Research on Fluoride Cathode Electrochemical Window Expansion
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoride Cathode Development Background and Objectives
Fluoride-based cathode materials have emerged as promising candidates for next-generation energy storage systems due to their high theoretical energy density, abundant resources, and potential environmental benefits. The development of fluoride cathodes can be traced back to the early 2000s when researchers began exploring alternatives to traditional lithium-ion battery technologies to overcome energy density limitations. Initial research focused primarily on metal fluorides such as FeF3, CoF3, and CuF2, which demonstrated theoretical capacities exceeding 500 mAh/g, significantly higher than conventional cathode materials.
The evolution of fluoride cathode technology has been marked by significant challenges, particularly related to their narrow electrochemical stability window. This limitation has historically restricted their practical application despite their promising theoretical performance. The electrochemical window, defined as the potential range within which the electrolyte remains stable without decomposition, is a critical parameter that determines battery safety, cycle life, and energy density.
Recent technological advancements have renewed interest in fluoride cathodes, particularly in the context of expanding their electrochemical windows. This expansion is crucial for unlocking the full potential of these materials and enabling their integration into commercial energy storage solutions. The primary objective of current research efforts is to develop novel approaches to widen the electrochemical stability window of fluoride cathodes without compromising their inherent advantages.
Several technological trends are shaping the future direction of fluoride cathode development. These include the exploration of nanostructured fluoride materials to enhance kinetics and stability, the development of composite cathodes that combine fluorides with conductive additives, and the investigation of advanced electrolyte systems specifically designed for fluoride-based chemistries. Additionally, computational modeling and high-throughput screening methods are increasingly being employed to predict and optimize fluoride cathode performance.
The technical goals for fluoride cathode development are multifaceted and ambitious. They include expanding the electrochemical window by at least 1V compared to current limitations, achieving stable cycling performance over 1000 cycles with minimal capacity degradation, and developing manufacturing processes that are scalable and economically viable. Furthermore, researchers aim to integrate these advanced cathodes into practical devices that can operate under a wide range of environmental conditions.
Understanding the fundamental mechanisms that limit the electrochemical window of fluoride cathodes is essential for developing effective expansion strategies. This includes investigating the interfacial reactions between the cathode and electrolyte, studying the structural changes during cycling, and identifying the factors that contribute to electrolyte decomposition at high potentials.
The evolution of fluoride cathode technology has been marked by significant challenges, particularly related to their narrow electrochemical stability window. This limitation has historically restricted their practical application despite their promising theoretical performance. The electrochemical window, defined as the potential range within which the electrolyte remains stable without decomposition, is a critical parameter that determines battery safety, cycle life, and energy density.
Recent technological advancements have renewed interest in fluoride cathodes, particularly in the context of expanding their electrochemical windows. This expansion is crucial for unlocking the full potential of these materials and enabling their integration into commercial energy storage solutions. The primary objective of current research efforts is to develop novel approaches to widen the electrochemical stability window of fluoride cathodes without compromising their inherent advantages.
Several technological trends are shaping the future direction of fluoride cathode development. These include the exploration of nanostructured fluoride materials to enhance kinetics and stability, the development of composite cathodes that combine fluorides with conductive additives, and the investigation of advanced electrolyte systems specifically designed for fluoride-based chemistries. Additionally, computational modeling and high-throughput screening methods are increasingly being employed to predict and optimize fluoride cathode performance.
The technical goals for fluoride cathode development are multifaceted and ambitious. They include expanding the electrochemical window by at least 1V compared to current limitations, achieving stable cycling performance over 1000 cycles with minimal capacity degradation, and developing manufacturing processes that are scalable and economically viable. Furthermore, researchers aim to integrate these advanced cathodes into practical devices that can operate under a wide range of environmental conditions.
Understanding the fundamental mechanisms that limit the electrochemical window of fluoride cathodes is essential for developing effective expansion strategies. This includes investigating the interfacial reactions between the cathode and electrolyte, studying the structural changes during cycling, and identifying the factors that contribute to electrolyte decomposition at high potentials.
