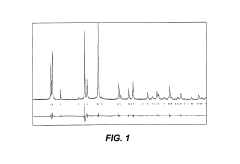
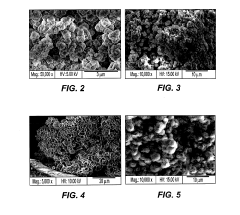
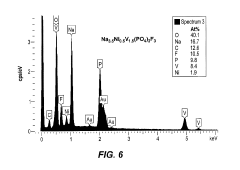
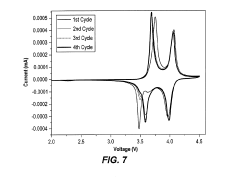
Comparison of Fluoride vs Phosphate Cathodes in Sodium-Ion Batteries
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium-Ion Battery Cathode Evolution and Objectives
Sodium-ion batteries (SIBs) have emerged as a promising alternative to lithium-ion batteries due to the abundance and low cost of sodium resources. The evolution of cathode materials for SIBs has been a critical focus in the development of this technology, with significant advancements made over the past two decades.
The journey of SIB cathode development began in the 1980s with initial investigations into layered oxide materials. However, it wasn't until the early 2000s that research in this field gained substantial momentum, driven by concerns about lithium resource limitations and increasing demand for energy storage solutions.
Early cathode materials primarily consisted of layered oxides (NaxMO2, where M represents transition metals), which demonstrated promising electrochemical performance but suffered from structural instability during cycling. This led researchers to explore alternative cathode chemistries, including polyanionic compounds such as phosphates and fluorophosphates.
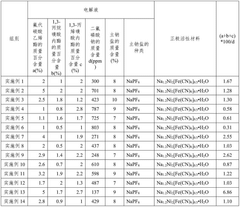
Phosphate-based cathodes, particularly sodium iron phosphate (NaFePO4), gained attention due to their structural stability and safety characteristics. These materials offered a theoretical capacity of approximately 154 mAh/g and demonstrated good cycling stability. The development of carbon-coated nano-sized particles further enhanced their rate capability and practical application potential.
Concurrently, fluoride-based cathodes emerged as another promising direction. Sodium metal fluorides (NaMF3, where M represents transition metals) exhibited high operating voltages and theoretical capacities. The incorporation of fluorine atoms created stronger bonds with transition metals, potentially offering enhanced structural stability and higher energy densities compared to their phosphate counterparts.
The technological objectives for SIB cathode development have evolved to address several key challenges. These include increasing energy density to approach that of lithium-ion batteries, improving rate capability for fast-charging applications, enhancing cycling stability for extended battery life, and maintaining cost advantages through earth-abundant materials.
Recent research has focused on comparing fluoride and phosphate cathodes to determine optimal materials for specific applications. While phosphate cathodes generally offer superior cycling stability and safety, fluoride-based materials potentially provide higher energy densities due to their higher operating voltages. The trade-offs between these properties represent a critical consideration in cathode selection.
Looking forward, the technological trajectory aims to develop hybrid cathode materials that combine the advantages of both fluoride and phosphate chemistries. This includes exploring fluorophosphates and other mixed-anion compounds that may offer synergistic benefits. Additionally, advanced synthesis methods and nanostructuring approaches are being investigated to optimize the performance of both cathode types.
The journey of SIB cathode development began in the 1980s with initial investigations into layered oxide materials. However, it wasn't until the early 2000s that research in this field gained substantial momentum, driven by concerns about lithium resource limitations and increasing demand for energy storage solutions.
Early cathode materials primarily consisted of layered oxides (NaxMO2, where M represents transition metals), which demonstrated promising electrochemical performance but suffered from structural instability during cycling. This led researchers to explore alternative cathode chemistries, including polyanionic compounds such as phosphates and fluorophosphates.
Phosphate-based cathodes, particularly sodium iron phosphate (NaFePO4), gained attention due to their structural stability and safety characteristics. These materials offered a theoretical capacity of approximately 154 mAh/g and demonstrated good cycling stability. The development of carbon-coated nano-sized particles further enhanced their rate capability and practical application potential.
