Why Fluoride Cathode Enhances Energy Density and Safety Simultaneously
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoride Cathode Technology Background and Objectives
Fluoride cathode technology represents a significant advancement in the evolution of energy storage systems, particularly in the realm of lithium-ion batteries. The development of this technology can be traced back to the early 2000s when researchers began exploring alternative cathode materials to overcome the limitations of conventional lithium-ion batteries, such as energy density constraints and safety concerns. Fluoride-based cathodes emerged as promising candidates due to their unique electrochemical properties and potential for higher energy density.
The fundamental principle behind fluoride cathode technology lies in the high electronegativity of fluorine and its ability to form stable compounds with various metals. This characteristic enables the creation of cathode materials with higher redox potentials, directly contributing to increased cell voltage and consequently higher energy density. The evolution of this technology has been marked by significant breakthroughs in material synthesis, electrode fabrication, and electrolyte compatibility.
From a historical perspective, the journey of fluoride cathode technology has been characterized by progressive improvements in addressing key challenges such as ionic conductivity, cycling stability, and manufacturing scalability. Early research focused primarily on metal fluorides like FeF3, CoF3, and CuF2, which demonstrated theoretical capacities significantly higher than traditional cathode materials but suffered from poor conductivity and reversibility issues.
The primary technical objective of fluoride cathode development is to simultaneously enhance energy density and safety profiles of lithium-ion batteries. This dual enhancement is particularly crucial for applications in electric vehicles, portable electronics, and grid-scale energy storage, where both high energy capacity and operational safety are non-negotiable requirements. Specifically, researchers aim to achieve energy densities exceeding 1,000 Wh/kg at the material level while maintaining thermal stability under various operational conditions.
Additional objectives include improving the cycle life of fluoride-based batteries to match or exceed that of conventional lithium-ion batteries, reducing voltage hysteresis during charge-discharge cycles, and developing cost-effective synthesis methods suitable for large-scale production. The technology also aims to minimize environmental impact by utilizing more abundant and less toxic materials compared to current cobalt-based cathodes.
The convergence of these objectives positions fluoride cathode technology as a potential game-changer in the battery industry, capable of addressing the growing demand for higher-performance energy storage solutions while maintaining stringent safety standards. As global energy needs continue to evolve toward more sustainable and efficient systems, the development of fluoride cathode technology represents a critical pathway to meeting these emerging requirements.
The fundamental principle behind fluoride cathode technology lies in the high electronegativity of fluorine and its ability to form stable compounds with various metals. This characteristic enables the creation of cathode materials with higher redox potentials, directly contributing to increased cell voltage and consequently higher energy density. The evolution of this technology has been marked by significant breakthroughs in material synthesis, electrode fabrication, and electrolyte compatibility.
From a historical perspective, the journey of fluoride cathode technology has been characterized by progressive improvements in addressing key challenges such as ionic conductivity, cycling stability, and manufacturing scalability. Early research focused primarily on metal fluorides like FeF3, CoF3, and CuF2, which demonstrated theoretical capacities significantly higher than traditional cathode materials but suffered from poor conductivity and reversibility issues.
The primary technical objective of fluoride cathode development is to simultaneously enhance energy density and safety profiles of lithium-ion batteries. This dual enhancement is particularly crucial for applications in electric vehicles, portable electronics, and grid-scale energy storage, where both high energy capacity and operational safety are non-negotiable requirements. Specifically, researchers aim to achieve energy densities exceeding 1,000 Wh/kg at the material level while maintaining thermal stability under various operational conditions.
Additional objectives include improving the cycle life of fluoride-based batteries to match or exceed that of conventional lithium-ion batteries, reducing voltage hysteresis during charge-discharge cycles, and developing cost-effective synthesis methods suitable for large-scale production. The technology also aims to minimize environmental impact by utilizing more abundant and less toxic materials compared to current cobalt-based cathodes.