Market Analysis for Advanced Battery Technologies
The global advanced battery market is experiencing unprecedented growth, driven by the increasing demand for high-energy density storage solutions across multiple sectors. The fluoride cathode technology represents a significant frontier in battery innovation, with potential market value projected to reach 25 billion USD by 2030 if technical challenges in electrochemical window expansion are successfully addressed.
Electric vehicle manufacturers constitute the primary market segment for advanced battery technologies, with automotive applications accounting for approximately 60% of the total addressable market. The push for longer-range electric vehicles with faster charging capabilities has intensified research interest in fluoride-based cathode materials, which theoretically offer energy densities 2-3 times higher than current lithium-ion technologies.
Consumer electronics represents the second largest market segment, where demand for longer-lasting, smaller, and lighter batteries continues to grow at 12% annually. The potential for fluoride cathode batteries to deliver higher energy density in compact form factors makes this technology particularly attractive to smartphone, laptop, and wearable device manufacturers seeking competitive advantages.
Grid-scale energy storage presents another significant growth opportunity, with market projections indicating a 25% compound annual growth rate through 2028. Utility companies are increasingly investing in advanced battery technologies to support renewable energy integration and grid stabilization, with particular interest in chemistries that offer improved safety profiles and longer cycle life.
Market analysis reveals regional variations in adoption potential, with Asia-Pacific leading manufacturing capacity development for advanced battery technologies. North America and Europe follow closely, with substantial investments in research and development focused on next-generation chemistries including fluoride-based systems.
Customer requirements across these markets consistently emphasize five key performance indicators: energy density, cycle life, safety, charging speed, and cost. Fluoride cathode technology shows particular promise in addressing the energy density and potentially safety aspects, though current limitations in electrochemical stability window restrict commercial viability.
Competitive analysis indicates that several major battery manufacturers and materials science companies have active research programs in fluoride-based cathode materials, with patent filings increasing by 35% annually over the past three years. This acceleration signals growing commercial interest despite the technical challenges that remain.
Market entry barriers include not only the technical hurdles of expanding the electrochemical window but also scaling manufacturing processes and establishing supply chains for novel materials. Early market adoption would likely target premium segments where performance advantages can command price premiums sufficient to offset higher production costs.
Electric vehicle manufacturers constitute the primary market segment for advanced battery technologies, with automotive applications accounting for approximately 60% of the total addressable market. The push for longer-range electric vehicles with faster charging capabilities has intensified research interest in fluoride-based cathode materials, which theoretically offer energy densities 2-3 times higher than current lithium-ion technologies.
Consumer electronics represents the second largest market segment, where demand for longer-lasting, smaller, and lighter batteries continues to grow at 12% annually. The potential for fluoride cathode batteries to deliver higher energy density in compact form factors makes this technology particularly attractive to smartphone, laptop, and wearable device manufacturers seeking competitive advantages.
Grid-scale energy storage presents another significant growth opportunity, with market projections indicating a 25% compound annual growth rate through 2028. Utility companies are increasingly investing in advanced battery technologies to support renewable energy integration and grid stabilization, with particular interest in chemistries that offer improved safety profiles and longer cycle life.
Market analysis reveals regional variations in adoption potential, with Asia-Pacific leading manufacturing capacity development for advanced battery technologies. North America and Europe follow closely, with substantial investments in research and development focused on next-generation chemistries including fluoride-based systems.
Customer requirements across these markets consistently emphasize five key performance indicators: energy density, cycle life, safety, charging speed, and cost. Fluoride cathode technology shows particular promise in addressing the energy density and potentially safety aspects, though current limitations in electrochemical stability window restrict commercial viability.
Competitive analysis indicates that several major battery manufacturers and materials science companies have active research programs in fluoride-based cathode materials, with patent filings increasing by 35% annually over the past three years. This acceleration signals growing commercial interest despite the technical challenges that remain.
Market entry barriers include not only the technical hurdles of expanding the electrochemical window but also scaling manufacturing processes and establishing supply chains for novel materials. Early market adoption would likely target premium segments where performance advantages can command price premiums sufficient to offset higher production costs.
