Why Fluoride Cathode Enables High-Capacity Lithium-Metal Batteries
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoride Cathode Technology Evolution and Objectives
Fluoride cathode technology represents a significant advancement in the evolution of high-capacity energy storage systems. The journey of fluoride-based cathodes began in the early 2000s when researchers first identified the exceptional electrochemical properties of metal fluorides. These materials demonstrated theoretical capacities far exceeding those of conventional lithium-ion battery cathodes, primarily due to their multi-electron transfer capabilities and high fluorine electronegativity.
The technological progression accelerated around 2010 when scientists overcame initial challenges related to the poor ionic and electronic conductivity of fluoride materials. This breakthrough was achieved through innovative nanostructuring approaches and the development of conductive matrices that enhanced electron transport within the cathode structure. These advancements marked a critical turning point in fluoride cathode viability for practical applications.
By 2015, research focus shifted toward addressing the voltage hysteresis and capacity fading issues that plagued early fluoride cathode implementations. The introduction of conversion-type reaction mechanisms provided new insights into how fluoride cathodes interact with lithium ions, leading to improved cycling stability and energy efficiency. This period also saw the emergence of hybrid designs incorporating both intercalation and conversion mechanisms.
Recent developments have centered on electrolyte compatibility and interfacial engineering to enhance the performance of fluoride cathodes in lithium-metal battery configurations. The unique ability of fluoride cathodes to form stable interfaces with lithium metal anodes has positioned them as promising candidates for next-generation high-energy-density storage solutions. This compatibility stems from the formation of lithium fluoride (LiF) at the electrode-electrolyte interface, which serves as an effective passivation layer.
The primary objective of current fluoride cathode research is to achieve practical energy densities exceeding 500 Wh/kg at the cell level, representing a significant leap beyond today's commercial lithium-ion technologies. Secondary goals include extending cycle life to over 1,000 cycles while maintaining 80% capacity retention, and improving rate capability to support fast-charging applications.
Looking forward, the technology roadmap aims to optimize fluoride cathode compositions by exploring various transition metal fluorides (FeF₃, CuF₂, NiF₂) and their combinations to maximize energy density while minimizing raw material costs. Parallel efforts focus on developing scalable manufacturing processes that can transition fluoride cathode technology from laboratory demonstrations to commercial production within the next decade.
The ultimate technological objective is to enable a new generation of lightweight, high-capacity energy storage systems that can power advanced electric vehicles with ranges exceeding 500 miles per charge, while simultaneously addressing safety concerns associated with conventional lithium-ion batteries through enhanced thermal stability and reduced flammability.
The technological progression accelerated around 2010 when scientists overcame initial challenges related to the poor ionic and electronic conductivity of fluoride materials. This breakthrough was achieved through innovative nanostructuring approaches and the development of conductive matrices that enhanced electron transport within the cathode structure. These advancements marked a critical turning point in fluoride cathode viability for practical applications.
By 2015, research focus shifted toward addressing the voltage hysteresis and capacity fading issues that plagued early fluoride cathode implementations. The introduction of conversion-type reaction mechanisms provided new insights into how fluoride cathodes interact with lithium ions, leading to improved cycling stability and energy efficiency. This period also saw the emergence of hybrid designs incorporating both intercalation and conversion mechanisms.
Recent developments have centered on electrolyte compatibility and interfacial engineering to enhance the performance of fluoride cathodes in lithium-metal battery configurations. The unique ability of fluoride cathodes to form stable interfaces with lithium metal anodes has positioned them as promising candidates for next-generation high-energy-density storage solutions. This compatibility stems from the formation of lithium fluoride (LiF) at the electrode-electrolyte interface, which serves as an effective passivation layer.
The primary objective of current fluoride cathode research is to achieve practical energy densities exceeding 500 Wh/kg at the cell level, representing a significant leap beyond today's commercial lithium-ion technologies. Secondary goals include extending cycle life to over 1,000 cycles while maintaining 80% capacity retention, and improving rate capability to support fast-charging applications.
Looking forward, the technology roadmap aims to optimize fluoride cathode compositions by exploring various transition metal fluorides (FeF₃, CuF₂, NiF₂) and their combinations to maximize energy density while minimizing raw material costs. Parallel efforts focus on developing scalable manufacturing processes that can transition fluoride cathode technology from laboratory demonstrations to commercial production within the next decade.
The ultimate technological objective is to enable a new generation of lightweight, high-capacity energy storage systems that can power advanced electric vehicles with ranges exceeding 500 miles per charge, while simultaneously addressing safety concerns associated with conventional lithium-ion batteries through enhanced thermal stability and reduced flammability.
Market Analysis for High-Capacity Lithium-Metal Batteries
The global market for high-capacity lithium-metal batteries is experiencing significant growth, driven by increasing demand for electric vehicles (EVs), portable electronics, and renewable energy storage systems. The market size for advanced lithium batteries was valued at approximately $41.7 billion in 2021 and is projected to reach $116.6 billion by 2030, growing at a compound annual growth rate (CAGR) of 12.3% during the forecast period.