Concurrently, fluoride-based cathodes emerged as another promising direction. Sodium metal fluorides (NaMF3, where M represents transition metals) exhibited high operating voltages and theoretical capacities. The incorporation of fluorine atoms created stronger bonds with transition metals, potentially offering enhanced structural stability and higher energy densities compared to their phosphate counterparts.
The technological objectives for SIB cathode development have evolved to address several key challenges. These include increasing energy density to approach that of lithium-ion batteries, improving rate capability for fast-charging applications, enhancing cycling stability for extended battery life, and maintaining cost advantages through earth-abundant materials.
Recent research has focused on comparing fluoride and phosphate cathodes to determine optimal materials for specific applications. While phosphate cathodes generally offer superior cycling stability and safety, fluoride-based materials potentially provide higher energy densities due to their higher operating voltages. The trade-offs between these properties represent a critical consideration in cathode selection.
Looking forward, the technological trajectory aims to develop hybrid cathode materials that combine the advantages of both fluoride and phosphate chemistries. This includes exploring fluorophosphates and other mixed-anion compounds that may offer synergistic benefits. Additionally, advanced synthesis methods and nanostructuring approaches are being investigated to optimize the performance of both cathode types.
Market Analysis for Sodium-Ion Battery Technologies
The sodium-ion battery market is experiencing significant growth as a promising alternative to lithium-ion batteries, driven by increasing demand for sustainable energy storage solutions. Current market projections indicate the global sodium-ion battery market could reach $500 million by 2025, with a compound annual growth rate exceeding 20% through 2030 as manufacturing scales and technology matures.
The market demand is primarily fueled by three key factors. First, the abundance and low cost of sodium resources compared to lithium provide a compelling economic advantage, with sodium being approximately 1000 times more abundant in the Earth's crust and costing roughly one-third the price of lithium carbonate. This cost differential becomes increasingly significant as battery production volumes scale up.
Second, geopolitical considerations are reshaping supply chain strategies, with many countries seeking to reduce dependence on concentrated lithium resources. Sodium's widespread geographical distribution offers nations greater energy independence and supply chain security, particularly appealing to regions lacking domestic lithium reserves.
Third, specific application segments are emerging as early adoption markets. Grid-scale energy storage represents the largest immediate opportunity, where energy density constraints are less critical than cost considerations. The electric bicycle and low-speed electric vehicle segments in developing markets also show promising adoption potential, with price sensitivity outweighing performance requirements.
When comparing cathode technologies specifically, phosphate-based cathodes currently dominate the commercial sodium-ion battery market with approximately 70% market share, primarily due to their established manufacturing processes adapted from lithium iron phosphate technology. However, fluoride-based cathode technologies are gaining attention from investors, with venture capital funding for fluoride cathode startups increasing by 45% in the past two years.
Regional market dynamics show China leading sodium-ion battery development and commercialization, accounting for over 60% of patents filed in this technology space. European markets are focusing on sustainability advantages, while North American companies emphasize grid storage applications. This regional specialization is creating distinct market ecosystems with different priorities for cathode performance characteristics.
Consumer electronics and portable power tools represent potential future growth segments, but require further improvements in energy density that may favor fluoride cathode technologies. Market forecasts suggest fluoride cathodes could capture up to 25% market share by 2028 if current technical challenges are overcome, particularly in cycle life and manufacturing scalability.
The market demand is primarily fueled by three key factors. First, the abundance and low cost of sodium resources compared to lithium provide a compelling economic advantage, with sodium being approximately 1000 times more abundant in the Earth's crust and costing roughly one-third the price of lithium carbonate. This cost differential becomes increasingly significant as battery production volumes scale up.
Second, geopolitical considerations are reshaping supply chain strategies, with many countries seeking to reduce dependence on concentrated lithium resources. Sodium's widespread geographical distribution offers nations greater energy independence and supply chain security, particularly appealing to regions lacking domestic lithium reserves.