Current Limitations in Fluoride Cathode Electrochemical Windows
Fluoride cathode materials have garnered significant attention in battery research due to their theoretical high energy density and potential for multi-electron reactions. However, the practical implementation of these materials faces substantial challenges, primarily related to their limited electrochemical stability windows. Current fluoride cathodes typically operate within a narrow voltage range, significantly constraining their energy density and practical applications.
The fundamental limitation stems from the high electronegativity of fluorine, which creates strong ionic bonds with metal atoms. This strong bonding results in high redox potentials that often exceed the stability limits of conventional electrolytes. When operating beyond these limits, parasitic reactions occur, leading to electrolyte decomposition, formation of solid-electrolyte interphase (SEI) layers, and eventual capacity fading.
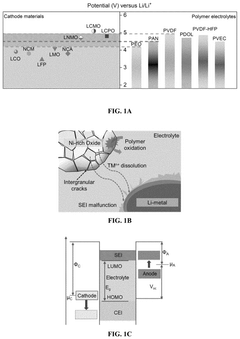
Most current fluoride cathode systems demonstrate stable cycling only within a 2.0-4.2V window versus Li/Li+. Attempts to push beyond 4.2V typically result in rapid capacity degradation due to accelerated side reactions. This narrow window severely limits the practical energy density achievable with fluoride-based systems, making them less competitive compared to commercial cathode materials like NMC (Nickel Manganese Cobalt oxide) or NCA (Nickel Cobalt Aluminum oxide).
Another critical limitation is the structural instability of fluoride cathodes during cycling. The insertion and extraction of charge carriers (like Li+ or F-) cause significant volume changes and phase transformations. These structural changes often lead to particle cracking, loss of electrical contact, and accelerated capacity fading, particularly when operating near the boundaries of the electrochemical window.
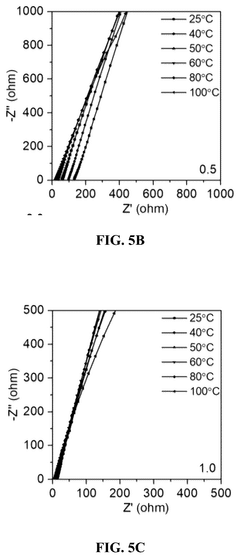
The electrolyte compatibility issue presents another major challenge. Conventional carbonate-based electrolytes undergo oxidative decomposition at high potentials when used with fluoride cathodes. Meanwhile, electrolytes specifically designed for fluoride systems often suffer from poor ionic conductivity or limited anodic stability, further constraining the operational voltage window.
Temperature sensitivity compounds these challenges, as the stability window of fluoride cathodes narrows significantly at elevated temperatures. This thermal instability limits their application in environments with varying temperature conditions and raises safety concerns for large-scale implementation.
The electronic conductivity of most fluoride materials is inherently low, requiring substantial amounts of conductive additives in electrode formulations. These additives reduce the overall energy density and complicate efforts to expand the electrochemical window, as they may catalyze unwanted side reactions at extreme potentials.
The fundamental limitation stems from the high electronegativity of fluorine, which creates strong ionic bonds with metal atoms. This strong bonding results in high redox potentials that often exceed the stability limits of conventional electrolytes. When operating beyond these limits, parasitic reactions occur, leading to electrolyte decomposition, formation of solid-electrolyte interphase (SEI) layers, and eventual capacity fading.
Most current fluoride cathode systems demonstrate stable cycling only within a 2.0-4.2V window versus Li/Li+. Attempts to push beyond 4.2V typically result in rapid capacity degradation due to accelerated side reactions. This narrow window severely limits the practical energy density achievable with fluoride-based systems, making them less competitive compared to commercial cathode materials like NMC (Nickel Manganese Cobalt oxide) or NCA (Nickel Cobalt Aluminum oxide).
Another critical limitation is the structural instability of fluoride cathodes during cycling. The insertion and extraction of charge carriers (like Li+ or F-) cause significant volume changes and phase transformations. These structural changes often lead to particle cracking, loss of electrical contact, and accelerated capacity fading, particularly when operating near the boundaries of the electrochemical window.
