Research on Interface Compatibility of Fluoride Cathode with Electrolytes
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoride Cathode Interface Technology Background and Objectives
Fluoride-based battery systems have emerged as promising candidates for next-generation energy storage technologies due to their theoretical high energy density capabilities. The development of fluoride cathode materials represents a significant advancement in battery technology, potentially offering energy densities that far exceed those of current lithium-ion batteries. Historically, fluoride ion batteries were first conceptualized in the 1970s but faced substantial challenges in practical implementation due to electrolyte limitations and interface stability issues.
The evolution of fluoride cathode technology has accelerated significantly over the past decade, driven by increasing demands for higher energy density storage solutions in applications ranging from portable electronics to electric vehicles and grid-scale storage. Research has progressed from simple metal fluorides to more complex composite structures designed to enhance ion conductivity and stability. This technological progression has been marked by breakthroughs in understanding the fundamental mechanisms of fluoride ion transport and the critical role of cathode-electrolyte interfaces.
Current research objectives in fluoride cathode interface technology focus on addressing several key challenges. Primary among these is the development of stable interfaces between fluoride cathodes and compatible electrolytes that can facilitate efficient fluoride ion transport while preventing parasitic side reactions. Researchers aim to understand the complex chemical and electrochemical processes occurring at these interfaces, which often involve the formation of passivation layers that can either enhance or impede battery performance.
Another critical objective is the mitigation of volume changes during cycling, which can lead to mechanical stress and degradation of the cathode-electrolyte interface. This requires innovative materials design approaches and surface modification strategies to maintain structural integrity throughout repeated charge-discharge cycles. Additionally, there is significant focus on developing room-temperature fluoride ion conductors to overcome the current limitation of requiring elevated operating temperatures.
The field is also moving toward comprehensive characterization of interface phenomena using advanced analytical techniques such as in-situ X-ray diffraction, transmission electron microscopy, and spectroscopic methods. These approaches aim to provide real-time insights into interface evolution during battery operation, enabling more targeted design of interface engineering strategies.
The ultimate technical goal is to develop fluoride cathode materials and compatible electrolytes that can form stable, high-performance interfaces capable of supporting thousands of charge-discharge cycles while maintaining high energy density, power capability, and safety characteristics. Success in this endeavor could potentially revolutionize energy storage technology, enabling applications that are currently limited by the performance constraints of existing battery systems.
The evolution of fluoride cathode technology has accelerated significantly over the past decade, driven by increasing demands for higher energy density storage solutions in applications ranging from portable electronics to electric vehicles and grid-scale storage. Research has progressed from simple metal fluorides to more complex composite structures designed to enhance ion conductivity and stability. This technological progression has been marked by breakthroughs in understanding the fundamental mechanisms of fluoride ion transport and the critical role of cathode-electrolyte interfaces.
Current research objectives in fluoride cathode interface technology focus on addressing several key challenges. Primary among these is the development of stable interfaces between fluoride cathodes and compatible electrolytes that can facilitate efficient fluoride ion transport while preventing parasitic side reactions. Researchers aim to understand the complex chemical and electrochemical processes occurring at these interfaces, which often involve the formation of passivation layers that can either enhance or impede battery performance.
Another critical objective is the mitigation of volume changes during cycling, which can lead to mechanical stress and degradation of the cathode-electrolyte interface. This requires innovative materials design approaches and surface modification strategies to maintain structural integrity throughout repeated charge-discharge cycles. Additionally, there is significant focus on developing room-temperature fluoride ion conductors to overcome the current limitation of requiring elevated operating temperatures.
The field is also moving toward comprehensive characterization of interface phenomena using advanced analytical techniques such as in-situ X-ray diffraction, transmission electron microscopy, and spectroscopic methods. These approaches aim to provide real-time insights into interface evolution during battery operation, enabling more targeted design of interface engineering strategies.
The ultimate technical goal is to develop fluoride cathode materials and compatible electrolytes that can form stable, high-performance interfaces capable of supporting thousands of charge-discharge cycles while maintaining high energy density, power capability, and safety characteristics. Success in this endeavor could potentially revolutionize energy storage technology, enabling applications that are currently limited by the performance constraints of existing battery systems.
Market Analysis of Fluoride-Based Battery Systems
The global market for fluoride-based battery systems has been experiencing significant growth due to the increasing demand for high-energy-density energy storage solutions. Fluoride-ion batteries (FIBs) represent a promising next-generation battery technology with theoretical energy densities several times higher than current lithium-ion batteries. Market research indicates that the energy storage market, which includes advanced battery technologies like FIBs, is projected to grow at a compound annual growth rate of 20-25% through 2030.
