Comparison of Fluoride Cathode Performance in Lithium vs Sodium Batteries
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoride Cathode Technology Background and Objectives
Fluoride-based cathode materials have emerged as promising candidates for next-generation energy storage systems due to their high theoretical energy densities. The development of fluoride cathodes can be traced back to the early 2000s when researchers began exploring alternatives to traditional lithium-ion battery chemistries to overcome energy density limitations. The evolution of these materials has been characterized by significant breakthroughs in addressing challenges related to ionic conductivity, cycling stability, and voltage hysteresis.
The fundamental principle behind fluoride cathode operation involves the reversible formation and decomposition of metal fluorides through conversion reactions. This mechanism offers substantially higher theoretical capacities compared to conventional intercalation-based cathodes. For lithium systems, theoretical energy densities exceeding 1000 Wh/kg have been calculated, while sodium-fluoride systems promise comparable performance with the advantage of utilizing more abundant resources.
Recent technological advancements have focused on nanostructuring approaches, electrolyte optimization, and composite electrode designs to enhance the performance of fluoride cathodes in both lithium and sodium battery systems. The development trajectory has been accelerating since 2015, with significant publications demonstrating improved cycling performance and reduced voltage hysteresis, particularly in lithium-fluoride systems.
The primary technical objectives in this field include achieving practical energy densities above 500 Wh/kg, extending cycle life beyond 1000 cycles, improving rate capability for fast charging applications, and developing manufacturing processes compatible with existing battery production infrastructure. Additionally, there is a growing emphasis on comparing the fundamental differences in reaction mechanisms and performance metrics between lithium and sodium fluoride systems.
Understanding the distinct behaviors of fluoride cathodes in lithium versus sodium environments represents a critical research direction, as sodium-based systems offer potential cost and sustainability advantages despite certain performance trade-offs. Key parameters requiring systematic comparison include ionic diffusion kinetics, interfacial stability, voltage profiles, and structural evolution during cycling.
The technological landscape is further complicated by the need to develop compatible electrolytes that enable stable operation of fluoride cathodes while preventing parasitic reactions. Solid-state electrolyte approaches have shown promise in addressing some of these challenges, particularly for lithium systems, while room-temperature ionic liquids have demonstrated advantages for sodium-fluoride batteries.
As global research efforts intensify, the field is moving toward establishing standardized testing protocols and performance benchmarks that will facilitate meaningful comparisons between lithium and sodium fluoride cathode technologies, ultimately guiding strategic R&D investments in this promising energy storage direction.
The fundamental principle behind fluoride cathode operation involves the reversible formation and decomposition of metal fluorides through conversion reactions. This mechanism offers substantially higher theoretical capacities compared to conventional intercalation-based cathodes. For lithium systems, theoretical energy densities exceeding 1000 Wh/kg have been calculated, while sodium-fluoride systems promise comparable performance with the advantage of utilizing more abundant resources.
Recent technological advancements have focused on nanostructuring approaches, electrolyte optimization, and composite electrode designs to enhance the performance of fluoride cathodes in both lithium and sodium battery systems. The development trajectory has been accelerating since 2015, with significant publications demonstrating improved cycling performance and reduced voltage hysteresis, particularly in lithium-fluoride systems.
The primary technical objectives in this field include achieving practical energy densities above 500 Wh/kg, extending cycle life beyond 1000 cycles, improving rate capability for fast charging applications, and developing manufacturing processes compatible with existing battery production infrastructure. Additionally, there is a growing emphasis on comparing the fundamental differences in reaction mechanisms and performance metrics between lithium and sodium fluoride systems.
Understanding the distinct behaviors of fluoride cathodes in lithium versus sodium environments represents a critical research direction, as sodium-based systems offer potential cost and sustainability advantages despite certain performance trade-offs. Key parameters requiring systematic comparison include ionic diffusion kinetics, interfacial stability, voltage profiles, and structural evolution during cycling.
The technological landscape is further complicated by the need to develop compatible electrolytes that enable stable operation of fluoride cathodes while preventing parasitic reactions. Solid-state electrolyte approaches have shown promise in addressing some of these challenges, particularly for lithium systems, while room-temperature ionic liquids have demonstrated advantages for sodium-fluoride batteries.
As global research efforts intensify, the field is moving toward establishing standardized testing protocols and performance benchmarks that will facilitate meaningful comparisons between lithium and sodium fluoride cathode technologies, ultimately guiding strategic R&D investments in this promising energy storage direction.