The electrolyte compatibility issue presents another major challenge. Conventional carbonate-based electrolytes undergo oxidative decomposition at high potentials when used with fluoride cathodes. Meanwhile, electrolytes specifically designed for fluoride systems often suffer from poor ionic conductivity or limited anodic stability, further constraining the operational voltage window.
Temperature sensitivity compounds these challenges, as the stability window of fluoride cathodes narrows significantly at elevated temperatures. This thermal instability limits their application in environments with varying temperature conditions and raises safety concerns for large-scale implementation.
The electronic conductivity of most fluoride materials is inherently low, requiring substantial amounts of conductive additives in electrode formulations. These additives reduce the overall energy density and complicate efforts to expand the electrochemical window, as they may catalyze unwanted side reactions at extreme potentials.
Current Approaches to Electrochemical Window Expansion
01 Fluoride-based cathode materials for enhanced electrochemical stability
Fluoride-based cathode materials can provide improved electrochemical stability and wider operating voltage windows in battery systems. These materials typically contain metal fluorides or fluorinated compounds that offer higher redox potentials and better resistance to degradation during cycling. The incorporation of fluoride ions in the cathode structure helps maintain structural integrity and prevents capacity fading during charge-discharge cycles, thereby extending the electrochemical window of operation.- Fluoride-based cathode materials for wide electrochemical window: Fluoride-based cathode materials can provide a wide electrochemical window, which is beneficial for high-energy density batteries. These materials typically contain metal fluorides that offer high theoretical capacities and operating voltages. The wide electrochemical stability window allows for operation at higher voltages without electrolyte decomposition, leading to improved energy density and cycling stability in battery applications.
- Electrolyte compositions compatible with fluoride cathodes: Specialized electrolyte compositions are developed to be compatible with fluoride cathode materials, enhancing the electrochemical window of the battery system. These electrolytes often contain fluorinated solvents or additives that form stable interfaces with the cathode surface, preventing unwanted side reactions and extending the upper voltage limit of operation. The electrolyte composition plays a crucial role in determining the practical electrochemical window of fluoride-based battery systems.
- Conversion-type fluoride cathode mechanisms: Conversion-type fluoride cathodes operate through a different mechanism than intercalation materials, involving the breaking and forming of chemical bonds during cycling. This mechanism allows for multi-electron transfers, potentially enabling higher energy densities. The electrochemical window of these materials is influenced by the metal-fluorine bond strength and the reversibility of the conversion reaction, with proper engineering allowing for stable operation within a defined voltage range.
- Surface modification of fluoride cathodes: Surface modification techniques are employed to enhance the electrochemical stability window of fluoride cathodes. These modifications include coatings, dopants, or composite structures that protect the cathode surface from direct contact with the electrolyte at high voltages. By mitigating unwanted side reactions at the cathode-electrolyte interface, these modifications allow for operation at higher voltages, effectively widening the usable electrochemical window of the battery system.
- Nanostructured fluoride cathodes for enhanced performance: Nanostructuring of fluoride cathode materials can significantly impact their electrochemical window and overall performance. Reducing particle size to the nanoscale improves ionic and electronic conductivity, facilitates faster reaction kinetics, and can mitigate volume changes during cycling. These benefits allow nanostructured fluoride cathodes to maintain stability over a wider voltage range, effectively expanding their electrochemical window and enabling higher energy density battery systems.
