoride cathode materials
Composite and hybrid fluoride cathode materials combine fluorides with carbon, polymers, or other active materials to enhance conductivity and structural stability. These composites can buffer volume changes during cycling and provide conductive networks for electron transport. The synergistic effects between different components in these composite structures lead to improved electrochemical performance and mitigated structural degradation during charge-discharge processes.Expand Specific Solutions05 In-situ and ex-situ characterization of fluoride cathode transformations
Advanced characterization techniques including in-situ and ex-situ X-ray diffraction, electron microscopy, and spectroscopic methods are employed to study the structural transformations in fluoride cathodes during electrochemical cycling. These techniques provide valuable insights into the reaction mechanisms, phase evolution, and degradation processes, guiding the rational design of improved fluoride cathode materials with enhanced structural stability and electrochemical performance.Expand Specific Solutions
Leading Research Groups and Industrial Partners
The fluoride cathode structural transformation research field is currently in an early growth phase, characterized by significant academic involvement and emerging commercial interest. The market for fluoride-based battery technologies is projected to expand as demand for high-energy density storage solutions increases, though it remains smaller than established lithium-ion technologies. Research institutions like Centre National de la Recherche Scientifique, California Institute of Technology, and Karlsruher Institut für Technologie lead fundamental investigations, while companies including Sila Nanotechnologies, Faradion, and Alsym Energy are developing commercial applications. The technology sits at mid-level maturity, with significant breakthroughs in understanding cycling mechanisms but remaining challenges in stability and performance optimization before widespread commercialization can occur.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed advanced in-situ and operando characterization techniques to analyze structural transformations in fluoride cathodes during cycling. Their approach combines synchrotron-based X-ray diffraction (XRD), X-ray absorption spectroscopy (XAS), and transmission electron microscopy (TEM) to monitor phase transitions and structural changes in real-time. They've pioneered the use of solid-state NMR spectroscopy specifically adapted for fluoride materials, allowing for detailed analysis of local atomic environments and fluorine migration pathways during charge-discharge cycles. CNRS researchers have identified key degradation mechanisms in fluoride cathodes, including the formation of inactive phases and the role of electrolyte decomposition products in capacity fading. Their work has established correlations between synthesis parameters, structural stability, and electrochemical performance of various metal fluoride cathode materials.
Strengths: World-class characterization facilities and expertise in operando techniques provide unprecedented insights into dynamic structural changes. Their multidisciplinary approach combines complementary analytical methods for comprehensive understanding. Weaknesses: Their fundamental research focus sometimes lacks immediate industrial applicability, and the specialized equipment required limits widespread adoption of their analytical approaches.
California Institute of Technology
Technical Solution: Caltech has developed a multi-scale computational framework for analyzing structural transformations in fluoride cathodes during cycling. Their approach combines density functional theory (DFT) calculations with phase-field modeling to predict phase boundaries, ion diffusion pathways, and structural evolution during fluoride insertion/extraction. They've created advanced algorithms that can simulate the formation of conversion reaction products and predict voltage profiles based on calculated formation energies. Caltech researchers have also developed machine learning models trained on experimental data to identify critical structural parameters that influence cycling stability. Their computational methods have successfully predicted previously unobserved metastable phases that form during cycling of metal fluoride cathodes, providing insights into capacity fading mechanisms and guiding the design of more stable fluoride-based battery materials.
Strengths: Their computational approach enables rapid screening of potential fluoride cathode materials before experimental synthesis, accelerating materials discovery. The multi-scale modeling provides insights across atomic to mesoscale structural changes. Weaknesses: Computational predictions still require experimental validation, and the models have limitations in accurately representing complex interfacial phenomena that occur during cycling.
Critical Patents in Fluoride-Based Energy Storage
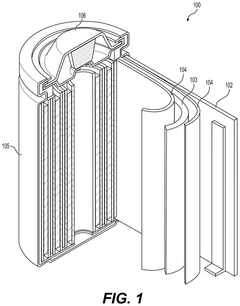
Cathode having doped metal fluoride core-shell particle and batteries comprising the same
PatentPendingUS20250158057A1
Innovation
- The development of a cathode comprising a composite core-shell particle with a conversion-type metal fluoride core and a cation-doped metal oxide or metal oxyfluoride shell, which enhances electrical and ionic conductivity, reduces volume changes, and improves cycle stability.