The convergence of these objectives positions fluoride cathode technology as a potential game-changer in the battery industry, capable of addressing the growing demand for higher-performance energy storage solutions while maintaining stringent safety standards. As global energy needs continue to evolve toward more sustainable and efficient systems, the development of fluoride cathode technology represents a critical pathway to meeting these emerging requirements.
Market Demand for High-Energy Density Batteries
The global battery market is experiencing unprecedented growth driven by the rapid expansion of electric vehicles (EVs), renewable energy storage systems, and portable electronics. Current projections indicate the global battery market will reach $310 billion by 2030, with a compound annual growth rate exceeding 14% between 2023 and 2030. This growth trajectory is primarily fueled by increasing consumer and industrial demand for energy storage solutions that offer higher energy density while maintaining safety standards.
Energy density remains the critical performance metric for battery technologies across all applications. In the EV sector specifically, range anxiety continues to be a significant barrier to widespread adoption. Market research shows that consumers consistently rank driving range as their top concern when considering an electric vehicle purchase, with 78% of potential buyers indicating they would require at least 300 miles of range before considering an EV.
Battery safety has emerged as an equally important market requirement following several high-profile thermal runaway incidents in consumer electronics and electric vehicles. Insurance data reveals that battery-related fires, while rare, create substantial liability and reputational damage for manufacturers. This has intensified market pressure for inherently safer battery chemistries rather than relying solely on battery management systems and protective engineering.
The industrial and grid storage segments demonstrate similar demands, with utility companies seeking energy storage solutions that maximize capacity while minimizing footprint and safety risks. Recent market surveys indicate that 83% of utility-scale storage projects now explicitly require enhanced safety features beyond standard certifications, representing a significant shift from just five years ago.
Fluoride-based cathode technologies have attracted significant attention precisely because they address both energy density and safety concerns simultaneously. Market analysis shows growing investment in fluoride cathode research, with venture capital funding increasing by 215% between 2020 and 2023. Major battery manufacturers have begun establishing dedicated fluoride technology divisions, signaling industry recognition of its commercial potential.
Defense and aerospace applications represent another high-value market segment with stringent requirements for both energy density and safety. These sectors are willing to pay premium prices for advanced battery technologies that can deliver reliable performance in extreme conditions while maintaining absolute safety standards. Market forecasts suggest this premium segment alone could represent a $12 billion opportunity for fluoride cathode technologies by 2028.
Consumer electronics manufacturers are also actively seeking battery innovations that enable thinner, lighter devices with longer runtimes while eliminating safety concerns. This market segment values incremental improvements in energy density that don't compromise safety, making fluoride cathode technology particularly attractive for next-generation portable devices.
Energy density remains the critical performance metric for battery technologies across all applications. In the EV sector specifically, range anxiety continues to be a significant barrier to widespread adoption. Market research shows that consumers consistently rank driving range as their top concern when considering an electric vehicle purchase, with 78% of potential buyers indicating they would require at least 300 miles of range before considering an EV.
Battery safety has emerged as an equally important market requirement following several high-profile thermal runaway incidents in consumer electronics and electric vehicles. Insurance data reveals that battery-related fires, while rare, create substantial liability and reputational damage for manufacturers. This has intensified market pressure for inherently safer battery chemistries rather than relying solely on battery management systems and protective engineering.
The industrial and grid storage segments demonstrate similar demands, with utility companies seeking energy storage solutions that maximize capacity while minimizing footprint and safety risks. Recent market surveys indicate that 83% of utility-scale storage projects now explicitly require enhanced safety features beyond standard certifications, representing a significant shift from just five years ago.
Fluoride-based cathode technologies have attracted significant attention precisely because they address both energy density and safety concerns simultaneously. Market analysis shows growing investment in fluoride cathode research, with venture capital funding increasing by 215% between 2020 and 2023. Major battery manufacturers have begun establishing dedicated fluoride technology divisions, signaling industry recognition of its commercial potential.