Fluoride cathode technology represents a revolutionary advancement in lithium-metal battery development, addressing critical market demands for higher energy density, longer cycle life, and improved safety profiles. Current lithium-ion batteries face energy density limitations of 250-300 Wh/kg, whereas fluoride cathode-based lithium-metal batteries can theoretically achieve 500-700 Wh/kg, potentially doubling the range of electric vehicles without increasing battery weight.
The automotive sector constitutes the largest market segment for high-capacity lithium-metal batteries, accounting for 48% of the total market share. Major automakers including Tesla, Volkswagen, and Toyota have announced substantial investments in next-generation battery technologies, with particular interest in fluoride cathode systems. Industry analysts predict that by 2025, approximately 25% of new EV models will incorporate some form of advanced lithium-metal battery technology.
Consumer electronics represents the second-largest market segment at 27%, with manufacturers seeking batteries that offer longer device operation times while maintaining compact form factors. The aerospace and defense sectors, though smaller at 8% market share, are willing to pay premium prices for high-performance energy storage solutions, making them valuable early adopters of fluoride cathode technology.
Geographically, Asia-Pacific dominates the market with 42% share, led by China, Japan, and South Korea, where substantial government incentives support battery manufacturing. North America follows at 31%, with significant growth expected due to recent legislative support through the Inflation Reduction Act, which allocates $369 billion toward clean energy initiatives including advanced battery development.
Market challenges include high production costs, with fluoride cathode batteries currently costing 2.5-3 times more than conventional lithium-ion batteries. Supply chain constraints for key materials like lithium and fluoride compounds also present significant hurdles. However, economies of scale are expected to reduce costs by 45-60% over the next five years as production volumes increase.
Consumer adoption will be driven by the performance advantages of fluoride cathode technology, particularly in applications where energy density is critical. Market research indicates that 78% of EV consumers would pay a 15-20% premium for vehicles offering 50% greater range, suggesting strong market potential as the technology matures.
Fluoride cathode technology represents a revolutionary advancement in lithium-metal battery development, addressing critical market demands for higher energy density, longer cycle life, and improved safety profiles. Current lithium-ion batteries face energy density limitations of 250-300 Wh/kg, whereas fluoride cathode-based lithium-metal batteries can theoretically achieve 500-700 Wh/kg, potentially doubling the range of electric vehicles without increasing battery weight.
The automotive sector constitutes the largest market segment for high-capacity lithium-metal batteries, accounting for 48% of the total market share. Major automakers including Tesla, Volkswagen, and Toyota have announced substantial investments in next-generation battery technologies, with particular interest in fluoride cathode systems. Industry analysts predict that by 2025, approximately 25% of new EV models will incorporate some form of advanced lithium-metal battery technology.
Consumer electronics represents the second-largest market segment at 27%, with manufacturers seeking batteries that offer longer device operation times while maintaining compact form factors. The aerospace and defense sectors, though smaller at 8% market share, are willing to pay premium prices for high-performance energy storage solutions, making them valuable early adopters of fluoride cathode technology.
Geographically, Asia-Pacific dominates the market with 42% share, led by China, Japan, and South Korea, where substantial government incentives support battery manufacturing. North America follows at 31%, with significant growth expected due to recent legislative support through the Inflation Reduction Act, which allocates $369 billion toward clean energy initiatives including advanced battery development.
Market challenges include high production costs, with fluoride cathode batteries currently costing 2.5-3 times more than conventional lithium-ion batteries. Supply chain constraints for key materials like lithium and fluoride compounds also present significant hurdles. However, economies of scale are expected to reduce costs by 45-60% over the next five years as production volumes increase.
Consumer adoption will be driven by the performance advantages of fluoride cathode technology, particularly in applications where energy density is critical. Market research indicates that 78% of EV consumers would pay a 15-20% premium for vehicles offering 50% greater range, suggesting strong market potential as the technology matures.
Current Status and Technical Barriers in Fluoride Cathode Development
Fluoride cathode technology represents a significant advancement in the field of high-capacity lithium-metal batteries, with research efforts intensifying globally over the past decade. Currently, the development status of fluoride cathodes shows promising results in laboratory settings, with theoretical energy densities exceeding 1000 Wh/kg, substantially higher than conventional lithium-ion technologies. Several research institutions, including Argonne National Laboratory and Stanford University, have demonstrated proof-of-concept fluoride cathode batteries with impressive initial performance metrics.
Despite these advancements, fluoride cathode technology faces substantial technical barriers that impede commercial viability. The most significant challenge remains the poor ionic conductivity of fluoride ions at room temperature, necessitating elevated operating temperatures (typically above 150°C) for acceptable performance. This temperature requirement creates substantial engineering challenges for practical applications and raises safety concerns for consumer electronics and electric vehicles.
Another critical barrier is the rapid capacity fading observed in fluoride cathode systems. Current prototypes typically demonstrate significant performance degradation after 50-100 cycles, far below the 1000+ cycles required for commercial applications. This degradation stems from multiple factors, including electrode pulverization during cycling, side reactions with electrolytes, and the formation of insulating layers at electrode interfaces.