02 Electrolyte compositions compatible with fluoride cathodes
Specialized electrolyte formulations are essential for maximizing the electrochemical window of fluoride cathodes. These electrolytes typically contain fluoride salts or fluorinated solvents that are stable at high voltages and compatible with fluoride-containing electrode materials. The electrolyte compositions can include additives that form protective surface films on the cathode, preventing unwanted side reactions and extending the upper voltage limit of the electrochemical window while maintaining ionic conductivity throughout the operating range.Expand Specific Solutions03 Nanostructured fluoride cathode designs
Nanostructuring of fluoride cathode materials can significantly expand their electrochemical window by improving reaction kinetics and reducing diffusion distances. These designs include nanoparticles, nanocomposites, and core-shell structures that enhance the electrochemical performance of fluoride-based cathodes. The increased surface area and engineered interfaces facilitate faster ion transport and more complete utilization of active materials, allowing operation at higher voltages without significant degradation or capacity loss.Expand Specific Solutions04 Fluoride conversion cathode mechanisms
Fluoride conversion cathodes operate through a distinct electrochemical mechanism involving the breaking and forming of chemical bonds during cycling, which can provide higher energy densities and wider electrochemical windows. These cathodes typically undergo conversion reactions where metal fluorides are reduced to metals and fluoride ions during discharge. The reversibility of these reactions determines the practical electrochemical window, with recent advances focusing on improving the reaction kinetics and cycle stability through various material modifications and electrode engineering approaches.Expand Specific Solutions05 Protective coatings and interface engineering for fluoride cathodes
Surface modifications and interface engineering techniques can extend the electrochemical window of fluoride cathodes by preventing unwanted side reactions with the electrolyte. These approaches include applying protective coatings, creating artificial solid electrolyte interfaces, and introducing buffer layers that stabilize the cathode surface at high voltages. Such modifications help mitigate degradation mechanisms like fluoride dissolution and structural collapse that typically limit the upper boundary of the electrochemical window, enabling operation at higher potentials with improved cycling stability.Expand Specific Solutions
Leading Research Groups and Industrial Players
The fluoride cathode electrochemical window expansion technology market is in an early growth phase, characterized by intensive research activities primarily led by academic institutions like University of Maryland and California Institute of Technology, alongside industrial players. The market size remains relatively modest but is expanding rapidly due to increasing demand for high-energy-density battery technologies. Technical maturity varies significantly across competitors, with companies like Wildcat Discovery Technologies and Solvay Specialty Polymers demonstrating advanced capabilities in materials development. Samsung Display, Honda Motor, and 3M are leveraging their manufacturing expertise to scale promising technologies, while specialized firms like Membrane Technology & Research are focusing on niche applications. University-industry collaborations are accelerating innovation in this competitive landscape.
California Institute of Technology
Technical Solution: Caltech has developed an innovative approach to fluoride cathode electrochemical window expansion through their work on liquid electrolyte systems compatible with metal fluoride cathodes. Their research focuses on room-temperature fluoride ion batteries using specifically designed organic solvents with dissolved fluoride salts that maintain stability at higher operating voltages. The institute has pioneered the use of fluorinated ether-based electrolytes that resist oxidation at the cathode interface, effectively expanding the electrochemical stability window. Their cathode materials incorporate nanoscale engineering of conversion-type metal fluorides with protective surface coatings that prevent unwanted side reactions while allowing fluoride ion transport. This architecture has demonstrated stable cycling at potentials exceeding traditional limitations by approximately 0.7V. Caltech researchers have also explored the fundamental thermodynamics of fluoride ion insertion mechanisms to optimize cathode composition for maximum voltage range without triggering electrolyte decomposition.
Strengths: World-class fundamental research capabilities and interdisciplinary approach combining materials science, electrochemistry, and computational modeling. Weaknesses: Their solutions may prioritize scientific novelty over practical manufacturing considerations, potentially limiting near-term commercial applicability.
Wildcat Discovery Technologies, Inc.
Technical Solution: Wildcat Discovery Technologies has leveraged their high-throughput experimentation platform to systematically explore fluoride cathode materials with expanded electrochemical windows. Their proprietary approach involves parallel testing of hundreds of cathode formulations with varying dopants, coatings, and electrolyte combinations specifically designed for fluoride systems. The company has developed composite cathode structures incorporating stabilizing agents that prevent fluoride dissolution at higher voltages, effectively expanding the usable voltage window. Their research has yielded fluoride cathode materials with up to 30% higher energy density through careful engineering of the cathode-electrolyte interface. Wildcat's technology includes specialized conductive additives compatible with fluoride chemistry that maintain electronic pathways without catalyzing electrolyte degradation at elevated potentials. Their systematic materials discovery process has identified several promising fluoride cathode compositions that demonstrate stable cycling above 4.5V versus conventional reference electrodes, representing a significant expansion of the traditional electrochemical window for these materials.