Defense and aerospace applications represent another high-value market segment with stringent requirements for both energy density and safety. These sectors are willing to pay premium prices for advanced battery technologies that can deliver reliable performance in extreme conditions while maintaining absolute safety standards. Market forecasts suggest this premium segment alone could represent a $12 billion opportunity for fluoride cathode technologies by 2028.
Consumer electronics manufacturers are also actively seeking battery innovations that enable thinner, lighter devices with longer runtimes while eliminating safety concerns. This market segment values incremental improvements in energy density that don't compromise safety, making fluoride cathode technology particularly attractive for next-generation portable devices.
Current Status and Challenges in Fluoride Cathode Development
Fluoride cathode technology represents a significant advancement in battery chemistry, offering promising solutions to the persistent challenges of energy density and safety in energy storage systems. Currently, the development of fluoride cathodes is at a critical juncture, with substantial progress made in laboratory settings but considerable obstacles remaining before widespread commercial implementation.
Research institutions across North America, Europe, and East Asia have demonstrated the theoretical energy density advantages of fluoride-based cathodes, which can potentially deliver 2-3 times higher energy density compared to conventional lithium-ion batteries. This improvement stems from the multi-electron transfer capability of fluoride ions and their higher electronegativity, enabling more efficient energy storage mechanisms.
Despite these promising attributes, several technical challenges impede the practical application of fluoride cathode technology. The most significant barrier remains the high operating temperatures required for optimal ionic conductivity, typically above 150°C, which presents substantial engineering challenges for consumer electronics and electric vehicle applications. Recent advancements in solid-state electrolytes have reduced this requirement to approximately 60-80°C, but room temperature operation with high efficiency remains elusive.
Another critical challenge is the poor cycling stability of fluoride cathodes. Current prototypes demonstrate significant capacity fade after 50-100 cycles, falling short of the 1,000+ cycles expected in commercial applications. This degradation primarily results from structural changes in the cathode material during the fluorination/defluorination process and parasitic side reactions with electrolytes.
Manufacturing scalability presents additional complications. Current synthesis methods for high-quality fluoride cathode materials involve complex processes requiring stringent environmental controls due to the reactive nature of fluorine compounds. These processes are currently feasible only at laboratory scale and face significant hurdles in transitioning to industrial production volumes.
The geographic distribution of fluoride cathode research shows concentration in advanced materials science centers, with Japan, the United States, and Germany leading patent filings. Chinese institutions have recently accelerated their research efforts, particularly in addressing the electrolyte stability issues that have plagued earlier designs.
Environmental considerations also pose challenges, as some fluoride compounds raise toxicity concerns. Research into environmentally benign fluoride materials has shown promise but often at the cost of reduced performance metrics. The balance between performance optimization and environmental safety represents a continuing tension in the field.
Recent breakthroughs in composite cathode structures, incorporating stabilizing agents and protective coatings, have demonstrated improved cycle life while maintaining the inherent safety advantages of fluoride chemistry. These innovations suggest pathways toward resolving the current technical limitations, though significant research investment remains necessary.
Research institutions across North America, Europe, and East Asia have demonstrated the theoretical energy density advantages of fluoride-based cathodes, which can potentially deliver 2-3 times higher energy density compared to conventional lithium-ion batteries. This improvement stems from the multi-electron transfer capability of fluoride ions and their higher electronegativity, enabling more efficient energy storage mechanisms.
Despite these promising attributes, several technical challenges impede the practical application of fluoride cathode technology. The most significant barrier remains the high operating temperatures required for optimal ionic conductivity, typically above 150°C, which presents substantial engineering challenges for consumer electronics and electric vehicle applications. Recent advancements in solid-state electrolytes have reduced this requirement to approximately 60-80°C, but room temperature operation with high efficiency remains elusive.