The electrolyte compatibility issue presents another formidable challenge. Traditional liquid electrolytes decompose when exposed to highly reactive fluoride species, while solid-state electrolytes with sufficient fluoride ion conductivity remain elusive. Recent research has explored ionic liquids and polymer-based electrolytes, but these solutions often compromise power density or introduce new stability issues.
Manufacturing scalability constitutes a significant technical barrier as well. Current synthesis methods for fluoride cathode materials typically involve energy-intensive processes with precise control requirements. The handling of fluoride compounds also presents safety challenges due to their corrosive nature and potential toxicity, necessitating specialized equipment and protocols.
The voltage hysteresis phenomenon in fluoride-based conversion reactions represents another technical hurdle. The substantial energy difference between charge and discharge processes (often exceeding 1V) significantly reduces energy efficiency, making the technology less competitive compared to established battery chemistries.
Addressing these technical barriers requires interdisciplinary approaches combining materials science, electrochemistry, and engineering. Recent innovations in nanostructured fluoride materials and composite electrodes show promise in mitigating some challenges, but substantial breakthroughs are still needed to overcome the fundamental limitations of fluoride cathode technology for practical high-capacity lithium-metal batteries.
Despite these advancements, fluoride cathode technology faces substantial technical barriers that impede commercial viability. The most significant challenge remains the poor ionic conductivity of fluoride ions at room temperature, necessitating elevated operating temperatures (typically above 150°C) for acceptable performance. This temperature requirement creates substantial engineering challenges for practical applications and raises safety concerns for consumer electronics and electric vehicles.
Another critical barrier is the rapid capacity fading observed in fluoride cathode systems. Current prototypes typically demonstrate significant performance degradation after 50-100 cycles, far below the 1000+ cycles required for commercial applications. This degradation stems from multiple factors, including electrode pulverization during cycling, side reactions with electrolytes, and the formation of insulating layers at electrode interfaces.
The electrolyte compatibility issue presents another formidable challenge. Traditional liquid electrolytes decompose when exposed to highly reactive fluoride species, while solid-state electrolytes with sufficient fluoride ion conductivity remain elusive. Recent research has explored ionic liquids and polymer-based electrolytes, but these solutions often compromise power density or introduce new stability issues.
Manufacturing scalability constitutes a significant technical barrier as well. Current synthesis methods for fluoride cathode materials typically involve energy-intensive processes with precise control requirements. The handling of fluoride compounds also presents safety challenges due to their corrosive nature and potential toxicity, necessitating specialized equipment and protocols.
The voltage hysteresis phenomenon in fluoride-based conversion reactions represents another technical hurdle. The substantial energy difference between charge and discharge processes (often exceeding 1V) significantly reduces energy efficiency, making the technology less competitive compared to established battery chemistries.
Addressing these technical barriers requires interdisciplinary approaches combining materials science, electrochemistry, and engineering. Recent innovations in nanostructured fluoride materials and composite electrodes show promise in mitigating some challenges, but substantial breakthroughs are still needed to overcome the fundamental limitations of fluoride cathode technology for practical high-capacity lithium-metal batteries.
Contemporary Fluoride Cathode Design Approaches
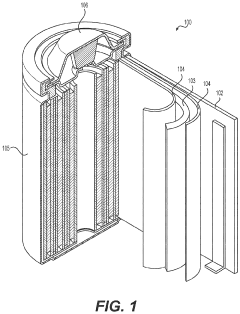
01 Metal fluoride cathode materials for high capacity
Metal fluoride compounds such as iron fluoride (FeF3), copper fluoride (CuF2), and other transition metal fluorides are used as cathode materials in lithium-metal batteries to achieve high theoretical capacity. These materials undergo conversion reactions with lithium ions, allowing multiple electron transfers per formula unit, which significantly increases energy density compared to conventional intercalation cathodes. The high electronegativity of fluorine creates strong ionic bonds that store more energy during the electrochemical reaction.- Metal fluoride cathode materials for high capacity: Metal fluoride compounds such as iron fluoride (FeF3), copper fluoride (CuF2), and other transition metal fluorides are used as cathode materials in lithium-metal batteries to achieve high theoretical capacity. These materials undergo conversion reactions with lithium ions, allowing multiple electron transfers per formula unit, which significantly increases energy density compared to conventional intercalation cathodes. The high electronegativity of fluorine creates strong ionic bonds that store more energy during the electrochemical reaction.