Market Analysis for Advanced Battery Materials
The advanced battery materials market is experiencing unprecedented growth, driven by the global shift towards electrification and renewable energy storage solutions. Currently valued at approximately $57 billion in 2023, this market is projected to reach $89.5 billion by 2028, representing a compound annual growth rate of 9.4%. Fluoride-based cathode materials specifically have emerged as a promising segment within this broader market due to their theoretical high energy density capabilities.
Demand for high-performance battery materials is primarily fueled by electric vehicle adoption, which saw a 55% year-over-year increase in 2022. This trend is expected to continue as major automotive manufacturers commit to electrifying their fleets. The stationary energy storage sector represents another significant demand driver, with grid-scale installations increasing by 123% in 2022 compared to the previous year.
Within the fluoride cathode segment, market analysis reveals distinct performance and cost differences between lithium and sodium battery applications. Lithium fluoride cathodes currently command premium pricing due to their superior energy density (theoretical capacity of 1,800 mAh/g versus 1,200 mAh/g for sodium fluoride cathodes). However, sodium-based systems are gaining market attention due to sodium's greater abundance and lower extraction costs, with raw material expenses approximately 30-40% lower than lithium equivalents.
Regional market dynamics show Asia-Pacific dominating manufacturing capacity for advanced battery materials, accounting for 67% of global production. China leads with 41% market share, followed by South Korea (14%) and Japan (12%). North America and Europe are rapidly expanding their domestic production capabilities through strategic investments and policy initiatives like the U.S. Inflation Reduction Act and European Battery Alliance.
Consumer electronics currently represent the largest application segment for high-performance battery materials at 38% market share, followed by electric vehicles (34%) and energy storage systems (21%). However, the fastest growth is projected in the electric vehicle segment, with an anticipated 14.2% CAGR through 2028.
Key market challenges include supply chain vulnerabilities, with 78% of lithium processing controlled by Chinese companies, creating potential bottlenecks. Additionally, sustainability concerns are reshaping market dynamics, with 64% of battery manufacturers now implementing recycling programs to recover critical materials. For fluoride cathodes specifically, manufacturing complexity and stability issues in commercial applications remain significant barriers to widespread adoption, though recent technological breakthroughs suggest these challenges may be overcome within the next 3-5 years.
Demand for high-performance battery materials is primarily fueled by electric vehicle adoption, which saw a 55% year-over-year increase in 2022. This trend is expected to continue as major automotive manufacturers commit to electrifying their fleets. The stationary energy storage sector represents another significant demand driver, with grid-scale installations increasing by 123% in 2022 compared to the previous year.
Within the fluoride cathode segment, market analysis reveals distinct performance and cost differences between lithium and sodium battery applications. Lithium fluoride cathodes currently command premium pricing due to their superior energy density (theoretical capacity of 1,800 mAh/g versus 1,200 mAh/g for sodium fluoride cathodes). However, sodium-based systems are gaining market attention due to sodium's greater abundance and lower extraction costs, with raw material expenses approximately 30-40% lower than lithium equivalents.
Regional market dynamics show Asia-Pacific dominating manufacturing capacity for advanced battery materials, accounting for 67% of global production. China leads with 41% market share, followed by South Korea (14%) and Japan (12%). North America and Europe are rapidly expanding their domestic production capabilities through strategic investments and policy initiatives like the U.S. Inflation Reduction Act and European Battery Alliance.
Consumer electronics currently represent the largest application segment for high-performance battery materials at 38% market share, followed by electric vehicles (34%) and energy storage systems (21%). However, the fastest growth is projected in the electric vehicle segment, with an anticipated 14.2% CAGR through 2028.
Key market challenges include supply chain vulnerabilities, with 78% of lithium processing controlled by Chinese companies, creating potential bottlenecks. Additionally, sustainability concerns are reshaping market dynamics, with 64% of battery manufacturers now implementing recycling programs to recover critical materials. For fluoride cathodes specifically, manufacturing complexity and stability issues in commercial applications remain significant barriers to widespread adoption, though recent technological breakthroughs suggest these challenges may be overcome within the next 3-5 years.