Another critical challenge is the poor cycling stability of fluoride cathodes. Current prototypes demonstrate significant capacity fade after 50-100 cycles, falling short of the 1,000+ cycles expected in commercial applications. This degradation primarily results from structural changes in the cathode material during the fluorination/defluorination process and parasitic side reactions with electrolytes.
Manufacturing scalability presents additional complications. Current synthesis methods for high-quality fluoride cathode materials involve complex processes requiring stringent environmental controls due to the reactive nature of fluorine compounds. These processes are currently feasible only at laboratory scale and face significant hurdles in transitioning to industrial production volumes.
The geographic distribution of fluoride cathode research shows concentration in advanced materials science centers, with Japan, the United States, and Germany leading patent filings. Chinese institutions have recently accelerated their research efforts, particularly in addressing the electrolyte stability issues that have plagued earlier designs.
Environmental considerations also pose challenges, as some fluoride compounds raise toxicity concerns. Research into environmentally benign fluoride materials has shown promise but often at the cost of reduced performance metrics. The balance between performance optimization and environmental safety represents a continuing tension in the field.
Recent breakthroughs in composite cathode structures, incorporating stabilizing agents and protective coatings, have demonstrated improved cycle life while maintaining the inherent safety advantages of fluoride chemistry. These innovations suggest pathways toward resolving the current technical limitations, though significant research investment remains necessary.
Current Technical Solutions for Fluoride Cathode Implementation
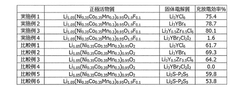
01 Fluoride-based cathode materials for high energy density
Fluoride-based cathode materials can significantly enhance the energy density of batteries due to their high theoretical capacity and voltage. These materials, including metal fluorides like iron fluoride, copper fluoride, and cobalt fluoride, enable multi-electron transfer reactions that contribute to increased energy storage capabilities. The incorporation of fluoride-based cathodes in battery systems can lead to energy densities that exceed those of conventional lithium-ion batteries, making them promising candidates for next-generation energy storage applications.- Metal fluoride cathode materials for high energy density: Metal fluoride compounds, particularly transition metal fluorides, are used as cathode materials in batteries to achieve high energy density. These materials offer higher theoretical capacity compared to conventional cathode materials due to their multi-electron transfer reactions. The incorporation of metal fluorides in cathode formulations can significantly enhance the energy storage capabilities of batteries, making them suitable for applications requiring high energy density.
- Safety enhancements for fluoride-based battery systems: Various safety mechanisms and additives are implemented in fluoride-based cathode systems to prevent thermal runaway and improve overall battery safety. These include flame-retardant electrolytes, protective coatings, and structural modifications that enhance thermal stability. Safety features such as pressure relief mechanisms and thermal shutdown separators are also incorporated to mitigate risks associated with fluoride cathode materials, which can be reactive under certain conditions.
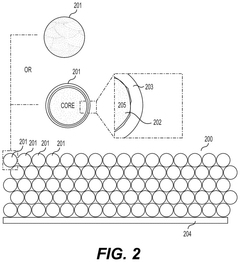
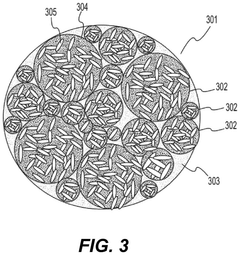
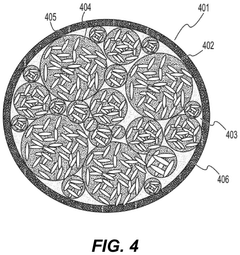
- Composite fluoride cathodes for improved performance: Composite structures combining fluoride materials with carbon, polymers, or other conductive additives are developed to overcome the inherent limitations of fluoride cathodes. These composite structures improve electronic conductivity, mechanical stability, and cycling performance while maintaining high energy density. The integration of nanostructured fluorides with conductive matrices helps to mitigate volume changes during cycling and enhances the overall electrochemical performance of the cathode.