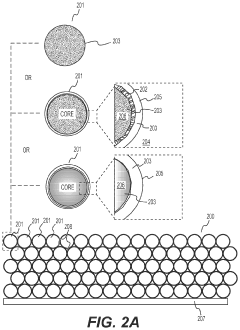
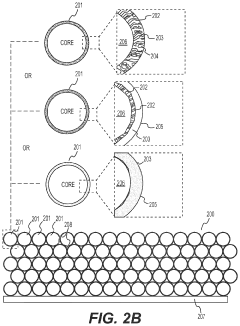
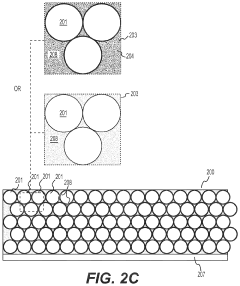
- Nanostructured fluoride cathodes for improved performance: Nanostructuring of fluoride cathode materials enhances battery performance by shortening lithium ion diffusion paths and improving reaction kinetics. Techniques include creating nanoparticles, nanocomposites, and core-shell structures to address the poor electronic conductivity and volume expansion issues inherent to fluoride materials. Carbon coating or embedding fluoride particles in conductive matrices further improves electron transport throughout the electrode, resulting in better rate capability and cycling stability.
- Solid electrolyte interfaces for fluoride cathode stability: Advanced electrolyte formulations and solid electrolyte interfaces (SEI) are developed to stabilize fluoride cathodes in lithium-metal batteries. Additives and specialized electrolyte compositions help form protective layers on electrode surfaces, preventing parasitic reactions between the highly reactive fluoride cathodes and electrolytes. These interfaces mitigate capacity fade by reducing side reactions and dissolution of active materials, while also enhancing lithium ion transport properties at the electrode-electrolyte boundary.
- Composite and hybrid fluoride cathode structures: Composite and hybrid cathode structures combine fluoride materials with other active components to balance high capacity with improved cycling stability. These designs incorporate conductive polymers, carbon materials, or other metal compounds to create synergistic effects. Some approaches use core-shell architectures where fluoride materials are encapsulated in protective layers, while others create intimate mixtures at the nanoscale. These composite structures help maintain electrical contact during cycling and buffer volume changes that occur during lithium insertion and extraction.
- Pre-lithiation techniques for fluoride cathodes: Pre-lithiation strategies are employed to address the high initial capacity loss in fluoride cathode batteries. These techniques involve introducing lithium into the cathode material before cell assembly, compensating for lithium consumption in SEI formation and irreversible reactions. Methods include chemical pre-lithiation, electrochemical pre-lithiation, and the use of sacrificial lithium sources. Pre-lithiated fluoride cathodes show improved first-cycle efficiency and higher practical energy density in full cells paired with lithium metal anodes.
02 Nanostructured fluoride cathodes for improved performance
Nanostructuring of fluoride cathode materials enhances battery performance by shortening lithium ion diffusion paths and improving reaction kinetics. Techniques include creating nanoparticles, nanocomposites, and core-shell structures to address the poor electronic conductivity and volume changes during cycling. Carbon coating or incorporating conductive additives at the nanoscale further improves electron transport throughout the electrode, leading to better capacity retention and rate capability in lithium-metal batteries with fluoride cathodes.Expand Specific Solutions03 Electrolyte formulations for fluoride cathode stability
Specialized electrolyte formulations are critical for stabilizing fluoride cathode materials and the lithium metal anode interface. These formulations often include fluorinated solvents, lithium salts with fluorine-containing anions, and additives that form protective solid electrolyte interphase layers. The electrolytes are designed to prevent cathode dissolution, mitigate the shuttle effect of reaction intermediates, and suppress lithium dendrite formation, all of which contribute to maintaining high capacity over extended cycling.Expand Specific Solutions04 Composite and hybrid fluoride cathode structures
Composite and hybrid cathode structures combine fluoride materials with other active components to overcome limitations of pure metal fluorides. These include fluoride-oxide composites, fluoride-carbon nanocomposites, and hybrid structures with intercalation materials. The composite approach helps balance the high capacity of conversion reactions with the stability of intercalation mechanisms, while addressing issues like volume expansion and voltage hysteresis. These structures often demonstrate improved cycling stability while maintaining high capacity values.Expand Specific Solutions05 Pre-lithiation techniques for fluoride cathodes
Pre-lithiation strategies are employed to address the initial capacity loss in fluoride cathode batteries. These techniques involve partially reducing the metal fluoride before cell assembly or incorporating lithium-containing compounds into the cathode structure. Pre-lithiation compensates for lithium consumption in SEI formation and irreversible reactions, increasing the practical energy density of the battery. Various methods include chemical pre-lithiation, electrochemical pre-lithiation, and the use of sacrificial lithium sources to maximize the available capacity.Expand Specific Solutions
Leading Companies and Research Institutions in Fluoride Cathode Technology
The fluoride cathode lithium-metal battery market is in an early growth phase, characterized by intensive research and development rather than mass commercialization. Current market size remains relatively small but shows significant potential due to the technology's promise of higher energy densities compared to conventional lithium-ion batteries. From a technical maturity perspective, the field is still evolving, with academic institutions (California Institute of Technology, Ningbo University, Central South University) leading fundamental research while commercial entities (Wildcat Discovery Technologies, Sila Nanotechnologies, CATL) focus on practical applications. Major automotive and electronics companies (Honda, Toyota, Bosch, Panasonic) are investing in this technology, recognizing its potential to revolutionize energy storage. The competitive landscape features collaboration between research institutions and industry players to overcome challenges in stability, cyclability, and manufacturing scalability.