Surface modified cathode with improved lithium intercalation behavior
PatentActiveUS10224539B2
Innovation
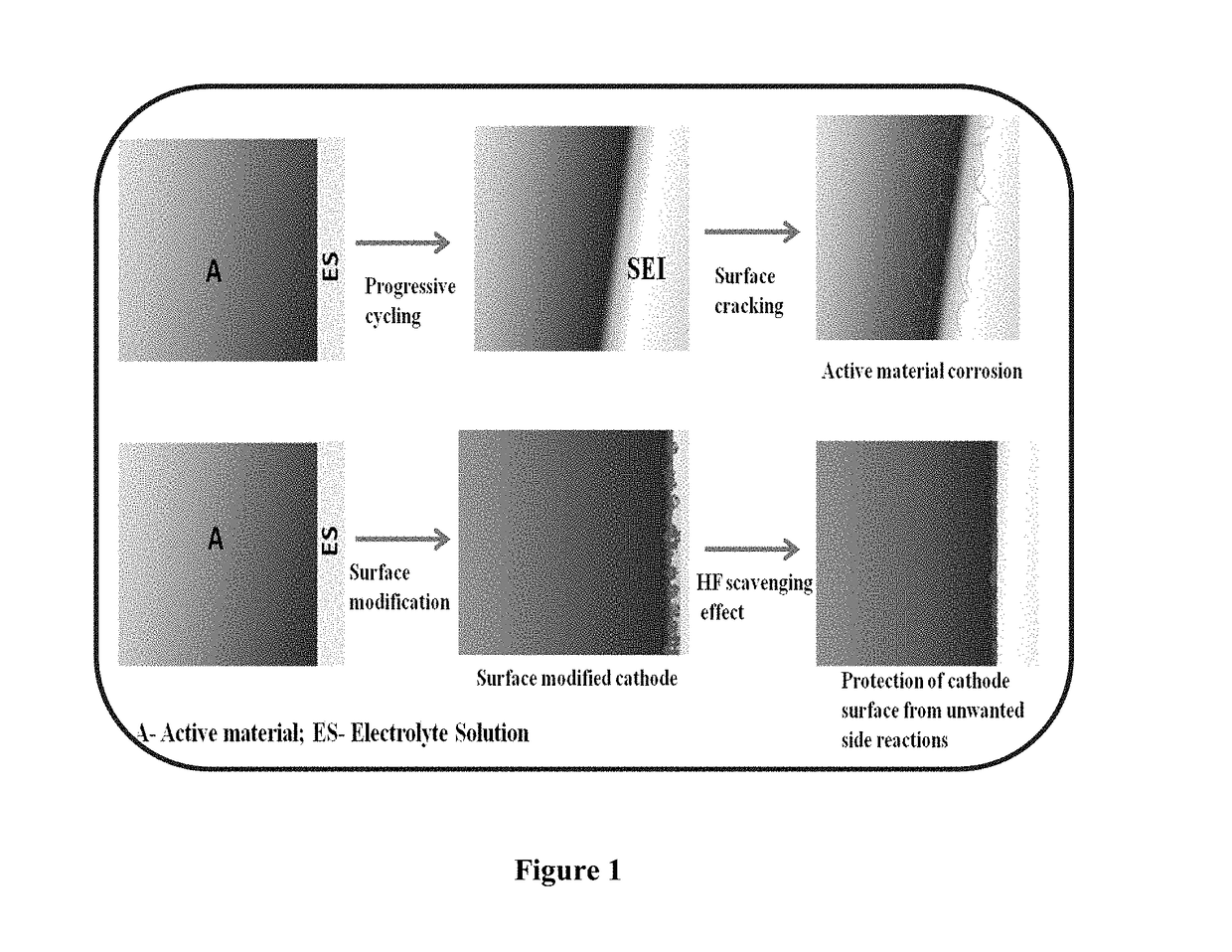
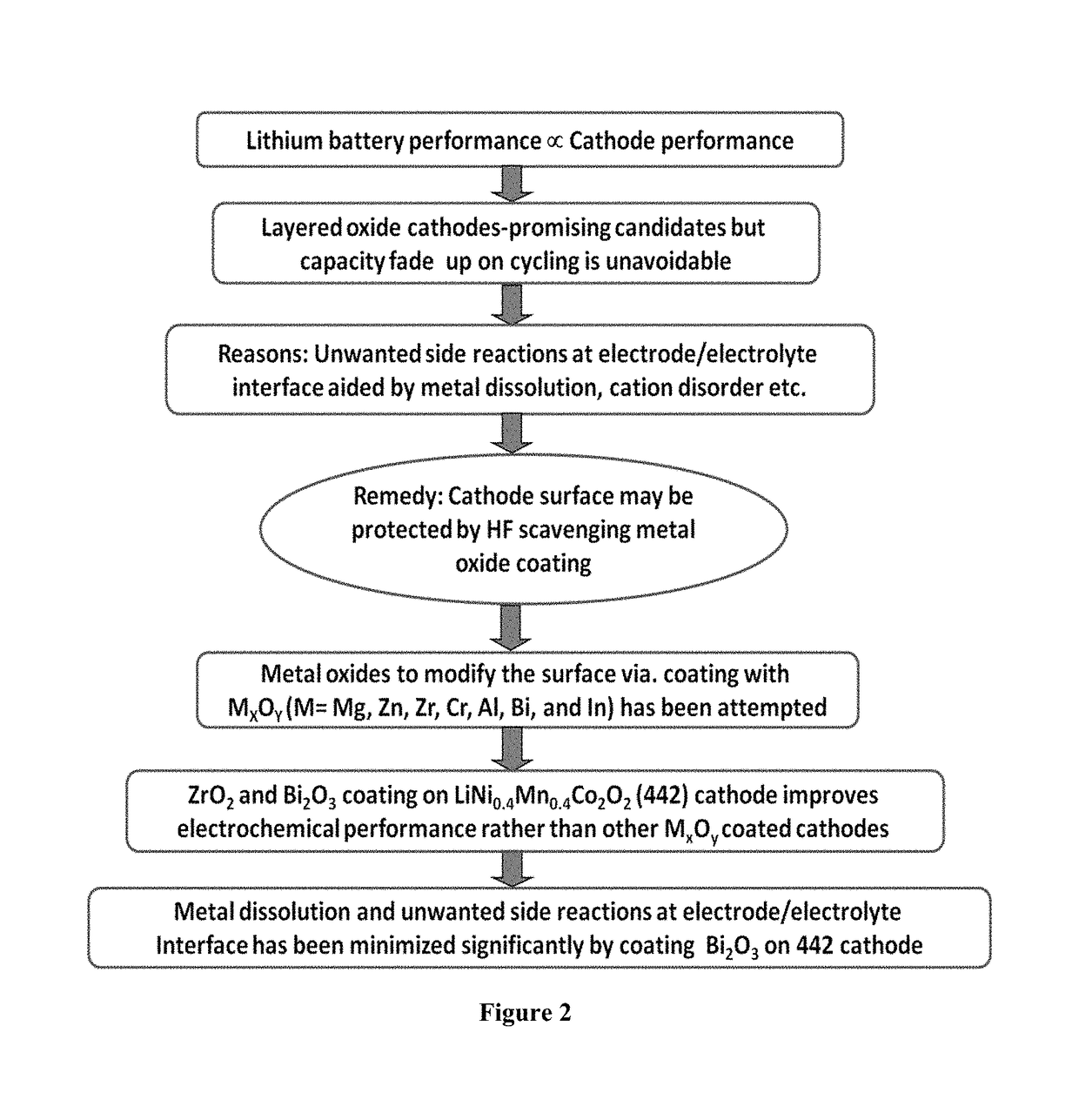
- Surface modification of the LiNi0.4Mn0.4Co0.2O2 cathode with MxOy type metal oxides such as Al2O3, Bi2O3, In2O3, Cr2O3, ZrO2, and MgO, which impart HF scavenging effects, suppress phase transitions, and block active sites for electrolyte decomposition, thereby improving electrochemical performance.