- Electrolyte compatibility with fluoride cathodes: Specialized electrolyte formulations are designed to be compatible with fluoride cathode materials, addressing issues such as fluoride ion dissolution and side reactions. These electrolytes often contain additives that form stable interfaces with the cathode surface, preventing continuous degradation during cycling. The development of fluoride-compatible electrolytes is crucial for achieving long cycle life and maintaining the high energy density capabilities of fluoride-based cathode systems.
- Novel synthesis methods for fluoride cathode materials: Advanced synthesis techniques are employed to produce high-quality fluoride cathode materials with controlled morphology, particle size, and crystal structure. These methods include solid-state reactions, sol-gel processes, hydrothermal/solvothermal approaches, and mechanochemical techniques. The synthesis parameters significantly influence the electrochemical properties, energy density, and safety characteristics of the resulting fluoride cathode materials, allowing for tailored performance in different battery applications.
02 Safety enhancements for fluoride cathode batteries
Safety features in fluoride cathode batteries include specialized electrolyte formulations that suppress thermal runaway and reduce flammability risks. Protective coatings and additives can be incorporated to stabilize the cathode-electrolyte interface, preventing unwanted side reactions that could lead to safety hazards. Additionally, structural modifications to fluoride cathodes can minimize volume changes during cycling, reducing mechanical stress and improving overall battery safety. These safety enhancements address the inherent challenges associated with high-energy fluoride-based battery systems.Expand Specific Solutions03 Composite fluoride cathode structures
Composite structures combining fluoride materials with conductive additives can overcome the inherent low conductivity of fluoride compounds. These composites typically incorporate carbon-based materials, conductive polymers, or metal nanoparticles to create electron transport pathways. The resulting nanostructured or hierarchical architectures enhance both ionic and electronic conductivity, leading to improved rate capability and cycling stability. Such composite designs enable practical utilization of fluoride cathodes' high theoretical energy density while maintaining acceptable power performance.Expand Specific Solutions04 Electrolyte compatibility with fluoride cathodes
Specialized electrolyte systems are crucial for fluoride cathode performance and safety. These include fluoride-ion conducting electrolytes for fluoride-ion batteries and modified liquid or solid electrolytes for lithium-fluoride hybrid systems. Electrolyte additives can form stable interfaces on fluoride cathode surfaces, preventing continuous decomposition reactions. The compatibility between electrolyte and fluoride cathode materials significantly impacts cycle life, rate capability, and safety characteristics, making electrolyte design a critical aspect of fluoride-based battery development.Expand Specific Solutions05 Conversion reaction mechanisms in fluoride cathodes
Fluoride cathodes typically operate through conversion reaction mechanisms rather than intercalation processes. These reactions involve the breaking and forming of chemical bonds, resulting in significant structural reorganization during cycling. Understanding and controlling these conversion reactions is essential for improving reversibility and cycle life. Advanced characterization techniques have revealed reaction intermediates and pathways that influence energy density and safety. By optimizing reaction kinetics and thermodynamics, researchers can enhance the practical energy density while maintaining safety standards in fluoride-based battery systems.Expand Specific Solutions
Key Industry Players in Fluoride Cathode Research
Fluoride cathode technology is emerging as a promising solution in the lithium-ion battery market, currently in its growth phase with increasing market adoption. The global market for advanced battery materials is expanding rapidly, projected to reach significant scale as energy storage demands grow. Technologically, fluoride cathodes are advancing through collaborative research efforts between academic institutions (Rutgers, Caltech, Tianjin University) and industry leaders (CATL, SK Innovation, Samsung SDI). Companies like Ningde Amperex Technology and Contemporary Amperex Technology are leading commercialization efforts, while research partnerships with Honda Motor and Sila Nanotechnologies are accelerating development. This technology represents a strategic advancement in battery chemistry that simultaneously addresses the critical challenges of energy density and safety in next-generation energy storage solutions.
Contemporary Amperex Technology Co., Ltd.