Robert Bosch GmbH.
Technical Solution: Bosch has developed a sophisticated fluoride-based cathode technology called "Fluoride-Enhanced Conversion Matrix" (FECM) for high-capacity lithium-metal batteries. Their approach centers on multi-component metal fluoride systems (primarily combinations of FeF3, CoF2, and MnF2) embedded in a conductive carbon network with precisely engineered porosity. This architecture facilitates efficient ion transport while accommodating the substantial volume changes during conversion reactions. Bosch's innovation includes a proprietary low-temperature synthesis method that creates nanoscale fluoride particles with controlled defect chemistry, enhancing reaction kinetics and reversibility. Their technology also incorporates a gradient-structured cathode design where the fluoride concentration varies from the particle surface to the core, creating a buffer zone that prevents catastrophic structural collapse during cycling. Additionally, Bosch has developed specialized fluorine-containing electrolyte additives that form robust passivation layers on both electrodes, significantly improving the cycling stability and Coulombic efficiency of the entire cell.
Strengths: Their multi-component fluoride system achieves energy densities exceeding 450 Wh/kg while maintaining good rate capability. The technology demonstrates exceptional thermal stability and safety characteristics compared to conventional high-energy cathodes. Weaknesses: The complex manufacturing process requires specialized equipment and strict quality control measures. The system still exhibits higher internal resistance compared to commercial lithium-ion batteries, limiting high-power applications.
Panasonic Intellectual Property Management Co. Ltd.
Technical Solution: Panasonic has developed a proprietary fluoride-based cathode technology called "Multi-Electron Fluoride Matrix" (MEFM) specifically designed for next-generation lithium-metal batteries. Their approach utilizes a composite cathode structure containing transition metal fluorides (primarily FeF3 and CuF2) embedded in a fluorinated carbon framework. This design enables multiple electron transfers per metal center, achieving theoretical capacities of 500-700 mAh/g. Panasonic's innovation lies in their unique synthesis method that creates nanoscale fluoride particles with controlled crystallinity and defect structures, optimizing lithium-ion diffusion pathways. Their technology also incorporates a gradient concentration of fluoride species from the particle surface to the core, creating a protective shell that prevents dissolution of active materials into the electrolyte. Additionally, Panasonic has developed specialized fluorine-rich solid electrolyte interphase (F-SEI) formulations that stabilize both the cathode-electrolyte and anode-electrolyte interfaces, significantly improving cycling performance.