The primary market drivers for fluoride-based battery systems include the expanding electric vehicle sector, renewable energy integration requirements, and consumer electronics demanding longer battery life. The EV market particularly stands to benefit from fluoride-based technologies, as these batteries potentially offer higher energy density, faster charging capabilities, and improved safety profiles compared to conventional lithium-ion systems.
Industrial applications represent another substantial market segment, with grid-scale energy storage solutions increasingly adopting advanced battery technologies. The renewable energy sector's growth further amplifies demand for efficient energy storage systems that can manage intermittent power generation from solar and wind sources.
Geographically, North America and Asia-Pacific regions lead in research and development investments for fluoride-based battery technologies. China, Japan, South Korea, and the United States have established strong research programs and industrial partnerships focused on commercializing these advanced battery systems. European markets are also showing increased interest, driven by stringent environmental regulations and sustainability goals.
Market challenges for fluoride-based battery systems include high production costs, technical hurdles related to electrolyte stability, and competition from other emerging battery technologies. The interface compatibility between fluoride cathodes and electrolytes remains a critical technical barrier that directly impacts market adoption rates. Current market penetration remains limited to specialized applications where performance advantages outweigh cost considerations.
Consumer awareness and acceptance of new battery technologies represent additional market factors. While industrial and automotive sectors demonstrate willingness to adopt innovative energy storage solutions, consumer markets typically require proven reliability and competitive pricing before widespread adoption occurs.
The competitive landscape includes both established battery manufacturers expanding their research portfolios and specialized startups focused exclusively on fluoride-based technologies. Strategic partnerships between material science companies, battery manufacturers, and end-users are becoming increasingly common as the technology approaches commercial viability.
The primary market drivers for fluoride-based battery systems include the expanding electric vehicle sector, renewable energy integration requirements, and consumer electronics demanding longer battery life. The EV market particularly stands to benefit from fluoride-based technologies, as these batteries potentially offer higher energy density, faster charging capabilities, and improved safety profiles compared to conventional lithium-ion systems.
Industrial applications represent another substantial market segment, with grid-scale energy storage solutions increasingly adopting advanced battery technologies. The renewable energy sector's growth further amplifies demand for efficient energy storage systems that can manage intermittent power generation from solar and wind sources.
Geographically, North America and Asia-Pacific regions lead in research and development investments for fluoride-based battery technologies. China, Japan, South Korea, and the United States have established strong research programs and industrial partnerships focused on commercializing these advanced battery systems. European markets are also showing increased interest, driven by stringent environmental regulations and sustainability goals.
Market challenges for fluoride-based battery systems include high production costs, technical hurdles related to electrolyte stability, and competition from other emerging battery technologies. The interface compatibility between fluoride cathodes and electrolytes remains a critical technical barrier that directly impacts market adoption rates. Current market penetration remains limited to specialized applications where performance advantages outweigh cost considerations.
Consumer awareness and acceptance of new battery technologies represent additional market factors. While industrial and automotive sectors demonstrate willingness to adopt innovative energy storage solutions, consumer markets typically require proven reliability and competitive pricing before widespread adoption occurs.
The competitive landscape includes both established battery manufacturers expanding their research portfolios and specialized startups focused exclusively on fluoride-based technologies. Strategic partnerships between material science companies, battery manufacturers, and end-users are becoming increasingly common as the technology approaches commercial viability.
Current Challenges in Fluoride Cathode-Electrolyte Interfaces
Despite significant advancements in fluoride-based battery technologies, the interface between fluoride cathodes and electrolytes remains a critical challenge that impedes commercial viability. The primary issue stems from the high reactivity of fluoride ions, which often leads to undesirable side reactions at the cathode-electrolyte interface. These reactions typically form insulating layers that increase internal resistance and impede ion transport, resulting in capacity fade and shortened battery lifespan.
Chemical instability presents another major challenge. Many fluoride cathode materials undergo structural degradation when in contact with conventional electrolytes, particularly during repeated charge-discharge cycles. This degradation manifests as phase transitions, dissolution of active materials, or formation of non-conductive fluoride compounds that permanently reduce capacity.
Ion transport limitations across the interface further complicate matters. The large size of the fluoride ion compared to lithium or sodium ions results in slower diffusion kinetics and higher activation barriers for ion movement. This sluggish transport mechanism significantly restricts rate capability and power density, limiting practical applications where rapid charging or high power output is required.