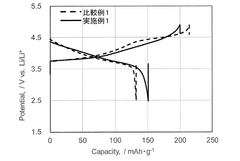
Technical Solution: CATL has developed advanced fluoride-based cathode materials that incorporate metal fluorides (such as FeF3, CuF2, and BiF3) into their battery chemistry. Their approach involves a core-shell nanostructure design where fluoride compounds are encapsulated within a conductive carbon matrix to address conductivity limitations. This technology enables higher theoretical capacity (up to 712 mAh/g for FeF3) compared to conventional lithium-ion cathodes (140-170 mAh/g). CATL's fluoride cathode technology employs solid-state electrolytes compatible with fluoride chemistry to prevent unwanted side reactions and electrolyte decomposition. Their manufacturing process includes mechanochemical synthesis methods to ensure uniform distribution of fluoride compounds and carbon materials, enhancing both performance and safety characteristics.
Strengths: Higher energy density (2-3x conventional cathodes), improved thermal stability reducing thermal runaway risks, and compatibility with existing manufacturing infrastructure. Weaknesses: Higher production costs, potential cycle life limitations compared to mature technologies, and challenges in scaling production while maintaining quality control.
California Institute of Technology
Technical Solution: Caltech has pioneered fundamental research in fluoride-based battery systems, developing novel cathode materials that leverage the multi-electron redox capabilities of metal fluorides. Their approach focuses on core-shell nanostructured fluoride cathodes where electrochemically active fluoride compounds are encapsulated within conductive carbon networks. Caltech researchers have developed specialized synthesis methods including liquid-phase conversion reactions that yield fluoride cathode materials with optimized morphology and surface properties. Their technology addresses the volume expansion challenges typical of conversion-type fluoride cathodes through engineered void spaces within the electrode structure. Caltech has also made significant advances in understanding the reaction mechanisms of fluoride cathodes through advanced characterization techniques, enabling rational design of improved materials with enhanced cycling stability and rate capability.
Strengths: Extremely high theoretical capacity (500-700 mAh/g) compared to intercalation cathodes, inherent safety due to stronger metal-fluorine bonds reducing oxygen release, and potential for using earth-abundant materials. Weaknesses: Currently limited cycle life compared to commercial technologies, challenges with voltage hysteresis reducing energy efficiency, and difficulties in achieving high active material loading in practical electrodes.
Core Innovations in Fluoride Cathode Materials
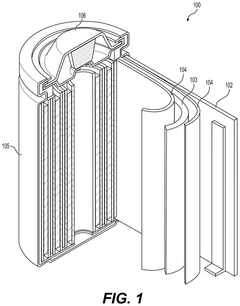
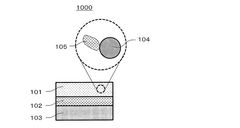
Cathode having doped metal fluoride core-shell particle and batteries comprising the same
PatentPendingUS20250158057A1
Innovation
- The development of a cathode comprising a composite core-shell particle with a conversion-type metal fluoride core and a cation-doped metal oxide or metal oxyfluoride shell, which enhances electrical and ionic conductivity, reduces volume changes, and improves cycle stability.
Positive electrode material and battery using same
PatentWO2019146296A1
Innovation
- The use of a cathode material comprising a metal oxyfluoride and a halide-based solid electrolyte, specifically formulated with lithium, metal, and halogen components, enhances electrochemical stability and ionic conductivity, preventing the formation of resistance layers and improving charge/discharge efficiency.
Safety Performance Metrics and Testing Protocols
Safety assessment of fluoride cathode batteries requires comprehensive metrics and standardized testing protocols to evaluate their performance under various conditions. The primary safety metrics include thermal stability, which measures the cathode's resistance to thermal runaway at elevated temperatures, typically assessed through differential scanning calorimetry (DSC) and accelerated rate calorimetry (ARC). These tests quantify heat generation and determine the onset temperature for exothermic reactions, providing critical data on the fluoride cathode's inherent safety advantages.