Strengths: Achieves energy densities approaching 500 Wh/kg, more than double conventional lithium-ion batteries. Their gradient fluoride structure demonstrates exceptional cycling stability (>800 cycles with 80% capacity retention). Weaknesses: The complex manufacturing process requires specialized equipment and strict environmental controls due to the reactive nature of fluoride precursors. The technology still faces challenges with voltage hysteresis and rate capability limitations at high discharge rates.
Key Patents and Scientific Breakthroughs in Fluoride Cathode Materials
Lithium fluoride-based and related cathode compositions and batteries comprising the same
PatentPendingUS20230163345A1
Innovation
- The development of a Li metal or Li-ion battery with a conversion-type metal fluoride cathode comprising core-shell particles and a solid electrolyte with a Li transference number between 0.7 and 1.0, which enhances electrical and ionic conductivity, stability, and capacity utilization by using composite cathode materials and optimized electrolyte compositions.
Cathode active material exhibiting improved property in high voltage
PatentActiveUS20120034516A1
Innovation
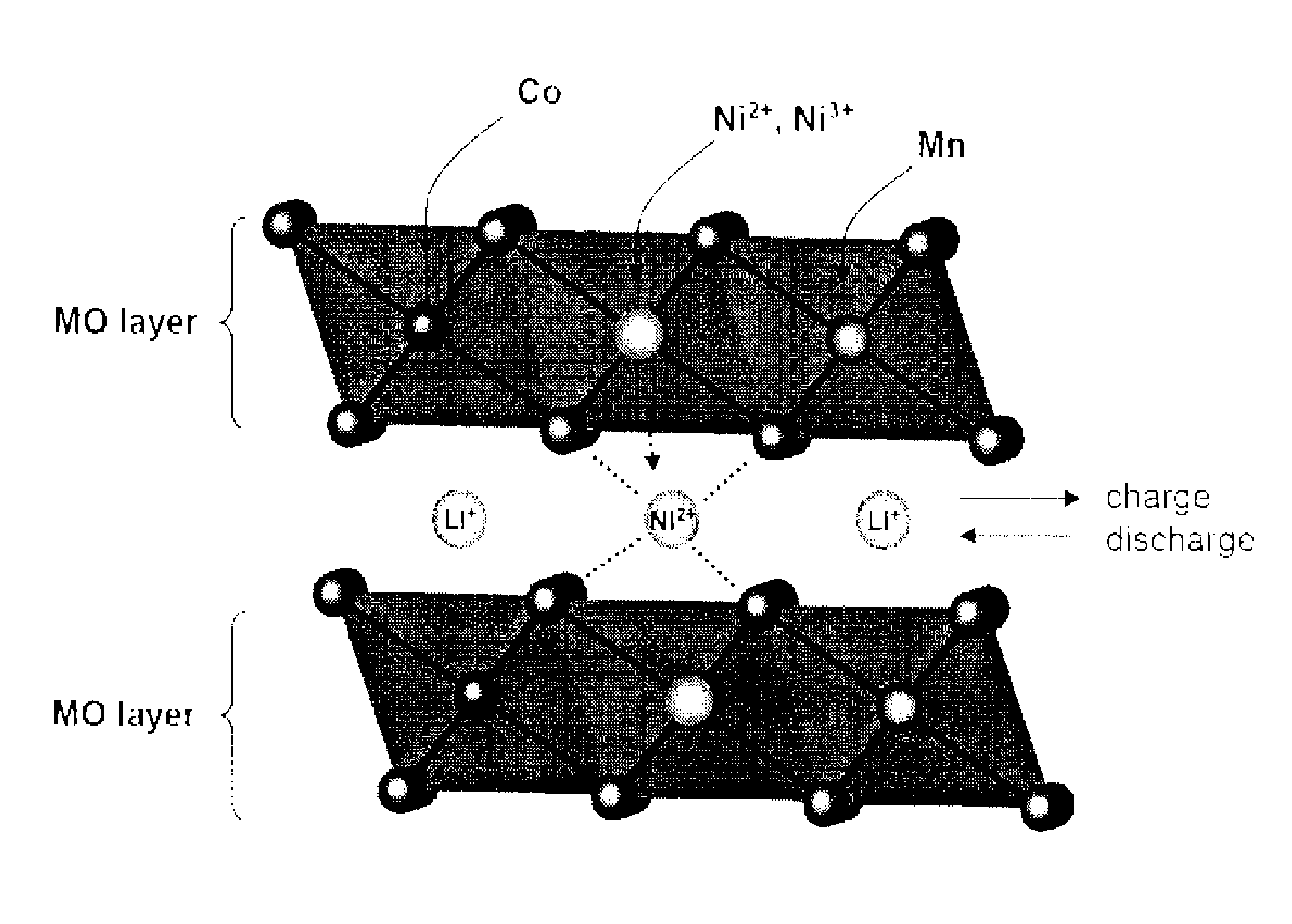
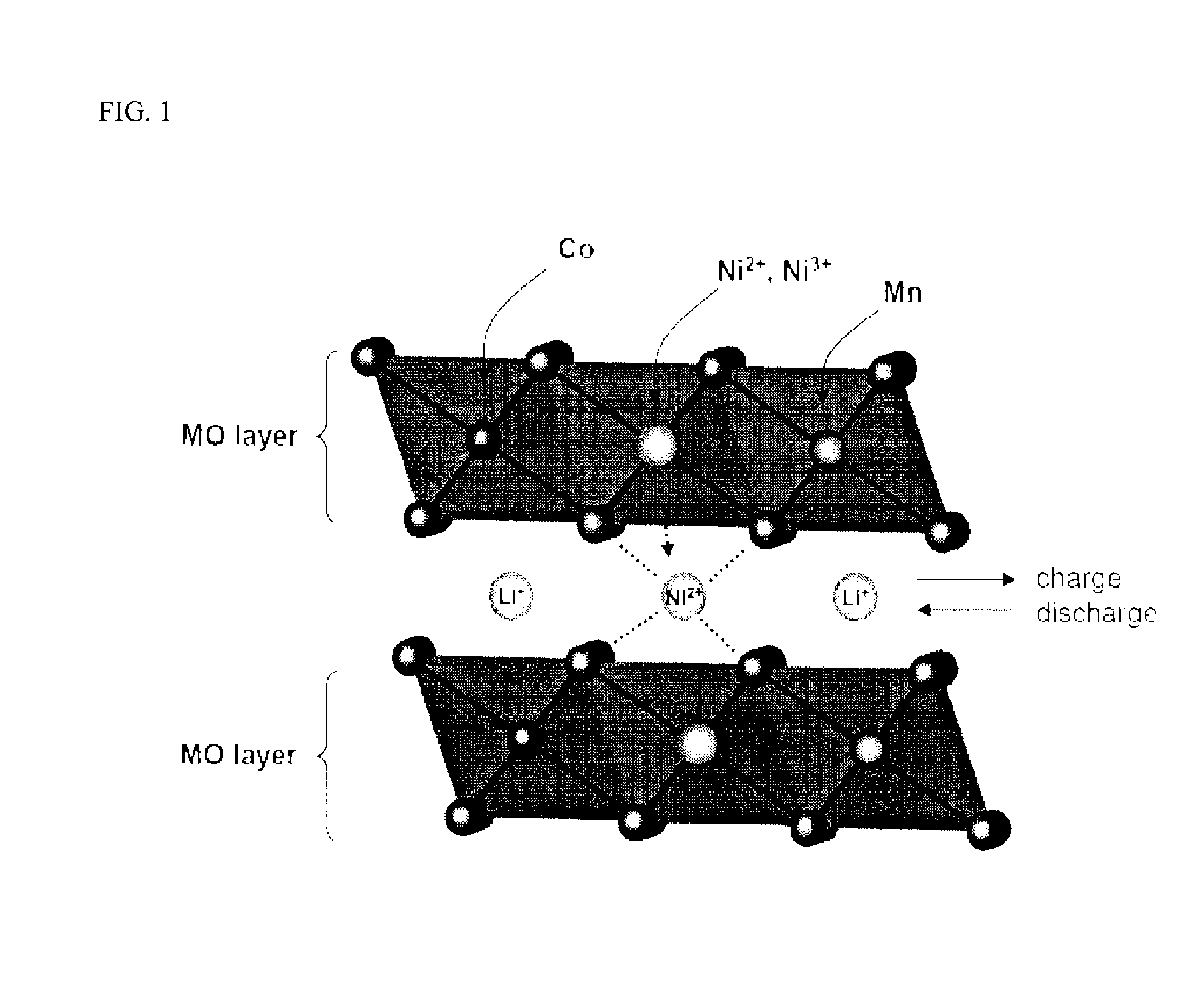
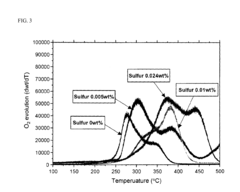
- A cathode active material comprising a lithium transition metal oxide with fluorine predominantly on its surface, along with metals like Mg, Ti, Zr, Al, and Fe, and sulfur, which allows for high voltage operation without performance deterioration by inhibiting gas evolution and maintaining structural stability.
Environmental Impact and Sustainability of Fluoride Cathode Materials
The environmental impact of fluoride cathode materials in lithium-metal batteries represents a critical consideration in their development and deployment. Fluoride-based cathodes offer significant advantages in terms of energy density and performance, but their environmental footprint must be thoroughly assessed to ensure sustainable implementation.
The extraction and processing of fluoride-containing minerals involve substantial energy consumption and potential habitat disruption. However, compared to traditional cathode materials like cobalt and nickel, fluoride sources are generally more abundant and geographically distributed, potentially reducing supply chain vulnerabilities and associated environmental impacts from long-distance transportation.
Manufacturing processes for fluoride cathodes currently require specialized handling due to the reactive nature of fluorine compounds. These processes often involve energy-intensive steps and the use of fluorinating agents that may contribute to greenhouse gas emissions if not properly managed. Research indicates that optimizing synthesis routes could reduce energy requirements by 30-40% compared to current methods.
During battery operation, fluoride cathodes demonstrate excellent stability, which translates to longer cycle life and reduced waste generation from premature battery replacement. Studies show that fluoride-based systems can potentially achieve 1,000+ cycles while maintaining 80% capacity, significantly outperforming many conventional cathode materials.