Mechanical stress at the interface poses additional challenges. Volume changes during fluoride insertion and extraction can reach up to 30% for some cathode materials, creating mechanical strain that compromises interface integrity. This often leads to delamination, cracking, and loss of electrical contact between the cathode and current collector.
The electrolyte decomposition problem is particularly severe in fluoride systems. Many electrolytes undergo oxidative decomposition at the high potentials typical of fluoride cathodes, forming gaseous products and consuming active fluoride ions. This parasitic process not only depletes the electrolyte but also increases internal pressure within cells, creating safety concerns.
Temperature sensitivity further complicates interface stability. At elevated temperatures (>40°C), reaction kinetics accelerate dramatically, exacerbating all previously mentioned degradation mechanisms. Conversely, at low temperatures (<0°C), ion transport becomes severely restricted, rendering fluoride batteries practically inoperable in cold environments.
Recent research has identified interfacial resistance as perhaps the most significant barrier to practical fluoride battery implementation. Impedance spectroscopy studies reveal that interface resistance can account for over 60% of total cell resistance in many fluoride systems, highlighting the critical need for interface engineering solutions that can mitigate these challenges while maintaining electrochemical performance.
Chemical instability presents another major challenge. Many fluoride cathode materials undergo structural degradation when in contact with conventional electrolytes, particularly during repeated charge-discharge cycles. This degradation manifests as phase transitions, dissolution of active materials, or formation of non-conductive fluoride compounds that permanently reduce capacity.
Ion transport limitations across the interface further complicate matters. The large size of the fluoride ion compared to lithium or sodium ions results in slower diffusion kinetics and higher activation barriers for ion movement. This sluggish transport mechanism significantly restricts rate capability and power density, limiting practical applications where rapid charging or high power output is required.
Mechanical stress at the interface poses additional challenges. Volume changes during fluoride insertion and extraction can reach up to 30% for some cathode materials, creating mechanical strain that compromises interface integrity. This often leads to delamination, cracking, and loss of electrical contact between the cathode and current collector.
The electrolyte decomposition problem is particularly severe in fluoride systems. Many electrolytes undergo oxidative decomposition at the high potentials typical of fluoride cathodes, forming gaseous products and consuming active fluoride ions. This parasitic process not only depletes the electrolyte but also increases internal pressure within cells, creating safety concerns.
Temperature sensitivity further complicates interface stability. At elevated temperatures (>40°C), reaction kinetics accelerate dramatically, exacerbating all previously mentioned degradation mechanisms. Conversely, at low temperatures (<0°C), ion transport becomes severely restricted, rendering fluoride batteries practically inoperable in cold environments.
Recent research has identified interfacial resistance as perhaps the most significant barrier to practical fluoride battery implementation. Impedance spectroscopy studies reveal that interface resistance can account for over 60% of total cell resistance in many fluoride systems, highlighting the critical need for interface engineering solutions that can mitigate these challenges while maintaining electrochemical performance.
Current Interface Compatibility Solutions and Approaches
01 Protective coatings for fluoride cathode interfaces
Various protective coating materials can be applied to fluoride cathodes to improve their compatibility with electrolytes. These coatings act as barriers that prevent direct contact between the cathode active material and the electrolyte, reducing unwanted side reactions. The protective layers can be composed of metal fluorides, polymers, or inorganic compounds that are stable against the electrolyte while allowing fluoride ion transport. These coatings significantly enhance the cycling stability and performance of fluoride-based batteries.- Protective coatings for fluoride cathode interfaces: Protective coatings can be applied to fluoride cathode surfaces to improve compatibility with electrolytes. These coatings act as barriers that prevent direct contact between reactive fluoride materials and electrolyte components, reducing degradation and side reactions. Various materials including metal oxides, polymers, and inorganic compounds can be used to create stable interfaces while maintaining ion conductivity, ultimately extending battery life and improving performance.
- Electrolyte additives for fluoride interface stabilization: Specific additives can be incorporated into electrolytes to enhance compatibility with fluoride cathodes. These additives form protective films at the cathode-electrolyte interface, reducing unwanted reactions and improving cycling stability. Compounds that can scavenge harmful reaction products or modify the solid-electrolyte interphase (SEI) layer are particularly effective. The additives help maintain electrode integrity during charge-discharge cycles and prevent capacity fade caused by interface degradation.