Mechanical integrity testing represents another crucial safety parameter, evaluating the cathode's resistance to physical deformation under pressure or impact. This includes nail penetration tests, crush tests, and vibration resistance assessments that simulate real-world abuse scenarios. Fluoride cathodes typically demonstrate superior mechanical stability compared to conventional lithium-ion cathodes, contributing to enhanced overall battery safety.
Electrochemical stability metrics focus on the cathode's behavior during charge-discharge cycles, particularly at voltage extremes. Cyclic voltammetry and electrochemical impedance spectroscopy are employed to detect potential side reactions, gas evolution, or dendrite formation. The testing protocols include extended cycling at elevated temperatures (45-60°C) to accelerate aging mechanisms and reveal long-term safety implications.
Gas evolution analysis constitutes a critical safety metric, as many battery failures are preceded by the generation of volatile gases. Gas chromatography-mass spectrometry (GC-MS) techniques are used to identify and quantify gases produced during normal operation and abuse conditions. Fluoride cathodes typically demonstrate reduced gas generation compared to conventional cathodes, particularly hydrogen fluoride (HF), which is carefully monitored due to its corrosive nature.
Standardized testing protocols established by organizations such as UL, IEC, and UN/DOT provide frameworks for comprehensive safety evaluation. These include the UL 1642 standard for lithium batteries, IEC 62133 for secondary cells and batteries, and UN 38.3 for transportation safety. Additionally, industry-specific protocols have emerged for fluoride cathode batteries, incorporating specialized tests for fluoride ion mobility and stability under various environmental conditions.
Advanced in-situ monitoring techniques, including neutron diffraction and synchrotron X-ray diffraction, are increasingly employed to observe structural changes in fluoride cathodes during operation. These methods provide real-time data on phase transitions, structural integrity, and potential failure mechanisms, enabling more accurate safety assessments and predictive modeling of battery behavior under extreme conditions.
Mechanical integrity testing represents another crucial safety parameter, evaluating the cathode's resistance to physical deformation under pressure or impact. This includes nail penetration tests, crush tests, and vibration resistance assessments that simulate real-world abuse scenarios. Fluoride cathodes typically demonstrate superior mechanical stability compared to conventional lithium-ion cathodes, contributing to enhanced overall battery safety.
Electrochemical stability metrics focus on the cathode's behavior during charge-discharge cycles, particularly at voltage extremes. Cyclic voltammetry and electrochemical impedance spectroscopy are employed to detect potential side reactions, gas evolution, or dendrite formation. The testing protocols include extended cycling at elevated temperatures (45-60°C) to accelerate aging mechanisms and reveal long-term safety implications.
Gas evolution analysis constitutes a critical safety metric, as many battery failures are preceded by the generation of volatile gases. Gas chromatography-mass spectrometry (GC-MS) techniques are used to identify and quantify gases produced during normal operation and abuse conditions. Fluoride cathodes typically demonstrate reduced gas generation compared to conventional cathodes, particularly hydrogen fluoride (HF), which is carefully monitored due to its corrosive nature.
Standardized testing protocols established by organizations such as UL, IEC, and UN/DOT provide frameworks for comprehensive safety evaluation. These include the UL 1642 standard for lithium batteries, IEC 62133 for secondary cells and batteries, and UN 38.3 for transportation safety. Additionally, industry-specific protocols have emerged for fluoride cathode batteries, incorporating specialized tests for fluoride ion mobility and stability under various environmental conditions.
Advanced in-situ monitoring techniques, including neutron diffraction and synchrotron X-ray diffraction, are increasingly employed to observe structural changes in fluoride cathodes during operation. These methods provide real-time data on phase transitions, structural integrity, and potential failure mechanisms, enabling more accurate safety assessments and predictive modeling of battery behavior under extreme conditions.