Strengths: Industry-leading high-throughput experimentation capabilities allow rapid iteration and optimization of fluoride cathode formulations. Their commercial focus drives practical solutions with manufacturing scalability in mind. Weaknesses: As a specialized materials discovery company, they may lack the vertical integration capabilities for full battery system development and production.
Key Patents and Scientific Breakthroughs
High-voltage lithium-ion battery with a wide electrochemical window of polymer electrolytes
PatentPendingUS20250096321A1
Innovation
- A PVTF-based solid polymer electrolyte is developed, incorporating a polymer matrix with a sacrificial additive like lithium difluorophosphate, which expands the electrochemical window to 5.68 V and stabilizes Ni-rich cathodes, enabling consistent cycling performance and high-capacity retention.
Materials Science Challenges and Opportunities
The expansion of electrochemical windows for fluoride cathodes represents one of the most significant materials science challenges in advanced battery technology. Current fluoride-based cathode materials exhibit limited voltage stability ranges, typically constrained to 2.5-3.5V versus Li/Li+, which substantially restricts the energy density potential of these systems. This fundamental limitation stems from the intrinsic electronic structure of metal fluorides and their complex interaction with electrolyte systems.
At the molecular level, the strong ionic character of metal-fluorine bonds creates localized electronic states that are susceptible to redox activity at relatively narrow potential ranges. When pushed beyond these limits, fluoride cathodes often experience irreversible structural transformations, leading to capacity fading and shortened cycle life. The challenge is further compounded by the high electronegativity of fluorine, which can trigger aggressive side reactions with conventional electrolyte components.
Material scientists are exploring several promising approaches to address these limitations. Nanostructuring of fluoride materials has shown potential for stabilizing interfaces and modifying electronic properties, potentially expanding operational voltage windows by 0.3-0.5V. Additionally, surface modification strategies using atomic layer deposition of protective coatings have demonstrated improved stability at higher potentials, though often at the cost of increased internal resistance.
Computational materials science has emerged as a crucial tool in this research domain. Density functional theory calculations are helping researchers predict more stable fluoride compositions and identify potential dopants that could modify the band structure to enable wider electrochemical windows. These theoretical insights are guiding experimental efforts toward rational material design rather than traditional trial-and-error approaches.
The development of compatible electrolytes represents another critical research direction. Conventional carbonate-based electrolytes decompose at the surface of fluoride cathodes, forming unstable solid-electrolyte interphase layers. Novel fluorinated electrolytes and ionic liquids are being investigated for their potential to form more stable interfaces and withstand the highly oxidizing environment at higher potentials.
Hybrid materials combining fluorides with other anion chemistries (oxyfluorides, sulfur-fluoride composites) show particular promise for expanding electrochemical windows while maintaining the high energy density advantages of fluoride systems. These materials can potentially leverage synergistic effects between different anion groups to stabilize structures across wider voltage ranges.
At the molecular level, the strong ionic character of metal-fluorine bonds creates localized electronic states that are susceptible to redox activity at relatively narrow potential ranges. When pushed beyond these limits, fluoride cathodes often experience irreversible structural transformations, leading to capacity fading and shortened cycle life. The challenge is further compounded by the high electronegativity of fluorine, which can trigger aggressive side reactions with conventional electrolyte components.
Material scientists are exploring several promising approaches to address these limitations. Nanostructuring of fluoride materials has shown potential for stabilizing interfaces and modifying electronic properties, potentially expanding operational voltage windows by 0.3-0.5V. Additionally, surface modification strategies using atomic layer deposition of protective coatings have demonstrated improved stability at higher potentials, though often at the cost of increased internal resistance.
Computational materials science has emerged as a crucial tool in this research domain. Density functional theory calculations are helping researchers predict more stable fluoride compositions and identify potential dopants that could modify the band structure to enable wider electrochemical windows. These theoretical insights are guiding experimental efforts toward rational material design rather than traditional trial-and-error approaches.
The development of compatible electrolytes represents another critical research direction. Conventional carbonate-based electrolytes decompose at the surface of fluoride cathodes, forming unstable solid-electrolyte interphase layers. Novel fluorinated electrolytes and ionic liquids are being investigated for their potential to form more stable interfaces and withstand the highly oxidizing environment at higher potentials.