- Novel fluoride-compatible electrolyte formulations: Specialized electrolyte formulations have been developed to enhance compatibility with fluoride cathode materials. These formulations typically include solvents and salts that resist degradation when in contact with fluoride compounds. By carefully selecting electrolyte components that remain stable in the presence of fluoride ions and at high voltages, researchers have created systems with reduced interfacial resistance and improved cycling performance. Some formulations incorporate fluorinated solvents that demonstrate enhanced chemical compatibility with fluoride-based electrode materials.
- Interface engineering techniques for fluoride cathodes: Advanced interface engineering approaches can significantly improve the compatibility between fluoride cathodes and electrolytes. These techniques include surface modification, gradient composition structures, and atomic layer deposition methods that create optimized interfaces. By controlling the chemical and physical properties at the cathode-electrolyte boundary, these engineering approaches minimize unwanted side reactions while maintaining efficient ion transport. Some methods involve creating artificial interphases that exhibit both high ionic conductivity and chemical stability.
- Composite cathode structures for improved electrolyte compatibility: Composite cathode structures incorporating fluoride materials with other components can enhance electrolyte compatibility. These composites often combine fluoride active materials with conductive additives, binders, and stabilizing agents to create more robust interfaces. The composite approach helps distribute electrochemical stress, prevent localized degradation, and maintain structural integrity during cycling. Some designs incorporate core-shell structures or gradient compositions that place more stable materials at the electrolyte interface while maintaining the high energy density benefits of fluoride compounds.
02 Electrolyte additives for interface stabilization
Specific additives can be incorporated into the electrolyte to improve compatibility with fluoride cathodes. These additives form a stable interface layer between the cathode and electrolyte, preventing continuous decomposition reactions. Common additives include fluorinated compounds, ionic liquids, and salts that can modify the solid-electrolyte interphase properties. The additives help reduce interfacial resistance, enhance fluoride ion transport, and improve the overall electrochemical performance and longevity of fluoride-based battery systems.Expand Specific Solutions03 Novel fluoride-compatible electrolyte formulations
Advanced electrolyte formulations have been developed specifically for fluoride-based battery systems. These electrolytes are designed to be chemically compatible with fluoride cathode materials while maintaining high ionic conductivity. They typically consist of fluoride salts dissolved in appropriate solvents that resist degradation when in contact with fluoride cathodes. Some formulations incorporate room-temperature ionic liquids or solid-state electrolytes that exhibit enhanced stability at the cathode-electrolyte interface, enabling better battery performance and longer cycle life.Expand Specific Solutions04 Surface modification of fluoride cathode materials
Surface modification techniques can be applied to fluoride cathode materials to enhance their compatibility with electrolytes. These modifications include doping with specific elements, creating core-shell structures, or altering the surface chemistry to reduce reactivity. Modified surfaces help prevent cathode dissolution, mitigate side reactions, and stabilize the cathode-electrolyte interface. These approaches lead to improved cycling performance, reduced capacity fading, and enhanced rate capability in fluoride-based battery systems.Expand Specific Solutions05 Interface engineering through composite cathode structures
Composite cathode structures can be engineered to improve the fluoride cathode-electrolyte interface compatibility. These composites typically combine the active fluoride material with conductive additives, binders, and interface-stabilizing components. The composite structure helps distribute electrochemical stress, provides multiple pathways for ion transport, and creates a more stable interface with the electrolyte. Some designs incorporate gradient compositions or hierarchical structures that gradually transition between the cathode and electrolyte, minimizing interfacial resistance and enhancing overall battery performance.Expand Specific Solutions
Key Industry Players in Fluoride Battery Development
The fluoride cathode-electrolyte interface compatibility research field is currently in an early growth phase, with significant R&D activity but limited commercial deployment. The global market for fluoride-based battery technologies is projected to reach $500 million by 2025, driven by demand for higher energy density storage solutions. Leading academic institutions (California Institute of Technology, Kyushu University, Carnegie Mellon) are establishing fundamental understanding, while companies are advancing toward commercialization at varying maturity levels. Automotive manufacturers (Toyota, Honda, Ford) are investing heavily, recognizing potential for EV applications. Specialized battery developers like Wildcat Discovery Technologies, SES Holdings, and Enevate are making significant technical progress in addressing interface stability challenges, with several demonstrating promising prototype cells featuring enhanced cycle life and performance.