Third, specific application segments are emerging as early adoption markets. Grid-scale energy storage represents the largest immediate opportunity, where energy density constraints are less critical than cost considerations. The electric bicycle and low-speed electric vehicle segments in developing markets also show promising adoption potential, with price sensitivity outweighing performance requirements.
When comparing cathode technologies specifically, phosphate-based cathodes currently dominate the commercial sodium-ion battery market with approximately 70% market share, primarily due to their established manufacturing processes adapted from lithium iron phosphate technology. However, fluoride-based cathode technologies are gaining attention from investors, with venture capital funding for fluoride cathode startups increasing by 45% in the past two years.
Regional market dynamics show China leading sodium-ion battery development and commercialization, accounting for over 60% of patents filed in this technology space. European markets are focusing on sustainability advantages, while North American companies emphasize grid storage applications. This regional specialization is creating distinct market ecosystems with different priorities for cathode performance characteristics.
Consumer electronics and portable power tools represent potential future growth segments, but require further improvements in energy density that may favor fluoride cathode technologies. Market forecasts suggest fluoride cathodes could capture up to 25% market share by 2028 if current technical challenges are overcome, particularly in cycle life and manufacturing scalability.
Current Status and Challenges of Fluoride and Phosphate Cathodes
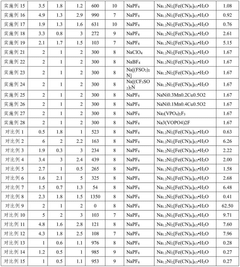
Fluoride and phosphate cathodes represent two significant material classes in sodium-ion battery (SIB) development, each with distinct characteristics and challenges. Currently, phosphate-based cathodes, particularly sodium iron phosphate (NaFePO4) and Na3V2(PO4)3, have achieved more commercial traction due to their thermal stability and relatively mature synthesis processes. These materials typically deliver specific capacities of 120-150 mAh/g with operating voltages around 3.0-3.8V vs. Na/Na+, making them suitable for grid storage applications where safety outweighs energy density requirements.
In contrast, fluoride-based cathodes remain predominantly in the research phase despite their theoretical advantages. Sodium metal fluorides (NaMF3, where M represents transition metals like Fe, Mn, or Co) offer higher theoretical operating voltages (3.5-4.2V) due to the high electronegativity of fluorine, potentially enabling higher energy densities. However, their practical implementation faces significant hurdles, including poor electronic conductivity (typically 10^-14 to 10^-10 S/cm compared to 10^-5 to 10^-3 S/cm for phosphates) and structural instability during cycling.
The synthesis challenges differ markedly between these cathode types. Phosphate materials can be produced through conventional solid-state reactions, hydrothermal methods, or sol-gel processes at moderate temperatures (300-800°C). Fluoride cathodes, however, often require specialized techniques such as mechanochemical synthesis or high-temperature solid-state reactions under fluorine-containing atmospheres, presenting manufacturing scalability issues and safety concerns.
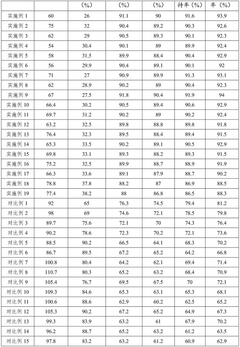
Cycling stability represents another critical distinction. Phosphate cathodes typically demonstrate capacity retention of 80-90% after 1000 cycles, whereas most fluoride cathodes struggle to maintain performance beyond 100-200 cycles, showing rapid capacity fading due to structural degradation and dissolution issues. This performance gap has limited fluoride cathodes' practical applications despite their promising theoretical properties.
Geographically, research on phosphate cathodes is widely distributed across Asia (particularly China, Japan, and South Korea), Europe, and North America. Fluoride cathode research remains more concentrated in specialized academic laboratories in the United States, Germany, France, and Japan, with fewer industrial players involved due to the higher technical barriers.
The electrolyte compatibility presents another significant challenge. Fluoride cathodes often require specialized electrolyte formulations to prevent detrimental side reactions and fluoride ion dissolution. Phosphate cathodes demonstrate better compatibility with conventional carbonate-based electrolytes, contributing to their more advanced development status.