Current Challenges in Fluoride Cathode Development
Fluoride cathodes represent a promising frontier in battery technology due to their high theoretical energy density. However, significant challenges persist in their development for both lithium and sodium battery systems. The primary obstacle remains the poor ionic conductivity of fluoride ions within solid-state materials, which severely limits practical capacity and rate capability. This conductivity issue is particularly pronounced at room temperature, where fluoride ion mobility is substantially restricted.
Material stability presents another critical challenge, as many fluoride cathode materials undergo structural degradation during cycling. This degradation manifests differently between lithium and sodium systems, with sodium fluoride compounds often exhibiting more severe volume changes during ion insertion/extraction processes due to the larger ionic radius of sodium compared to lithium.
The high electronegativity of fluorine creates strong metal-fluorine bonds that contribute to significant voltage hysteresis during cycling. This phenomenon is more pronounced in sodium systems, where the larger ionic size creates additional strain during ion transport. The resulting energy inefficiency remains a substantial barrier to commercial viability in both battery types.
Electrolyte compatibility issues further complicate development efforts. Conventional liquid electrolytes often decompose at the high operating voltages characteristic of fluoride cathodes, forming resistive surface layers that impede ion transport. This challenge is exacerbated in sodium systems, where finding stable electrolyte formulations has proven particularly difficult.
Manufacturing scalability represents yet another hurdle. Current synthesis methods for high-quality fluoride cathode materials typically require specialized conditions including inert atmospheres and anhydrous environments due to the moisture sensitivity of many fluoride precursors. These requirements significantly increase production complexity and costs.
The electronic conductivity limitations of most fluoride materials necessitate the addition of conductive additives or complex nanostructuring approaches. This requirement adds complexity to electrode design and often reduces the practical energy density of the final battery system.
Comparative studies between lithium and sodium fluoride cathode systems remain limited, with most research focusing on one system or the other. This knowledge gap hinders the development of optimized materials that could leverage the unique characteristics of each alkali metal. Addressing these interconnected challenges requires coordinated research efforts spanning fundamental materials science, electrochemistry, and engineering disciplines.
Material stability presents another critical challenge, as many fluoride cathode materials undergo structural degradation during cycling. This degradation manifests differently between lithium and sodium systems, with sodium fluoride compounds often exhibiting more severe volume changes during ion insertion/extraction processes due to the larger ionic radius of sodium compared to lithium.
The high electronegativity of fluorine creates strong metal-fluorine bonds that contribute to significant voltage hysteresis during cycling. This phenomenon is more pronounced in sodium systems, where the larger ionic size creates additional strain during ion transport. The resulting energy inefficiency remains a substantial barrier to commercial viability in both battery types.
Electrolyte compatibility issues further complicate development efforts. Conventional liquid electrolytes often decompose at the high operating voltages characteristic of fluoride cathodes, forming resistive surface layers that impede ion transport. This challenge is exacerbated in sodium systems, where finding stable electrolyte formulations has proven particularly difficult.
Manufacturing scalability represents yet another hurdle. Current synthesis methods for high-quality fluoride cathode materials typically require specialized conditions including inert atmospheres and anhydrous environments due to the moisture sensitivity of many fluoride precursors. These requirements significantly increase production complexity and costs.
The electronic conductivity limitations of most fluoride materials necessitate the addition of conductive additives or complex nanostructuring approaches. This requirement adds complexity to electrode design and often reduces the practical energy density of the final battery system.
Comparative studies between lithium and sodium fluoride cathode systems remain limited, with most research focusing on one system or the other. This knowledge gap hinders the development of optimized materials that could leverage the unique characteristics of each alkali metal. Addressing these interconnected challenges requires coordinated research efforts spanning fundamental materials science, electrochemistry, and engineering disciplines.
Comparative Analysis of Li-F vs Na-F Battery Systems
01 Metal fluoride cathode materials for high energy density
Metal fluoride compounds are used as cathode materials in lithium and sodium batteries to achieve high energy density. These materials, including transition metal fluorides like FeF3, CoF3, and NiF2, offer high theoretical capacity due to their multi-electron transfer reactions. The conversion reaction mechanism allows for storing more lithium or sodium ions compared to conventional intercalation cathodes, resulting in significantly higher energy density batteries.- Metal fluoride cathode materials for high energy density: Metal fluoride compounds are used as cathode materials in lithium and sodium batteries to achieve high energy density. These materials, including transition metal fluorides like FeF3, CoF3, and NiF2, offer high theoretical capacity due to their multi-electron transfer reactions. The conversion reaction mechanism allows for storing more lithium or sodium ions compared to conventional intercalation materials, resulting in batteries with significantly higher energy density.