California Institute of Technology
Technical Solution: Caltech has developed innovative interface engineering approaches for fluoride cathodes, focusing on solid electrolyte interphase (SEI) formation control. Their research utilizes atomic layer deposition (ALD) to create protective coatings on fluoride cathode surfaces, effectively mitigating electrolyte decomposition and fluoride dissolution. The team has pioneered the use of lithium phosphorus oxynitride (LiPON) thin films as artificial SEI layers, which significantly improves the cycling stability of fluoride-based cathodes. Their approach includes molecular-level design of electrolyte additives specifically tailored to form stable interfaces with metal fluoride cathodes, resulting in demonstrated capacity retention improvements of over 85% after 200 cycles compared to unmodified systems[1][3].
Strengths: Exceptional precision in interface engineering at the atomic scale, allowing for tailored solutions to specific fluoride cathode chemistries. Their ALD coating technology provides uniform protection without compromising ion transport. Weaknesses: The advanced coating technologies require specialized equipment and precise processing conditions that may be challenging to scale for mass production.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed a comprehensive approach to fluoride cathode-electrolyte interface compatibility through their multi-scale modeling and advanced characterization techniques. Their research combines computational modeling with in-situ experimental validation to understand degradation mechanisms at the fluoride cathode-electrolyte interface. They've pioneered