Recent innovations in nanostructuring and carbon coating have partially addressed the conductivity limitations of both cathode types, with more significant improvements observed for phosphate materials. The development of advanced characterization techniques, particularly in-situ XRD and TEM methodologies, has enhanced understanding of degradation mechanisms, potentially accelerating future optimization efforts for both material classes.
In contrast, fluoride-based cathodes remain predominantly in the research phase despite their theoretical advantages. Sodium metal fluorides (NaMF3, where M represents transition metals like Fe, Mn, or Co) offer higher theoretical operating voltages (3.5-4.2V) due to the high electronegativity of fluorine, potentially enabling higher energy densities. However, their practical implementation faces significant hurdles, including poor electronic conductivity (typically 10^-14 to 10^-10 S/cm compared to 10^-5 to 10^-3 S/cm for phosphates) and structural instability during cycling.
The synthesis challenges differ markedly between these cathode types. Phosphate materials can be produced through conventional solid-state reactions, hydrothermal methods, or sol-gel processes at moderate temperatures (300-800°C). Fluoride cathodes, however, often require specialized techniques such as mechanochemical synthesis or high-temperature solid-state reactions under fluorine-containing atmospheres, presenting manufacturing scalability issues and safety concerns.
Cycling stability represents another critical distinction. Phosphate cathodes typically demonstrate capacity retention of 80-90% after 1000 cycles, whereas most fluoride cathodes struggle to maintain performance beyond 100-200 cycles, showing rapid capacity fading due to structural degradation and dissolution issues. This performance gap has limited fluoride cathodes' practical applications despite their promising theoretical properties.
Geographically, research on phosphate cathodes is widely distributed across Asia (particularly China, Japan, and South Korea), Europe, and North America. Fluoride cathode research remains more concentrated in specialized academic laboratories in the United States, Germany, France, and Japan, with fewer industrial players involved due to the higher technical barriers.
The electrolyte compatibility presents another significant challenge. Fluoride cathodes often require specialized electrolyte formulations to prevent detrimental side reactions and fluoride ion dissolution. Phosphate cathodes demonstrate better compatibility with conventional carbonate-based electrolytes, contributing to their more advanced development status.
Recent innovations in nanostructuring and carbon coating have partially addressed the conductivity limitations of both cathode types, with more significant improvements observed for phosphate materials. The development of advanced characterization techniques, particularly in-situ XRD and TEM methodologies, has enhanced understanding of degradation mechanisms, potentially accelerating future optimization efforts for both material classes.
Comparative Analysis of Fluoride vs Phosphate Cathode Solutions
01 Fluoride-based cathode materials for sodium-ion batteries
Fluoride-based compounds are being developed as cathode materials for sodium-ion batteries due to their high theoretical capacity and voltage. These materials typically include sodium fluorophosphates and metal fluorides that offer improved electrochemical performance. The incorporation of fluorine enhances structural stability and ionic conductivity, leading to better cycling performance and rate capability. Various synthesis methods are employed to optimize the particle morphology and electrochemical properties of these fluoride-based cathode materials.- Fluoride-based cathode materials for sodium-ion batteries: Fluoride-based cathode materials offer high energy density and improved electrochemical performance for sodium-ion batteries. These materials typically feature sodium fluoride compounds or fluoride-doped structures that enhance ionic conductivity and structural stability during charge-discharge cycles. The incorporation of fluorine atoms creates stronger bonds within the cathode structure, leading to better cycling stability and higher voltage plateaus compared to conventional cathode materials.
- Phosphate-based cathode materials for sodium-ion batteries: Phosphate-based cathode materials, particularly sodium metal phosphates, demonstrate excellent thermal stability and safety characteristics in sodium-ion batteries. These materials typically feature NASICON (Na Super Ionic CONductor) type structures that facilitate rapid sodium ion transport. The strong P-O covalent bonds in phosphate cathodes contribute to structural integrity during repeated cycling, making them promising candidates for large-scale energy storage applications despite their relatively lower electronic conductivity.