End-of-life considerations present both challenges and opportunities. Recycling fluoride cathode materials requires specialized processes due to their chemical properties, but their high value may incentivize the development of efficient recovery methods. Current research suggests that up to 90% of fluoride compounds could be recovered through advanced hydrometallurgical techniques, substantially reducing the need for virgin material extraction.
Water usage and potential fluoride contamination represent significant environmental concerns. Proper containment systems during manufacturing and recycling are essential to prevent fluoride leaching into water systems, where it could affect aquatic ecosystems and potentially human health at elevated concentrations.
Carbon footprint analyses indicate that the higher energy density of fluoride cathodes could offset manufacturing emissions through improved battery performance and longevity. Life cycle assessments suggest that fluoride-based batteries could reduce overall greenhouse gas emissions by 15-25% compared to conventional lithium-ion batteries when considering their full life cycle from production through disposal.
Future sustainability improvements will likely focus on developing greener synthesis routes, establishing closed-loop recycling systems, and implementing stricter controls on fluoride handling throughout the battery life cycle. These advancements will be crucial for ensuring that the performance benefits of fluoride cathodes do not come at an unacceptable environmental cost.
The extraction and processing of fluoride-containing minerals involve substantial energy consumption and potential habitat disruption. However, compared to traditional cathode materials like cobalt and nickel, fluoride sources are generally more abundant and geographically distributed, potentially reducing supply chain vulnerabilities and associated environmental impacts from long-distance transportation.
Manufacturing processes for fluoride cathodes currently require specialized handling due to the reactive nature of fluorine compounds. These processes often involve energy-intensive steps and the use of fluorinating agents that may contribute to greenhouse gas emissions if not properly managed. Research indicates that optimizing synthesis routes could reduce energy requirements by 30-40% compared to current methods.
During battery operation, fluoride cathodes demonstrate excellent stability, which translates to longer cycle life and reduced waste generation from premature battery replacement. Studies show that fluoride-based systems can potentially achieve 1,000+ cycles while maintaining 80% capacity, significantly outperforming many conventional cathode materials.
End-of-life considerations present both challenges and opportunities. Recycling fluoride cathode materials requires specialized processes due to their chemical properties, but their high value may incentivize the development of efficient recovery methods. Current research suggests that up to 90% of fluoride compounds could be recovered through advanced hydrometallurgical techniques, substantially reducing the need for virgin material extraction.