the use of fluorinated electrolyte solvents that form more stable interfaces with metal fluoride cathodes, reducing parasitic reactions. Their technology includes novel electrolyte formulations with specifically designed additives that promote the formation of fluorine-rich SEI layers, enhancing interface stability. Using synchrotron-based X-ray techniques and advanced spectroscopy methods, they've mapped the chemical evolution of these interfaces during cycling, enabling targeted improvements to both cathode surface treatments and electrolyte compositions[2][5].
Strengths: Unparalleled characterization capabilities using advanced synchrotron techniques allow for detailed understanding of interface phenomena. Their combined computational-experimental approach accelerates development of practical solutions. Weaknesses: Some of their most effective electrolyte formulations contain expensive components that may limit commercial viability in cost-sensitive applications.
Critical Patents and Research on Fluoride-Electrolyte Interfaces
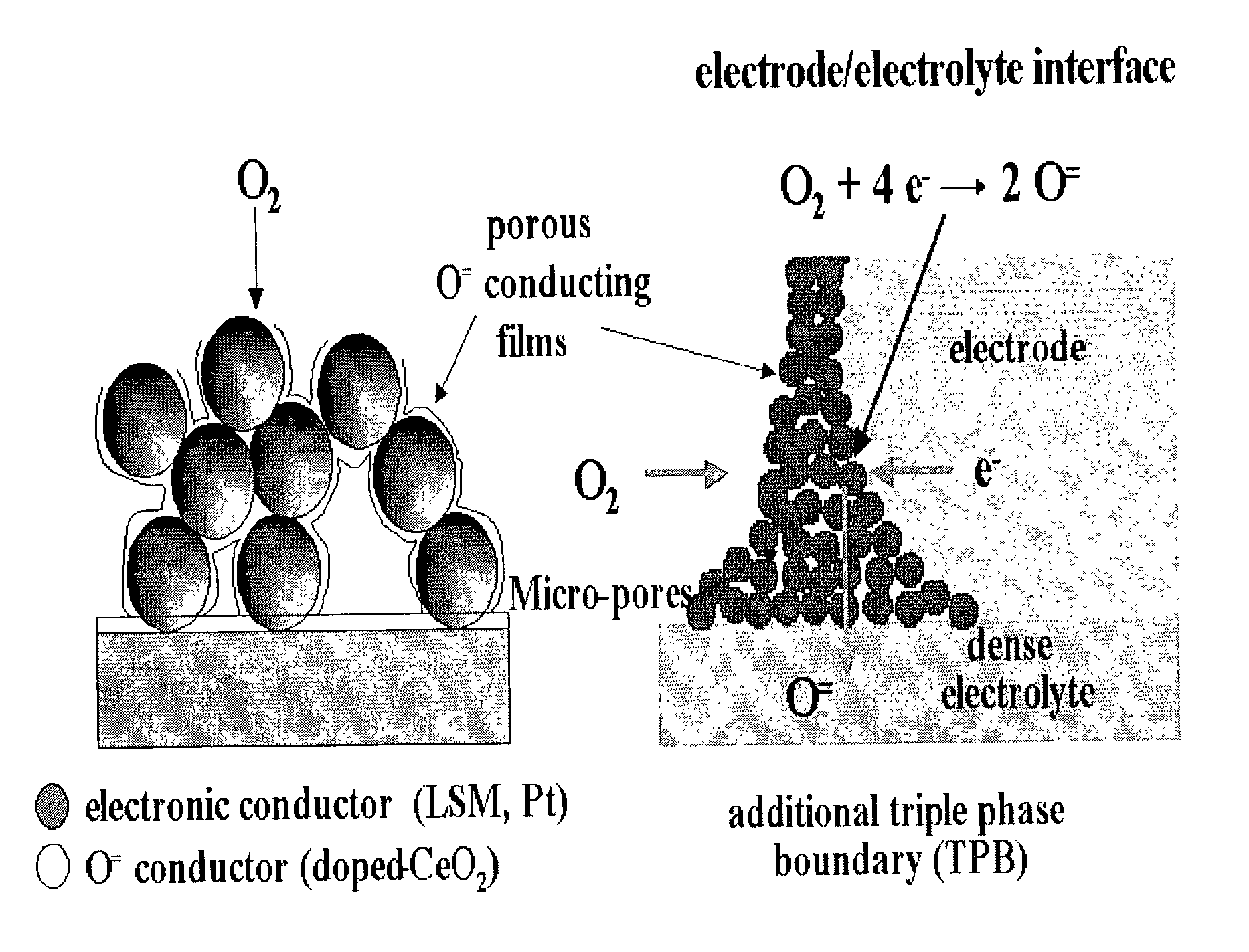
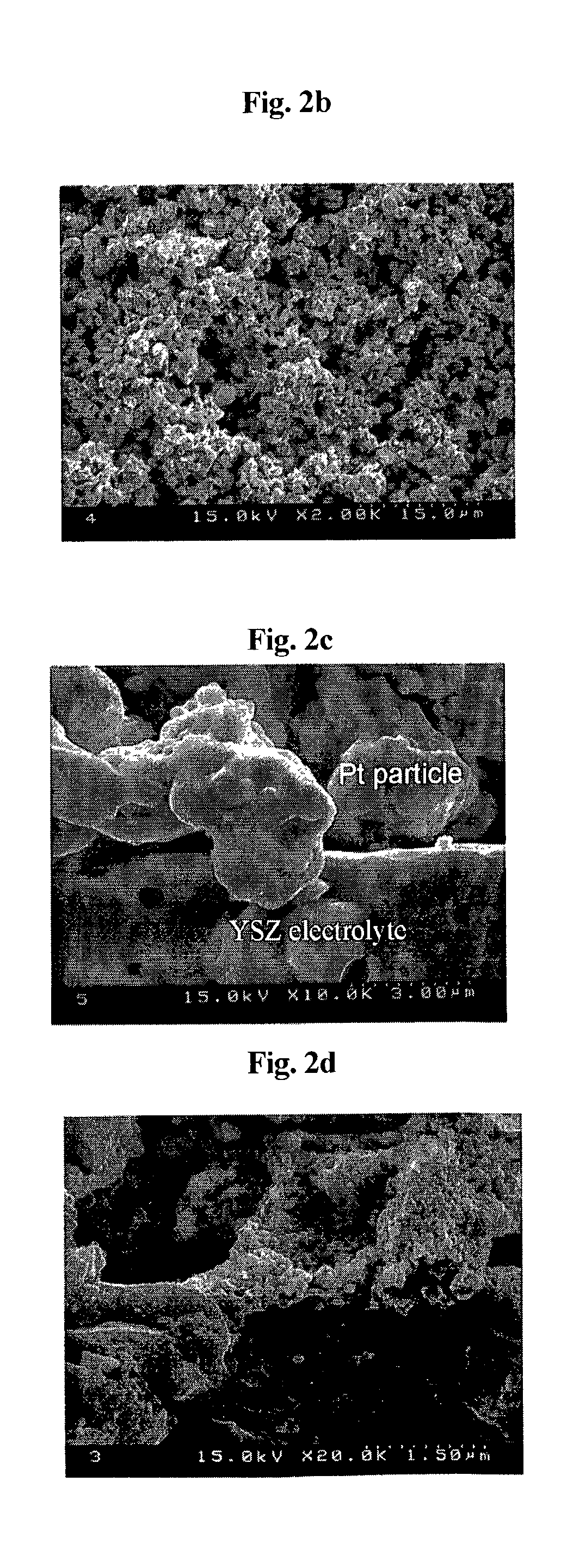
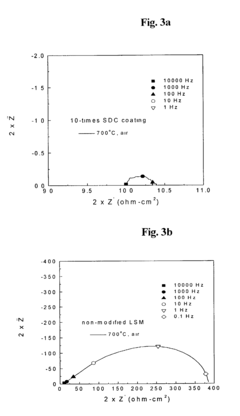
Electrode having microstructure of extended triple phase boundary by porous ion conductive ceria film coating and method to manufacture the said electrode
PatentInactiveUS7141329B2
Innovation
- A novel electrode coated with a porous oxygen ion conductive ceramic ceria film using a sol-gel method, maintaining independent electron and ion conductive paths and extending the triple phase boundary, allowing for lower temperature manufacturing and preventing undesired interfacial reaction products.