Hybrid materials combining fluorides with other anion chemistries (oxyfluorides, sulfur-fluoride composites) show particular promise for expanding electrochemical windows while maintaining the high energy density advantages of fluoride systems. These materials can potentially leverage synergistic effects between different anion groups to stabilize structures across wider voltage ranges.
Environmental Impact and Sustainability Considerations
The expansion of fluoride cathode electrochemical windows presents significant environmental implications that must be carefully considered alongside technological advancements. Fluoride-based battery systems offer promising energy density improvements compared to conventional lithium-ion technologies, potentially reducing the overall material footprint per unit of energy stored. This efficiency gain translates to fewer raw materials extracted from the environment, particularly reducing dependence on cobalt and nickel mining operations that have been associated with substantial ecological damage and human rights concerns.
However, fluoride compounds themselves present unique environmental challenges. The production, processing, and potential release of fluorinated materials require stringent controls due to their environmental persistence. Fluoride ions can accumulate in soil and water systems, potentially affecting aquatic ecosystems and entering food chains. Manufacturing processes for advanced fluoride cathodes typically involve energy-intensive synthesis methods and specialized handling protocols that may increase the carbon footprint of production facilities unless powered by renewable energy sources.
End-of-life management represents another critical environmental consideration. The recyclability of fluoride-based cathode materials remains underdeveloped compared to established lithium-ion recycling processes. Research into recovery methods that can efficiently separate and purify fluoride compounds from spent batteries is essential to creating closed-loop material systems and preventing potential environmental contamination from improper disposal.
From a sustainability perspective, the expanded electrochemical windows of fluoride cathodes could significantly extend battery lifespans, reducing replacement frequency and associated waste generation. This longevity factor represents a substantial sustainability advantage when considering total lifecycle impacts. Additionally, the higher energy density could enable more efficient electric transportation and renewable energy storage systems, contributing to broader decarbonization efforts.
Water usage in manufacturing processes presents another environmental concern, as fluoride production and processing typically require substantial quantities of ultrapure water. Implementing water recycling systems and developing less water-intensive synthesis methods will be crucial for sustainable scaling of this technology, particularly in water-stressed regions where manufacturing may occur.
Regulatory frameworks worldwide are increasingly focusing on the environmental footprint of battery technologies, with particular attention to potentially hazardous materials. Future development of fluoride cathode technologies must proactively address these regulatory considerations through green chemistry approaches, designing systems that minimize environmental hazards while maintaining performance advantages.
However, fluoride compounds themselves present unique environmental challenges. The production, processing, and potential release of fluorinated materials require stringent controls due to their environmental persistence. Fluoride ions can accumulate in soil and water systems, potentially affecting aquatic ecosystems and entering food chains. Manufacturing processes for advanced fluoride cathodes typically involve energy-intensive synthesis methods and specialized handling protocols that may increase the carbon footprint of production facilities unless powered by renewable energy sources.
End-of-life management represents another critical environmental consideration. The recyclability of fluoride-based cathode materials remains underdeveloped compared to established lithium-ion recycling processes. Research into recovery methods that can efficiently separate and purify fluoride compounds from spent batteries is essential to creating closed-loop material systems and preventing potential environmental contamination from improper disposal.
From a sustainability perspective, the expanded electrochemical windows of fluoride cathodes could significantly extend battery lifespans, reducing replacement frequency and associated waste generation. This longevity factor represents a substantial sustainability advantage when considering total lifecycle impacts. Additionally, the higher energy density could enable more efficient electric transportation and renewable energy storage systems, contributing to broader decarbonization efforts.
Water usage in manufacturing processes presents another environmental concern, as fluoride production and processing typically require substantial quantities of ultrapure water. Implementing water recycling systems and developing less water-intensive synthesis methods will be crucial for sustainable scaling of this technology, particularly in water-stressed regions where manufacturing may occur.
Regulatory frameworks worldwide are increasingly focusing on the environmental footprint of battery technologies, with particular attention to potentially hazardous materials. Future development of fluoride cathode technologies must proactively address these regulatory considerations through green chemistry approaches, designing systems that minimize environmental hazards while maintaining performance advantages.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!