Environmental Impact of Fluoride Battery Materials
The environmental impact of fluoride battery materials represents a critical consideration in the development and deployment of fluoride-based energy storage technologies. As structural transformations in fluoride cathodes during cycling directly influence the longevity and stability of these materials, they also have significant implications for environmental sustainability throughout the battery lifecycle.
Fluoride-based cathode materials offer promising advantages in terms of theoretical energy density and potential resource abundance compared to conventional lithium-ion technologies. However, the environmental footprint of these materials must be comprehensively assessed. The extraction processes for metal fluorides often involve energy-intensive mining operations and chemical processing that can generate hazardous waste streams and contribute to habitat disruption.
During battery operation, the structural transformations observed in fluoride cathodes may lead to material degradation that shortens battery lifespan, necessitating more frequent replacement and consequently increasing waste generation. The reversibility challenges in fluoride conversion reactions often result in capacity fading, which diminishes the overall environmental benefits these batteries might otherwise provide through extended service life.
The manufacturing processes for fluoride cathode materials typically require specialized handling due to the reactive nature of fluorides. These processes consume significant energy and may involve hazardous chemicals like hydrogen fluoride, presenting occupational and environmental risks that require stringent control measures. The carbon footprint associated with these manufacturing steps must be weighed against the potential environmental benefits of the final energy storage system.
End-of-life considerations for fluoride battery materials present both challenges and opportunities. The recovery and recycling of metal components from spent fluoride cathodes could significantly reduce the need for virgin material extraction. However, current recycling technologies for these specialized materials remain underdeveloped, with economic and technical barriers limiting widespread implementation.
Water usage and potential contamination represent additional environmental concerns. Fluoride compounds can be water-soluble, raising the risk of groundwater contamination if improper disposal occurs. Comprehensive lifecycle assessment studies are needed to quantify these impacts and identify mitigation strategies that can minimize environmental harm while maximizing the sustainability benefits of fluoride battery technologies.
Fluoride-based cathode materials offer promising advantages in terms of theoretical energy density and potential resource abundance compared to conventional lithium-ion technologies. However, the environmental footprint of these materials must be comprehensively assessed. The extraction processes for metal fluorides often involve energy-intensive mining operations and chemical processing that can generate hazardous waste streams and contribute to habitat disruption.
During battery operation, the structural transformations observed in fluoride cathodes may lead to material degradation that shortens battery lifespan, necessitating more frequent replacement and consequently increasing waste generation. The reversibility challenges in fluoride conversion reactions often result in capacity fading, which diminishes the overall environmental benefits these batteries might otherwise provide through extended service life.
The manufacturing processes for fluoride cathode materials typically require specialized handling due to the reactive nature of fluorides. These processes consume significant energy and may involve hazardous chemicals like hydrogen fluoride, presenting occupational and environmental risks that require stringent control measures. The carbon footprint associated with these manufacturing steps must be weighed against the potential environmental benefits of the final energy storage system.
End-of-life considerations for fluoride battery materials present both challenges and opportunities. The recovery and recycling of metal components from spent fluoride cathodes could significantly reduce the need for virgin material extraction. However, current recycling technologies for these specialized materials remain underdeveloped, with economic and technical barriers limiting widespread implementation.
Water usage and potential contamination represent additional environmental concerns. Fluoride compounds can be water-soluble, raising the risk of groundwater contamination if improper disposal occurs. Comprehensive lifecycle assessment studies are needed to quantify these impacts and identify mitigation strategies that can minimize environmental harm while maximizing the sustainability benefits of fluoride battery technologies.
Safety Considerations for Fluoride-Based Energy Systems
The safety aspects of fluoride-based energy systems require thorough consideration due to the reactive nature of fluoride compounds. Fluoride cathodes, while promising for high energy density applications, present unique safety challenges during cycling processes. The structural transformations that occur during charge-discharge cycles can lead to potential safety hazards if not properly managed.