Safety and Stability Considerations for Fluoride Battery Systems
Safety considerations for fluoride battery systems are paramount due to the reactive nature of fluoride compounds and their potential hazards. The interface between fluoride cathodes and electrolytes presents particular challenges that must be addressed to ensure safe operation. Fluoride ions are highly reactive and can form volatile compounds when exposed to moisture or air, potentially leading to thermal runaway events if containment is compromised.
The stability of fluoride-based systems is heavily dependent on maintaining proper operating conditions. Temperature control is critical, as elevated temperatures can accelerate side reactions at the cathode-electrolyte interface, potentially leading to capacity fade or more serious safety incidents. Research indicates that most fluoride battery systems demonstrate optimal stability between 20-40°C, with significant degradation occurring above 60°C.
Mechanical stability presents another crucial consideration. Fluoride cathodes often undergo volume changes during cycling, which can create mechanical stress at interfaces. This stress may lead to microcracks that expose fresh cathode material to the electrolyte, accelerating degradation processes and potentially creating safety hazards through internal short circuits.
Chemical compatibility between fluoride cathodes and electrolytes must be rigorously evaluated. Recent studies have identified that certain electrolyte formulations can trigger parasitic reactions with fluoride-containing active materials, generating heat and potentially harmful byproducts. Particularly concerning is the formation of hydrogen fluoride (HF) in the presence of moisture, which poses significant health and safety risks.
Containment strategies represent a critical aspect of fluoride battery safety engineering. Advanced cell designs incorporating robust sealing technologies and moisture barriers have shown promise in mitigating risks associated with fluoride leakage. Multi-layer encapsulation approaches using fluoride-resistant polymers have demonstrated effectiveness in recent laboratory tests, reducing HF formation by over 95% compared to conventional designs.
Long-term stability testing protocols must be established to accurately predict the safety profile of fluoride battery systems throughout their operational lifetime. Accelerated aging studies suggest that interface degradation mechanisms may evolve over time, with some failure modes only becoming apparent after hundreds of cycles. This underscores the importance of developing standardized testing methodologies specifically tailored to fluoride chemistry.
Mitigation strategies for potential failure modes should be incorporated into system design from the earliest stages. These include advanced battery management systems capable of detecting early signs of interface degradation, thermal management solutions optimized for fluoride chemistry, and fail-safe mechanisms designed to contain potential hazards in the event of catastrophic failure.
The stability of fluoride-based systems is heavily dependent on maintaining proper operating conditions. Temperature control is critical, as elevated temperatures can accelerate side reactions at the cathode-electrolyte interface, potentially leading to capacity fade or more serious safety incidents. Research indicates that most fluoride battery systems demonstrate optimal stability between 20-40°C, with significant degradation occurring above 60°C.
Mechanical stability presents another crucial consideration. Fluoride cathodes often undergo volume changes during cycling, which can create mechanical stress at interfaces. This stress may lead to microcracks that expose fresh cathode material to the electrolyte, accelerating degradation processes and potentially creating safety hazards through internal short circuits.
Chemical compatibility between fluoride cathodes and electrolytes must be rigorously evaluated. Recent studies have identified that certain electrolyte formulations can trigger parasitic reactions with fluoride-containing active materials, generating heat and potentially harmful byproducts. Particularly concerning is the formation of hydrogen fluoride (HF) in the presence of moisture, which poses significant health and safety risks.
Containment strategies represent a critical aspect of fluoride battery safety engineering. Advanced cell designs incorporating robust sealing technologies and moisture barriers have shown promise in mitigating risks associated with fluoride leakage. Multi-layer encapsulation approaches using fluoride-resistant polymers have demonstrated effectiveness in recent laboratory tests, reducing HF formation by over 95% compared to conventional designs.