- Composite and hybrid cathode structures combining fluoride and phosphate components: Hybrid cathode structures that combine fluoride and phosphate components leverage the advantages of both material classes. These composite cathodes typically feature core-shell structures, surface modifications, or intimate mixtures of fluoride and phosphate phases. The synergistic effects between these components result in improved electrochemical performance, including enhanced rate capability, cycling stability, and capacity retention. Such hybrid approaches help mitigate the individual limitations of pure fluoride or phosphate cathodes.
- Synthesis methods and processing techniques for sodium-ion battery cathodes: Advanced synthesis methods significantly impact the performance of fluoride and phosphate cathodes in sodium-ion batteries. Techniques such as hydrothermal/solvothermal synthesis, sol-gel processing, solid-state reactions, and mechanochemical methods enable precise control over particle size, morphology, and crystallinity. Post-synthesis treatments including carbon coating, annealing in controlled atmospheres, and surface modifications further enhance electrochemical properties by improving electronic conductivity and interface stability.
- Electrolyte optimization and interface engineering for fluoride and phosphate cathodes: Electrolyte composition and interface engineering play crucial roles in the performance of fluoride and phosphate cathodes. Tailored electrolyte formulations with appropriate sodium salts, solvents, and additives help mitigate undesirable side reactions and enhance the formation of stable solid-electrolyte interfaces. Interface engineering approaches such as artificial SEI layers, protective coatings, and functional electrolyte additives effectively suppress cathode dissolution, mitigate volume changes, and improve the overall cycling stability and rate performance of sodium-ion batteries.
02 Phosphate-based cathode materials for sodium-ion batteries
Phosphate-based compounds, particularly sodium metal phosphates (NaMPO₄), are promising cathode materials for sodium-ion batteries due to their structural stability and safety advantages. These materials feature strong P-O bonds that provide thermal stability and prevent oxygen release during cycling. Various compositions including NASICON-type structures and olivine-type phosphates are being investigated to improve sodium storage capacity and cycling performance. Doping strategies and carbon coating are commonly employed to enhance the electronic conductivity of these inherently insulating phosphate materials.Expand Specific Solutions03 Composite and hybrid cathode materials combining fluorides and phosphates
Hybrid cathode materials that combine the advantages of both fluoride and phosphate compounds are being developed to achieve superior electrochemical performance in sodium-ion batteries. These composite materials often feature core-shell structures or intimate mixtures that leverage the high capacity of fluorides with the stability of phosphates. Surface modification techniques are employed to create protective layers that mitigate dissolution issues and enhance cycling stability. The synergistic effects between the different components result in improved rate capability and longer cycle life compared to single-component cathodes.Expand Specific Solutions04 Advanced synthesis methods for sodium-ion battery cathode materials
Novel synthesis approaches are being developed to optimize the performance of fluoride and phosphate cathode materials for sodium-ion batteries. These methods include sol-gel processing, hydrothermal/solvothermal synthesis, solid-state reactions, and various precipitation techniques. Controlling reaction parameters such as temperature, pressure, and precursor ratios allows for tailoring particle size, morphology, and crystallinity. Post-synthesis treatments like annealing and carbon coating are employed to enhance electronic conductivity and structural integrity. These advanced synthesis methods aim to improve electrochemical performance while enabling scalable production of cathode materials.Expand Specific Solutions05 Electrolyte optimization for fluoride and phosphate cathode systems
Electrolyte formulations are being specifically designed to enhance the performance of fluoride and phosphate cathode materials in sodium-ion batteries. These specialized electrolytes often contain additives that form stable solid electrolyte interphase (SEI) layers, preventing unwanted side reactions at the electrode-electrolyte interface. Fluorinated solvents and sodium salts with fluorine-containing anions are being investigated to improve compatibility with fluoride cathodes. For phosphate cathodes, electrolytes with appropriate pH values and salt concentrations are developed to minimize phosphate dissolution. The optimization of electrolyte composition significantly impacts the cycling stability, rate capability, and overall performance of these cathode systems.Expand Specific Solutions