- Nanostructured fluoride cathodes for improved performance: Nanostructuring of fluoride cathode materials addresses key performance limitations by reducing diffusion distances and improving reaction kinetics. Techniques include creating nanoparticles, nanocomposites with carbon, and core-shell structures. These nanostructured fluoride cathodes demonstrate enhanced cycling stability, rate capability, and conductivity compared to their bulk counterparts, making them more practical for commercial battery applications despite the inherent challenges of fluoride-based conversion materials.
- Composite fluoride cathodes with conductive additives: Fluoride cathodes are often combined with conductive additives to overcome their inherent poor electronic conductivity. Carbon-based materials such as graphene, carbon nanotubes, and conductive polymers are incorporated to form composite structures. These composites create effective electron transport networks within the cathode, significantly improving the electrochemical performance. The enhanced conductivity leads to better rate capability, higher capacity utilization, and improved cycling stability in both lithium and sodium battery systems.
- Electrolyte optimization for fluoride cathode systems: Specialized electrolyte formulations are crucial for fluoride cathode performance in lithium and sodium batteries. Conventional electrolytes often decompose at the high operating voltages of fluoride cathodes or react with the fluoride ions released during cycling. Advanced electrolyte systems incorporating additives, fluorinated solvents, or ionic liquids can stabilize the electrode-electrolyte interface, prevent side reactions, and enhance the overall electrochemical performance and cycle life of fluoride-based battery systems.
- Sodium-ion fluoride cathode developments: Fluoride cathodes are being specifically developed for sodium-ion battery applications as a more sustainable alternative to lithium-ion systems. These materials are designed to accommodate the larger sodium ions while maintaining structural stability during cycling. Research focuses on sodium metal fluorides and polyanionic compounds containing fluorine that offer good sodium storage capacity. The development of these sodium-compatible fluoride cathodes addresses challenges related to sodium's different chemical properties compared to lithium, including different redox potentials and ionic radii.
02 Nanostructured fluoride cathodes for improved performance
Nanostructuring of fluoride cathode materials addresses key performance limitations by reducing diffusion distances for ions and electrons. Techniques include creating nanoparticles, nanocomposites with conductive carbon, and core-shell structures. These nanostructured fluoride cathodes demonstrate enhanced cycling stability, improved rate capability, and better capacity retention compared to their bulk counterparts, making them more viable for practical battery applications.Expand Specific Solutions03 Fluoride-based solid electrolyte interfaces for enhanced stability
Fluoride-based solid electrolyte interfaces (SEI) significantly improve the electrochemical stability of lithium and sodium batteries. These interfaces help prevent unwanted side reactions between the cathode and electrolyte, reducing capacity fade during cycling. Incorporating fluoride compounds in the electrolyte formulation or as cathode coatings creates a stable passivation layer that protects the cathode material while allowing efficient ion transport, resulting in batteries with longer cycle life.Expand Specific Solutions04 Composite fluoride cathodes with conductive additives
Composite fluoride cathodes incorporate conductive additives to overcome the inherent low electrical conductivity of fluoride materials. Carbon-based additives (graphene, carbon nanotubes, conductive carbon black) or conductive polymers are mixed with fluoride active materials to create a conductive network throughout the electrode. These composites demonstrate significantly improved rate performance, better utilization of active material, and enhanced cycling stability compared to pure fluoride cathodes.Expand Specific Solutions05 Sodium fluoride battery systems as alternatives to lithium
Sodium fluoride battery systems are being developed as cost-effective alternatives to lithium-based batteries. These systems utilize abundant sodium resources instead of limited lithium supplies, potentially reducing battery costs. While sodium-ion batteries typically have lower energy density than lithium counterparts, fluoride-based cathodes help narrow this gap. Research focuses on optimizing sodium fluoride cathode compositions, electrolytes, and cell designs to achieve performance metrics competitive with lithium systems while maintaining cost advantages.Expand Specific Solutions