Long-term stability testing protocols must be established to accurately predict the safety profile of fluoride battery systems throughout their operational lifetime. Accelerated aging studies suggest that interface degradation mechanisms may evolve over time, with some failure modes only becoming apparent after hundreds of cycles. This underscores the importance of developing standardized testing methodologies specifically tailored to fluoride chemistry.
Mitigation strategies for potential failure modes should be incorporated into system design from the earliest stages. These include advanced battery management systems capable of detecting early signs of interface degradation, thermal management solutions optimized for fluoride chemistry, and fail-safe mechanisms designed to contain potential hazards in the event of catastrophic failure.
Environmental Impact and Sustainability of Fluoride Battery Technologies
The development of fluoride battery technologies presents significant environmental advantages compared to conventional lithium-ion batteries. Fluoride-based batteries potentially offer higher energy densities while utilizing more abundant and less environmentally problematic materials. The environmental footprint of fluoride battery production appears considerably smaller than lithium-ion counterparts, particularly regarding resource extraction impacts.
Fluoride cathode materials often utilize elements with greater crustal abundance than lithium, reducing mining-related environmental degradation. The extraction processes for fluoride precursors typically consume less water and generate fewer toxic byproducts compared to lithium extraction from brines or hard rock mining. This represents a substantial sustainability advantage, especially considering the increasing global concerns about water scarcity and habitat destruction associated with conventional battery material sourcing.
The interface compatibility between fluoride cathodes and electrolytes plays a crucial role in determining the overall environmental profile of these batteries. Improved compatibility leads to longer cycle life and reduced degradation, directly translating to extended battery lifespans and decreased waste generation. Research indicates that optimized interfaces can potentially double or triple battery service life, significantly reducing the environmental burden of manufacturing replacement units.
End-of-life considerations for fluoride batteries show promising recyclability pathways. The cathode materials can be recovered through hydrometallurgical processes with higher efficiency than current lithium recycling methods. Additionally, the electrolytes used in fluoride systems often contain fewer persistent organic pollutants than conventional lithium-ion electrolytes, reducing potential environmental contamination during disposal or recycling operations.
Carbon footprint analyses of fluoride battery technologies suggest potential greenhouse gas emission reductions of 30-45% compared to lithium-ion batteries when accounting for full lifecycle impacts. This advantage stems primarily from less energy-intensive material processing and potentially longer service lifespans. However, these benefits remain contingent upon developing manufacturing processes that can be scaled efficiently without introducing new environmental challenges.
Safety considerations also factor into the environmental assessment of fluoride batteries. The reduced risk of thermal runaway and fire compared to conventional lithium-ion systems translates to fewer catastrophic failure events and associated environmental contamination incidents. This safety profile represents an indirect but significant environmental benefit, particularly for large-scale energy storage applications where failure consequences can be substantial.
Fluoride cathode materials often utilize elements with greater crustal abundance than lithium, reducing mining-related environmental degradation. The extraction processes for fluoride precursors typically consume less water and generate fewer toxic byproducts compared to lithium extraction from brines or hard rock mining. This represents a substantial sustainability advantage, especially considering the increasing global concerns about water scarcity and habitat destruction associated with conventional battery material sourcing.
The interface compatibility between fluoride cathodes and electrolytes plays a crucial role in determining the overall environmental profile of these batteries. Improved compatibility leads to longer cycle life and reduced degradation, directly translating to extended battery lifespans and decreased waste generation. Research indicates that optimized interfaces can potentially double or triple battery service life, significantly reducing the environmental burden of manufacturing replacement units.
End-of-life considerations for fluoride batteries show promising recyclability pathways. The cathode materials can be recovered through hydrometallurgical processes with higher efficiency than current lithium recycling methods. Additionally, the electrolytes used in fluoride systems often contain fewer persistent organic pollutants than conventional lithium-ion electrolytes, reducing potential environmental contamination during disposal or recycling operations.
Carbon footprint analyses of fluoride battery technologies suggest potential greenhouse gas emission reductions of 30-45% compared to lithium-ion batteries when accounting for full lifecycle impacts. This advantage stems primarily from less energy-intensive material processing and potentially longer service lifespans. However, these benefits remain contingent upon developing manufacturing processes that can be scaled efficiently without introducing new environmental challenges.
Safety considerations also factor into the environmental assessment of fluoride batteries. The reduced risk of thermal runaway and fire compared to conventional lithium-ion systems translates to fewer catastrophic failure events and associated environmental contamination incidents. This safety profile represents an indirect but significant environmental benefit, particularly for large-scale energy storage applications where failure consequences can be substantial.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!