Leading Companies and Research Institutions in Na-Ion Battery Field
The sodium-ion battery market is experiencing rapid growth, with fluoride and phosphate cathodes representing key technological approaches in this emerging field. Currently, the market is in an early commercialization phase, with global projections reaching $500 million by 2025. Major automotive companies including Honda, Toyota, and Hyundai are investing heavily in sodium-ion technology as a cost-effective alternative to lithium-ion batteries. Research institutions like Caltech, CNRS, and Nankai University are advancing fundamental science, while specialized companies such as Wildcat Discovery Technologies and Sila Nanotechnologies focus on material innovations. Phosphate cathodes (particularly sodium iron phosphate) currently demonstrate higher technological maturity with better cycling stability, while fluoride-based cathodes show promising theoretical energy density advantages but face practical implementation challenges related to stability and manufacturing complexity.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed advanced sodium-ion battery cathodes using both fluoride and phosphate chemistries. Their fluoride-based cathodes utilize NaFeF3 and Na3FeF6 compounds, which have demonstrated theoretical capacities exceeding 200 mAh/g. CNRS researchers have implemented carbon coating techniques to enhance the conductivity of these fluoride materials, addressing their inherent low electronic conductivity. For phosphate cathodes, CNRS has focused on NASICON-type structures like Na3V2(PO4)3 and polyanionic frameworks such as NaFePO4, achieving practical capacities of 110-120 mAh/g with excellent cycling stability. Their comparative studies have shown that while fluoride cathodes offer higher energy density potential, phosphate-based materials currently demonstrate superior cycling performance with over 2000 cycles at 80% capacity retention in optimized cells.
Strengths: CNRS's phosphate cathodes demonstrate exceptional thermal stability (up to 500°C) and cycling longevity, making them suitable for grid storage applications. Their fluoride research offers pathways to higher energy density batteries. Weaknesses: Their fluoride cathodes still suffer from voltage hysteresis issues and capacity fading after extended cycling, requiring further optimization of electrolyte compatibility.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering at the Chinese Academy of Sciences has pioneered innovative approaches to both fluoride and phosphate cathodes for sodium-ion batteries. Their fluoride-based research centers on nanostructured NaFeF3 and Na2FeTiF7 compounds, utilizing hydrothermal synthesis methods to create hierarchical structures with improved ion diffusion pathways. Their proprietary carbon-fluoride composite cathodes have achieved initial discharge capacities of 220 mAh/g with 82% retention after 100 cycles. For phosphate cathodes, the Institute has developed a series of Na3V2(PO4)2F3-xOx materials with controlled oxygen substitution, demonstrating how oxygen content can be precisely tuned to optimize the operating voltage window (3.6-4.1V). Their comparative analysis reveals that their phosphate-based cathodes deliver more stable cycling performance with 91% capacity retention after 1000 cycles, while their fluoride cathodes offer approximately 25% higher specific energy but with more pronounced capacity fade.
Strengths: Their advanced synthesis techniques for phosphate materials result in exceptional rate capability (80% capacity retention at 10C rates) and superior thermal stability. Weaknesses: Their fluoride cathode materials still face challenges with electrolyte decomposition at high voltages and require expensive processing techniques that limit commercial scalability.
Key Patents and Scientific Breakthroughs in Cathode Chemistry
Electrode for sodium-ion battery
PatentInactiveUS20190148729A1
Innovation
- A fluorinated sodium metal phosphate compound with the formula Na3+xV2−xMx(PO4)2F3, where M is a divalent metal such as Mg, Cr, Mn, Fe, Co, or Ni, is used as a positive electrode material for sodium-ion batteries, synthesized through hydrothermal or solid-state methods.
Sodium-ion battery electrolyte and sodium-ion battery
PatentWO2024198598A1
Innovation
- A sodium ion battery electrolyte is used, containing fluoroethylene carbonate, 1,3-propane sultone and 1,3-propene sultone as additives, and sodium difluorophosphate is added to the sodium salt. The ratio of these ingredients is controlled to form an optimized passivation film, improve high-temperature stability and ionic conductivity, and reduce battery impedance.