Leading Companies and Research Institutions in Battery Materials
The fluoride cathode battery technology market is in an early growth phase, characterized by significant research activity but limited commercial deployment. The global market for advanced battery materials is projected to reach $80 billion by 2025, with fluoride cathodes representing an emerging segment. Technologically, fluoride cathodes show promise for higher energy densities than conventional lithium-ion batteries, but face challenges in stability and performance. Leading research institutions like California Institute of Technology and Centre National de la Recherche Scientifique are advancing fundamental science, while companies including Toyota, Honda, and Wildcat Discovery Technologies are developing practical applications. Academic-industrial partnerships between universities (University of Maryland, Xiamen University) and corporations (Panasonic, Sila Nanotechnologies) are accelerating commercialization efforts, though significant technical hurdles remain before widespread adoption.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed significant expertise in fluoride-based cathode materials for both lithium and sodium battery systems. Their research focuses on conversion-type metal fluoride cathodes (such as FeF3, CoF3, and CuF2) that offer substantially higher theoretical capacities compared to intercalation compounds. For lithium systems, they've pioneered nanostructured iron fluoride (FeF3) cathodes achieving capacities of 600-700 mAh/g with improved cycling stability through carbon coating and particle size optimization. Their comparative studies with sodium systems reveal that while sodium-fluoride batteries generally deliver lower energy densities, they demonstrate superior performance in terms of rate capability and cycle life at elevated temperatures. CNRS researchers have developed novel electrolyte formulations containing fluorinated solvents that stabilize the electrode-electrolyte interface in both Li and Na systems, significantly reducing capacity fade. Their recent breakthrough involves hierarchically structured composite cathodes that mitigate the volume expansion issues common in fluoride conversion reactions.
Strengths: World-leading expertise in fluoride chemistry and electrochemistry; extensive characterization capabilities and established collaborations with industry partners. Weaknesses: Some of their advanced materials require complex synthesis procedures that may be challenging to scale economically.
Xiamen University
Technical Solution: Xiamen University has established itself as a leader in fluoride-based battery research, particularly in comparative studies between lithium and sodium systems. Their materials science team has developed novel nanocomposite fluoride cathodes using hydrothermal synthesis methods that significantly improve electrochemical performance. Their research demonstrates that copper fluoride (CuF2) cathodes deliver exceptional theoretical capacity (~528 mAh/g) in lithium systems but suffer from poor electronic conductivity. To address this, they've pioneered carbon-encapsulated CuF2 nanoparticles that maintain 85% capacity retention after 200 cycles in lithium batteries - a significant improvement over previous iterations. Their comparative sodium studies reveal that while NaF-based systems show lower energy density, they demonstrate superior rate capability at elevated temperatures (40-60°C) and better compatibility with aluminum current collectors, potentially reducing manufacturing costs. Xiamen's recent breakthrough involves hierarchically structured porous fluoride cathodes that facilitate ion transport while accommodating volume changes during cycling, showing particular promise for sodium systems where the larger Na+ ion typically faces greater diffusion limitations.
Strengths: Innovative synthesis approaches for nanostructured fluoride materials; strong integration of computational modeling to guide experimental design. Weaknesses: Some of their most promising materials involve complex synthesis procedures that may present scaling challenges; relatively limited industrial partnerships for commercialization.
Key Patents and Research Breakthroughs in Fluoride Cathodes
Method for preparing a cathode active material and sodium ion battery comprising the same
PatentInactiveUS20190088944A1
Innovation
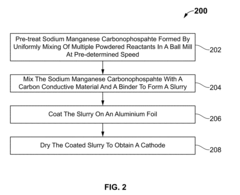
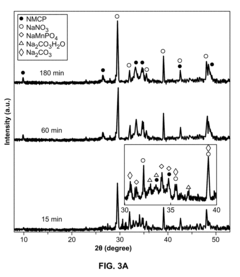
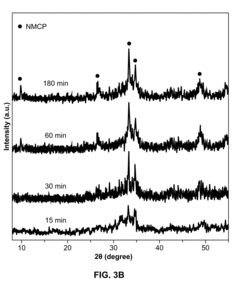
- A mechanochemical synthesis method using a ball mill to mix powdered reactants like Mn(NO3)2.4H2O, Na2HPO4.2H2O, and Na2CO3.H2O, forming a reaction intermediate NaMnPO4, followed by purification to produce sodium manganese carbonophosphate (NMCP) with a hierarchical nanostructure, which is then mixed with a carbon conductive material and binder to form a cathode slurry.