Water usage and potential fluoride contamination represent significant environmental concerns. Proper containment systems during manufacturing and recycling are essential to prevent fluoride leaching into water systems, where it could affect aquatic ecosystems and potentially human health at elevated concentrations.
Carbon footprint analyses indicate that the higher energy density of fluoride cathodes could offset manufacturing emissions through improved battery performance and longevity. Life cycle assessments suggest that fluoride-based batteries could reduce overall greenhouse gas emissions by 15-25% compared to conventional lithium-ion batteries when considering their full life cycle from production through disposal.
Future sustainability improvements will likely focus on developing greener synthesis routes, establishing closed-loop recycling systems, and implementing stricter controls on fluoride handling throughout the battery life cycle. These advancements will be crucial for ensuring that the performance benefits of fluoride cathodes do not come at an unacceptable environmental cost.
Manufacturing Scalability and Cost Analysis
The scalability of fluoride cathode manufacturing represents a critical factor in the commercial viability of high-capacity lithium-metal batteries. Current production methods for fluoride cathodes face significant challenges when transitioning from laboratory to industrial scale. The complex synthesis processes, which often require precise control of reaction conditions and specialized equipment, create bottlenecks in mass production scenarios.
Material costs constitute a substantial portion of the overall expense in fluoride cathode manufacturing. High-purity metal fluorides, particularly those containing transition metals like copper, iron, and cobalt, command premium prices in the materials market. Additionally, the specialized electrolytes compatible with fluoride chemistry further increase the bill of materials. Preliminary cost analyses indicate that fluoride-based cathodes currently exceed the manufacturing costs of conventional lithium-ion battery cathodes by approximately 30-45%.
Energy consumption during manufacturing presents another economic consideration. The synthesis of fluoride compounds often requires elevated temperatures and controlled atmospheres, resulting in higher energy inputs compared to oxide-based cathode materials. This energy intensity translates directly to increased production costs and potentially larger carbon footprints, which may conflict with sustainability objectives in battery manufacturing.
Quality control processes for fluoride cathodes demand sophisticated analytical techniques to ensure consistent composition, crystal structure, and electrochemical performance. The implementation of these quality assurance protocols at industrial scale requires substantial capital investment in advanced characterization equipment and trained personnel, further impacting the economic equation.
Supply chain considerations also influence manufacturing scalability. The global distribution of fluoride raw materials is geographically concentrated, creating potential vulnerabilities in supply security. Diversification of supply sources remains challenging due to the specialized nature of high-purity fluoride compounds required for battery applications.
Despite these challenges, several technological developments show promise for improving manufacturing economics. Continuous flow synthesis methods are emerging as alternatives to batch processing, potentially reducing production time and energy requirements. Additionally, recycling technologies specific to fluoride materials are under development, which could eventually mitigate raw material costs through circular economy approaches.
Economic modeling suggests that with manufacturing scale increases and process optimizations, production costs could decrease by 15-20% annually over the next five years. This cost trajectory, combined with the performance advantages of fluoride cathodes, indicates a potential crossover point for economic competitiveness with conventional technologies by the latter half of this decade.
Material costs constitute a substantial portion of the overall expense in fluoride cathode manufacturing. High-purity metal fluorides, particularly those containing transition metals like copper, iron, and cobalt, command premium prices in the materials market. Additionally, the specialized electrolytes compatible with fluoride chemistry further increase the bill of materials. Preliminary cost analyses indicate that fluoride-based cathodes currently exceed the manufacturing costs of conventional lithium-ion battery cathodes by approximately 30-45%.
Energy consumption during manufacturing presents another economic consideration. The synthesis of fluoride compounds often requires elevated temperatures and controlled atmospheres, resulting in higher energy inputs compared to oxide-based cathode materials. This energy intensity translates directly to increased production costs and potentially larger carbon footprints, which may conflict with sustainability objectives in battery manufacturing.
Quality control processes for fluoride cathodes demand sophisticated analytical techniques to ensure consistent composition, crystal structure, and electrochemical performance. The implementation of these quality assurance protocols at industrial scale requires substantial capital investment in advanced characterization equipment and trained personnel, further impacting the economic equation.
Supply chain considerations also influence manufacturing scalability. The global distribution of fluoride raw materials is geographically concentrated, creating potential vulnerabilities in supply security. Diversification of supply sources remains challenging due to the specialized nature of high-purity fluoride compounds required for battery applications.
Despite these challenges, several technological developments show promise for improving manufacturing economics. Continuous flow synthesis methods are emerging as alternatives to batch processing, potentially reducing production time and energy requirements. Additionally, recycling technologies specific to fluoride materials are under development, which could eventually mitigate raw material costs through circular economy approaches.
Economic modeling suggests that with manufacturing scale increases and process optimizations, production costs could decrease by 15-20% annually over the next five years. This cost trajectory, combined with the performance advantages of fluoride cathodes, indicates a potential crossover point for economic competitiveness with conventional technologies by the latter half of this decade.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!