Fluoride ion release during cathode degradation represents a primary safety concern. When fluoride cathodes undergo structural changes during cycling, particularly at elevated temperatures or under high current loads, they may release fluoride ions that can react with moisture to form hydrofluoric acid (HF). HF is highly corrosive and toxic, posing significant risks to both equipment integrity and human health.
Thermal stability issues constitute another critical safety consideration. Structural transformations in fluoride cathodes can sometimes be exothermic, potentially triggering thermal runaway events under certain conditions. Research indicates that some fluoride cathode materials exhibit phase transitions at specific state-of-charge levels that may compromise thermal stability, particularly when subjected to mechanical stress or electrical abuse.
Electrolyte compatibility presents ongoing challenges for fluoride-based systems. The highly reactive nature of fluoride ions necessitates specialized electrolyte formulations that can withstand potential side reactions. Conventional electrolytes may undergo degradation when exposed to fluoride ions released during cathode transformation, potentially generating volatile or flammable byproducts that increase safety risks.
Engineering controls and safety mechanisms must be implemented to mitigate these risks. These include robust cell designs with pressure relief mechanisms, thermal management systems, and advanced battery management systems capable of detecting early signs of abnormal structural transformations. Additionally, protective coatings for fluoride cathodes have shown promise in reducing unwanted side reactions while maintaining electrochemical performance.
Regulatory frameworks for fluoride-based energy systems remain under development. Current safety standards for lithium-ion batteries may not adequately address the unique characteristics of fluoride-based systems. Industry stakeholders are working to establish specific protocols for testing, handling, and disposing of fluoride-based energy storage devices, with particular attention to the structural transformation behaviors observed during cycling.
Future safety enhancements will likely focus on in-situ monitoring technologies capable of detecting structural changes in real-time, allowing for preventive measures before safety-critical conditions develop. Advanced computational models that can predict structural transformations under various operating conditions will also play a crucial role in designing inherently safer fluoride-based energy systems.
Fluoride ion release during cathode degradation represents a primary safety concern. When fluoride cathodes undergo structural changes during cycling, particularly at elevated temperatures or under high current loads, they may release fluoride ions that can react with moisture to form hydrofluoric acid (HF). HF is highly corrosive and toxic, posing significant risks to both equipment integrity and human health.
Thermal stability issues constitute another critical safety consideration. Structural transformations in fluoride cathodes can sometimes be exothermic, potentially triggering thermal runaway events under certain conditions. Research indicates that some fluoride cathode materials exhibit phase transitions at specific state-of-charge levels that may compromise thermal stability, particularly when subjected to mechanical stress or electrical abuse.
Electrolyte compatibility presents ongoing challenges for fluoride-based systems. The highly reactive nature of fluoride ions necessitates specialized electrolyte formulations that can withstand potential side reactions. Conventional electrolytes may undergo degradation when exposed to fluoride ions released during cathode transformation, potentially generating volatile or flammable byproducts that increase safety risks.
Engineering controls and safety mechanisms must be implemented to mitigate these risks. These include robust cell designs with pressure relief mechanisms, thermal management systems, and advanced battery management systems capable of detecting early signs of abnormal structural transformations. Additionally, protective coatings for fluoride cathodes have shown promise in reducing unwanted side reactions while maintaining electrochemical performance.
Regulatory frameworks for fluoride-based energy systems remain under development. Current safety standards for lithium-ion batteries may not adequately address the unique characteristics of fluoride-based systems. Industry stakeholders are working to establish specific protocols for testing, handling, and disposing of fluoride-based energy storage devices, with particular attention to the structural transformation behaviors observed during cycling.
Future safety enhancements will likely focus on in-situ monitoring technologies capable of detecting structural changes in real-time, allowing for preventive measures before safety-critical conditions develop. Advanced computational models that can predict structural transformations under various operating conditions will also play a crucial role in designing inherently safer fluoride-based energy systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!