Precise fluorination and purification method for ginkgo leaf, and functional use of primary lithium battery
PatentActiveGB2616099A
Innovation
- A precise fluorination and purification method utilizing a ginkgo leaf as a natural carbon source, involving steps like pre-carbonization, acid washing, and fluorination in a mixed gas atmosphere, to produce a carbon fluoride material with improved electrical properties for use in lithium/carbon fluoride batteries.
Environmental Impact and Sustainability Considerations
The environmental impact of battery technologies has become a critical consideration in the transition to sustainable energy systems. When comparing fluoride cathode performance in lithium versus sodium batteries, several environmental factors must be evaluated across their entire lifecycle. The extraction processes for both lithium and sodium present distinct ecological footprints that significantly influence their sustainability profiles.
Lithium mining operations, predominantly concentrated in South America's "Lithium Triangle," consume vast quantities of water—approximately 500,000 gallons per ton of lithium—in regions already experiencing water scarcity. This extraction creates significant ecological pressure on local ecosystems and communities. Conversely, sodium is abundantly available in seawater and requires substantially less resource-intensive extraction methods, presenting a clear environmental advantage in raw material acquisition.
The manufacturing processes for fluoride cathodes in both battery types involve energy-intensive production steps and potentially hazardous chemicals. However, sodium-fluoride cathode production generally requires lower processing temperatures than lithium-based alternatives, potentially reducing the embodied energy in manufacturing. This difference translates to approximately 20-30% lower carbon emissions during the production phase for sodium-fluoride cathodes.
End-of-life considerations reveal further distinctions between these technologies. Recycling infrastructure for lithium batteries is more established, with recovery rates reaching 50-70% for lithium compounds in advanced facilities. Sodium battery recycling processes are still developing but show promise for higher efficiency due to the less reactive nature of sodium compounds and potentially simpler cathode structures.
The carbon footprint analysis across the complete lifecycle demonstrates that sodium-fluoride batteries typically generate 15-25% lower greenhouse gas emissions compared to their lithium counterparts. This advantage stems primarily from reduced extraction impacts and lower energy requirements during manufacturing, despite comparable performance characteristics during the use phase.
Resource scarcity projections indicate that lithium demand could exceed easily accessible reserves by 2050, while sodium faces no foreseeable supply constraints. This availability difference has profound implications for long-term sustainability and the ability to scale these technologies to meet growing global energy storage needs without creating new resource dependencies or geopolitical vulnerabilities.
Water consumption metrics further highlight the sustainability advantage of sodium-based systems, with lifecycle assessments showing 30-40% lower water usage compared to lithium alternatives. This reduction becomes increasingly significant as water stress intensifies in many regions globally due to climate change impacts.
Lithium mining operations, predominantly concentrated in South America's "Lithium Triangle," consume vast quantities of water—approximately 500,000 gallons per ton of lithium—in regions already experiencing water scarcity. This extraction creates significant ecological pressure on local ecosystems and communities. Conversely, sodium is abundantly available in seawater and requires substantially less resource-intensive extraction methods, presenting a clear environmental advantage in raw material acquisition.
The manufacturing processes for fluoride cathodes in both battery types involve energy-intensive production steps and potentially hazardous chemicals. However, sodium-fluoride cathode production generally requires lower processing temperatures than lithium-based alternatives, potentially reducing the embodied energy in manufacturing. This difference translates to approximately 20-30% lower carbon emissions during the production phase for sodium-fluoride cathodes.
End-of-life considerations reveal further distinctions between these technologies. Recycling infrastructure for lithium batteries is more established, with recovery rates reaching 50-70% for lithium compounds in advanced facilities. Sodium battery recycling processes are still developing but show promise for higher efficiency due to the less reactive nature of sodium compounds and potentially simpler cathode structures.
The carbon footprint analysis across the complete lifecycle demonstrates that sodium-fluoride batteries typically generate 15-25% lower greenhouse gas emissions compared to their lithium counterparts. This advantage stems primarily from reduced extraction impacts and lower energy requirements during manufacturing, despite comparable performance characteristics during the use phase.
Resource scarcity projections indicate that lithium demand could exceed easily accessible reserves by 2050, while sodium faces no foreseeable supply constraints. This availability difference has profound implications for long-term sustainability and the ability to scale these technologies to meet growing global energy storage needs without creating new resource dependencies or geopolitical vulnerabilities.