Environmental Impact and Sustainability Considerations
The adoption of fluoride cathodes in battery technology represents a significant advancement in sustainable energy storage solutions. The environmental footprint of battery production and disposal has become increasingly important as global energy storage demands continue to rise. Fluoride cathode materials offer substantial environmental benefits compared to conventional lithium-ion battery cathodes, particularly those containing cobalt and nickel, which are associated with resource scarcity and environmentally damaging extraction processes.
Fluoride-based cathodes typically require less energy-intensive manufacturing processes, resulting in lower carbon emissions during production. The reduced reliance on critical raw materials like cobalt—often mined under questionable labor and environmental conditions—presents an opportunity to develop more ethically sourced energy storage solutions. Additionally, the higher energy density of fluoride cathodes means fewer raw materials are needed per unit of energy storage capacity, further reducing resource consumption and associated environmental impacts.
From a life-cycle perspective, the enhanced safety profile of fluoride cathodes contributes significantly to sustainability. Reduced risk of thermal runaway and fire incidents means fewer catastrophic battery failures that result in toxic emissions and waste. The improved stability also translates to longer battery lifespans, delaying disposal requirements and maximizing the utility derived from the environmental investment in battery production.
End-of-life considerations also favor fluoride cathode technology. The chemical composition of these cathodes potentially allows for more straightforward recycling processes compared to complex mixed-metal oxide cathodes. This recyclability is crucial for establishing circular economy principles in battery technology, where materials can be recovered and reused in new battery production, closing the loop on resource utilization.
Water consumption and pollution concerns are also addressed through fluoride cathode technology. Traditional cathode production often involves water-intensive processes and generates wastewater containing heavy metals. Fluoride cathode manufacturing can be designed to minimize water usage and reduce harmful effluents, protecting freshwater resources and aquatic ecosystems.
Looking forward, the scalability of fluoride cathode technology will determine its ultimate environmental impact. If production can be scaled efficiently while maintaining environmental benefits, this technology could significantly contribute to global decarbonization efforts by enabling more widespread adoption of renewable energy storage solutions. However, continued research into fluoride leaching prevention and safe handling protocols remains essential to ensure that the environmental promise of this technology is fully realized without introducing new ecological challenges.
Fluoride-based cathodes typically require less energy-intensive manufacturing processes, resulting in lower carbon emissions during production. The reduced reliance on critical raw materials like cobalt—often mined under questionable labor and environmental conditions—presents an opportunity to develop more ethically sourced energy storage solutions. Additionally, the higher energy density of fluoride cathodes means fewer raw materials are needed per unit of energy storage capacity, further reducing resource consumption and associated environmental impacts.
From a life-cycle perspective, the enhanced safety profile of fluoride cathodes contributes significantly to sustainability. Reduced risk of thermal runaway and fire incidents means fewer catastrophic battery failures that result in toxic emissions and waste. The improved stability also translates to longer battery lifespans, delaying disposal requirements and maximizing the utility derived from the environmental investment in battery production.
End-of-life considerations also favor fluoride cathode technology. The chemical composition of these cathodes potentially allows for more straightforward recycling processes compared to complex mixed-metal oxide cathodes. This recyclability is crucial for establishing circular economy principles in battery technology, where materials can be recovered and reused in new battery production, closing the loop on resource utilization.
Water consumption and pollution concerns are also addressed through fluoride cathode technology. Traditional cathode production often involves water-intensive processes and generates wastewater containing heavy metals. Fluoride cathode manufacturing can be designed to minimize water usage and reduce harmful effluents, protecting freshwater resources and aquatic ecosystems.
Looking forward, the scalability of fluoride cathode technology will determine its ultimate environmental impact. If production can be scaled efficiently while maintaining environmental benefits, this technology could significantly contribute to global decarbonization efforts by enabling more widespread adoption of renewable energy storage solutions. However, continued research into fluoride leaching prevention and safe handling protocols remains essential to ensure that the environmental promise of this technology is fully realized without introducing new ecological challenges.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!