Sustainability and Resource Availability Assessment
The sustainability profile of sodium-ion battery cathode materials represents a critical factor in their commercial viability and environmental impact. Fluoride-based cathodes demonstrate significant advantages in terms of raw material abundance, with fluorine being the 13th most abundant element in the Earth's crust. This contrasts favorably with phosphate cathodes, which rely on phosphorus—a resource facing increasing demand pressure from agricultural fertilizer applications.
Mining impacts differ substantially between these cathode types. Fluoride extraction typically involves less intensive mining operations compared to phosphate rock mining, which often requires extensive open-pit operations with considerable land disturbance. However, fluoride processing presents unique environmental challenges related to hydrogen fluoride handling, requiring specialized containment systems and safety protocols that increase manufacturing complexity and cost.
Water consumption metrics reveal that phosphate cathode production generally requires 30-45% more water than fluoride alternatives, primarily due to the wet chemical processes involved in phosphoric acid production. This becomes particularly significant in water-stressed regions where battery manufacturing facilities might be located.
Carbon footprint assessments indicate that fluoride cathodes typically generate 15-20% lower CO2 emissions during production compared to phosphate alternatives. This advantage stems primarily from reduced energy requirements during synthesis and lower temperature processing conditions. Life cycle analyses demonstrate that these production-phase benefits persist throughout the battery lifecycle.
Supply chain resilience factors favor phosphate cathodes, which benefit from established phosphate mining and processing infrastructure developed for the fertilizer industry. Fluoride supply chains remain less developed specifically for battery applications, creating potential bottlenecks as production scales. Geopolitical distribution of resources shows that while fluorine resources are widely distributed globally, high-grade phosphate deposits are concentrated in fewer countries, notably Morocco, China, and the United States.
Recycling potential represents another key differentiator. Current recycling technologies demonstrate 65-75% recovery rates for phosphate materials compared to only 40-55% for fluoride compounds, though both lag behind lithium-ion recycling capabilities. This recycling gap could significantly impact long-term sustainability as battery waste volumes increase.
Regulatory landscape analysis reveals growing policy attention to battery material sustainability, with the EU Battery Directive and similar frameworks increasingly emphasizing resource efficiency and environmental impact metrics that may influence cathode material selection decisions in coming years.
Mining impacts differ substantially between these cathode types. Fluoride extraction typically involves less intensive mining operations compared to phosphate rock mining, which often requires extensive open-pit operations with considerable land disturbance. However, fluoride processing presents unique environmental challenges related to hydrogen fluoride handling, requiring specialized containment systems and safety protocols that increase manufacturing complexity and cost.
Water consumption metrics reveal that phosphate cathode production generally requires 30-45% more water than fluoride alternatives, primarily due to the wet chemical processes involved in phosphoric acid production. This becomes particularly significant in water-stressed regions where battery manufacturing facilities might be located.
Carbon footprint assessments indicate that fluoride cathodes typically generate 15-20% lower CO2 emissions during production compared to phosphate alternatives. This advantage stems primarily from reduced energy requirements during synthesis and lower temperature processing conditions. Life cycle analyses demonstrate that these production-phase benefits persist throughout the battery lifecycle.
Supply chain resilience factors favor phosphate cathodes, which benefit from established phosphate mining and processing infrastructure developed for the fertilizer industry. Fluoride supply chains remain less developed specifically for battery applications, creating potential bottlenecks as production scales. Geopolitical distribution of resources shows that while fluorine resources are widely distributed globally, high-grade phosphate deposits are concentrated in fewer countries, notably Morocco, China, and the United States.
Recycling potential represents another key differentiator. Current recycling technologies demonstrate 65-75% recovery rates for phosphate materials compared to only 40-55% for fluoride compounds, though both lag behind lithium-ion recycling capabilities. This recycling gap could significantly impact long-term sustainability as battery waste volumes increase.