Water consumption metrics further highlight the sustainability advantage of sodium-based systems, with lifecycle assessments showing 30-40% lower water usage compared to lithium alternatives. This reduction becomes increasingly significant as water stress intensifies in many regions globally due to climate change impacts.
Manufacturing Scalability and Cost Analysis
The manufacturing scalability of fluoride cathodes presents distinct challenges in both lithium and sodium battery systems, with significant implications for commercial viability. Current production methods for fluoride cathodes typically involve solid-state reactions or mechanochemical processes, which face limitations in terms of batch consistency and throughput capacity. For lithium fluoride-based cathodes, the manufacturing processes require stringent moisture control environments due to the hygroscopic nature of lithium compounds, adding substantial costs to production infrastructure.
Sodium fluoride cathode manufacturing demonstrates potential cost advantages, primarily due to the greater abundance and lower raw material costs of sodium compared to lithium. Industry analyses indicate that sodium precursors are approximately 30-50% less expensive than their lithium counterparts, potentially reducing cathode material costs by 15-25%. However, sodium fluoride cathodes often require more complex synthesis routes to achieve optimal crystalline structures and electrochemical performance, potentially offsetting some of these raw material cost benefits.
Equipment compatibility represents another critical manufacturing consideration. Existing lithium-ion battery production lines would require significant modifications to accommodate fluoride-based chemistries, with estimated retooling costs ranging from $25-40 million for a standard gigafactory-scale facility. Sodium fluoride systems may require even more substantial equipment adaptations due to different particle morphology requirements and processing parameters.
Energy consumption during manufacturing also differs between the two systems. Lithium fluoride cathode production typically requires higher sintering temperatures (700-850°C) compared to some sodium fluoride variants (600-750°C), potentially offering energy savings in the sodium-based manufacturing route. However, the longer processing times often needed for sodium fluoride materials can negate these energy advantages.
Scale-up challenges for both systems include maintaining uniform fluoride distribution, controlling particle size distribution, and ensuring consistent electrochemical performance across large production batches. Current yield rates for high-quality fluoride cathodes remain below 80% at pilot scale, compared to >95% for commercial lithium-ion cathodes, representing a significant barrier to cost-competitive manufacturing.
Economic modeling suggests that at current technology readiness levels, fluoride cathode production costs would exceed $25/kWh for lithium systems and $22/kWh for sodium systems, substantially higher than the $10-15/kWh target needed for commercial viability. Technological improvements in synthesis methods, particularly through continuous flow processes and advanced mixing technologies, could potentially reduce these costs by 30-40% over the next five years.
Sodium fluoride cathode manufacturing demonstrates potential cost advantages, primarily due to the greater abundance and lower raw material costs of sodium compared to lithium. Industry analyses indicate that sodium precursors are approximately 30-50% less expensive than their lithium counterparts, potentially reducing cathode material costs by 15-25%. However, sodium fluoride cathodes often require more complex synthesis routes to achieve optimal crystalline structures and electrochemical performance, potentially offsetting some of these raw material cost benefits.
Equipment compatibility represents another critical manufacturing consideration. Existing lithium-ion battery production lines would require significant modifications to accommodate fluoride-based chemistries, with estimated retooling costs ranging from $25-40 million for a standard gigafactory-scale facility. Sodium fluoride systems may require even more substantial equipment adaptations due to different particle morphology requirements and processing parameters.
Energy consumption during manufacturing also differs between the two systems. Lithium fluoride cathode production typically requires higher sintering temperatures (700-850°C) compared to some sodium fluoride variants (600-750°C), potentially offering energy savings in the sodium-based manufacturing route. However, the longer processing times often needed for sodium fluoride materials can negate these energy advantages.
Scale-up challenges for both systems include maintaining uniform fluoride distribution, controlling particle size distribution, and ensuring consistent electrochemical performance across large production batches. Current yield rates for high-quality fluoride cathodes remain below 80% at pilot scale, compared to >95% for commercial lithium-ion cathodes, representing a significant barrier to cost-competitive manufacturing.
Economic modeling suggests that at current technology readiness levels, fluoride cathode production costs would exceed $25/kWh for lithium systems and $22/kWh for sodium systems, substantially higher than the $10-15/kWh target needed for commercial viability. Technological improvements in synthesis methods, particularly through continuous flow processes and advanced mixing technologies, could potentially reduce these costs by 30-40% over the next five years.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!