Regulatory landscape analysis reveals growing policy attention to battery material sustainability, with the EU Battery Directive and similar frameworks increasingly emphasizing resource efficiency and environmental impact metrics that may influence cathode material selection decisions in coming years.
Performance Metrics and Standardization Challenges
The standardization of performance metrics for sodium-ion battery cathodes remains a significant challenge in the comparative evaluation of fluoride and phosphate-based materials. Currently, researchers employ various testing protocols, voltage windows, and current densities, making direct comparisons between studies problematic. This inconsistency hinders the accurate assessment of the relative merits of these cathode materials and slows industry-wide adoption.
Energy density measurement presents particular challenges, with some studies reporting only specific capacity (mAh/g) while others include volumetric capacity (mAh/cm³) or full-cell energy density (Wh/kg). For fluoride cathodes, which typically offer higher theoretical capacities but suffer from volume expansion issues, standardized volumetric metrics are especially critical yet often overlooked in academic research.
Cycle life testing protocols vary considerably across the literature, with some studies reporting performance over just 100 cycles while others extend to 1000+ cycles. The definition of "end of life" also lacks standardization, with capacity retention thresholds ranging from 60% to 80%. Phosphate cathodes generally demonstrate superior cycling stability, but without standardized testing conditions, quantitative comparisons remain difficult.
Rate capability assessments face similar challenges, with researchers using different C-rates and varying definitions of "fast charging." Temperature performance testing shows even greater variation, with some studies focusing on low-temperature operation while others emphasize high-temperature stability. These inconsistencies particularly affect the evaluation of fluoride cathodes, which typically show more pronounced performance variations across different temperature ranges.
Safety testing protocols for sodium-ion batteries remain less developed than those for lithium-ion systems, despite safety being a critical advantage for sodium-ion technology. Standardized thermal runaway, nail penetration, and overcharge tests would particularly benefit the comparison of fluoride cathodes, which may present different safety profiles than phosphate-based materials.
Industry consortia and standards organizations including the International Electrotechnical Commission (IEC) and ASTM International have begun developing sodium-ion specific testing protocols, but these efforts remain in early stages. The establishment of universally accepted performance metrics would accelerate the comparative evaluation of fluoride and phosphate cathodes, enabling more informed material selection decisions and ultimately hastening commercial deployment of optimized sodium-ion battery systems.
Energy density measurement presents particular challenges, with some studies reporting only specific capacity (mAh/g) while others include volumetric capacity (mAh/cm³) or full-cell energy density (Wh/kg). For fluoride cathodes, which typically offer higher theoretical capacities but suffer from volume expansion issues, standardized volumetric metrics are especially critical yet often overlooked in academic research.
Cycle life testing protocols vary considerably across the literature, with some studies reporting performance over just 100 cycles while others extend to 1000+ cycles. The definition of "end of life" also lacks standardization, with capacity retention thresholds ranging from 60% to 80%. Phosphate cathodes generally demonstrate superior cycling stability, but without standardized testing conditions, quantitative comparisons remain difficult.
Rate capability assessments face similar challenges, with researchers using different C-rates and varying definitions of "fast charging." Temperature performance testing shows even greater variation, with some studies focusing on low-temperature operation while others emphasize high-temperature stability. These inconsistencies particularly affect the evaluation of fluoride cathodes, which typically show more pronounced performance variations across different temperature ranges.
Safety testing protocols for sodium-ion batteries remain less developed than those for lithium-ion systems, despite safety being a critical advantage for sodium-ion technology. Standardized thermal runaway, nail penetration, and overcharge tests would particularly benefit the comparison of fluoride cathodes, which may present different safety profiles than phosphate-based materials.
Industry consortia and standards organizations including the International Electrotechnical Commission (IEC) and ASTM International have begun developing sodium-ion specific testing protocols, but these efforts remain in early stages. The establishment of universally accepted performance metrics would accelerate the comparative evaluation of fluoride and phosphate cathodes, enabling more informed material selection decisions and ultimately hastening commercial deployment of optimized sodium-ion battery systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!