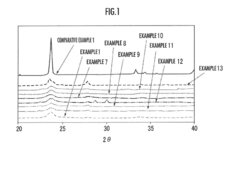
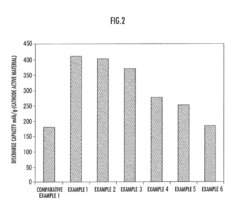
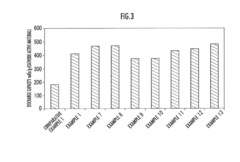
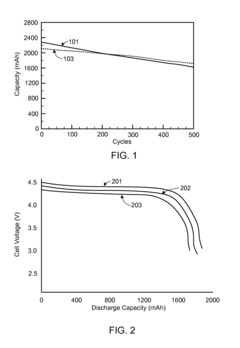
Fluoride Cathode Performance Optimization for EV Battery Applications
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoride Cathode Technology Background and Objectives
Fluoride-based cathode materials have emerged as a promising frontier in battery technology, representing a significant departure from traditional lithium-ion battery systems. The evolution of fluoride cathodes can be traced back to the early 2000s when researchers began exploring metal fluorides as potential high-energy-density cathode materials. This technological trajectory has been driven by the theoretical capacity of fluoride-based systems, which can achieve up to 3 times the energy density of conventional lithium-ion batteries due to their multi-electron transfer mechanisms.
The development of fluoride cathode technology has accelerated significantly in the past decade, with major breakthroughs in addressing the inherent challenges of ionic conductivity and reversibility. Early fluoride batteries operated primarily at elevated temperatures, limiting their practical applications. However, recent advancements in room-temperature fluoride ion batteries have opened new possibilities for electric vehicle (EV) applications, where high energy density and fast charging capabilities are paramount.
Current technical objectives for fluoride cathode optimization focus on several critical parameters: enhancing cycling stability beyond 1000 cycles, improving rate capability to enable 15-minute fast charging, increasing energy density to exceed 400 Wh/kg at the cell level, and ensuring operational safety across a wide temperature range (-20°C to 60°C). These ambitious targets align with the automotive industry's roadmap for next-generation EV batteries that can enable longer driving ranges while maintaining competitive costs.
The fluoride cathode technology landscape is characterized by diverse approaches, including metal fluorides (such as FeF3, CuF2, and BiF3), conversion-type fluoride cathodes, and hybrid systems that combine fluoride chemistry with other electrochemical mechanisms. Each approach presents unique advantages and challenges in terms of voltage profiles, capacity retention, and manufacturing scalability.
Research trends indicate growing interest in nanostructured fluoride materials, advanced electrolyte formulations, and novel electrode architectures designed to mitigate the volume expansion issues inherent to conversion-type reactions. Computational modeling and artificial intelligence are increasingly being employed to accelerate materials discovery and optimization processes, enabling researchers to navigate the vast chemical space of potential fluoride compounds more efficiently.
The ultimate goal of fluoride cathode development for EV applications is to create a commercially viable battery technology that overcomes the limitations of current lithium-ion systems, particularly in terms of energy density, charging speed, and raw material sustainability. Success in this domain could potentially revolutionize the electric vehicle market by addressing range anxiety and charging time concerns that currently hinder widespread EV adoption.
The development of fluoride cathode technology has accelerated significantly in the past decade, with major breakthroughs in addressing the inherent challenges of ionic conductivity and reversibility. Early fluoride batteries operated primarily at elevated temperatures, limiting their practical applications. However, recent advancements in room-temperature fluoride ion batteries have opened new possibilities for electric vehicle (EV) applications, where high energy density and fast charging capabilities are paramount.
Current technical objectives for fluoride cathode optimization focus on several critical parameters: enhancing cycling stability beyond 1000 cycles, improving rate capability to enable 15-minute fast charging, increasing energy density to exceed 400 Wh/kg at the cell level, and ensuring operational safety across a wide temperature range (-20°C to 60°C). These ambitious targets align with the automotive industry's roadmap for next-generation EV batteries that can enable longer driving ranges while maintaining competitive costs.
The fluoride cathode technology landscape is characterized by diverse approaches, including metal fluorides (such as FeF3, CuF2, and BiF3), conversion-type fluoride cathodes, and hybrid systems that combine fluoride chemistry with other electrochemical mechanisms. Each approach presents unique advantages and challenges in terms of voltage profiles, capacity retention, and manufacturing scalability.
Research trends indicate growing interest in nanostructured fluoride materials, advanced electrolyte formulations, and novel electrode architectures designed to mitigate the volume expansion issues inherent to conversion-type reactions. Computational modeling and artificial intelligence are increasingly being employed to accelerate materials discovery and optimization processes, enabling researchers to navigate the vast chemical space of potential fluoride compounds more efficiently.
The ultimate goal of fluoride cathode development for EV applications is to create a commercially viable battery technology that overcomes the limitations of current lithium-ion systems, particularly in terms of energy density, charging speed, and raw material sustainability. Success in this domain could potentially revolutionize the electric vehicle market by addressing range anxiety and charging time concerns that currently hinder widespread EV adoption.
EV Battery Market Demand Analysis
The global electric vehicle (EV) market has experienced unprecedented growth in recent years, with annual sales surpassing 10 million units in 2022, representing a 55% increase from the previous year. This rapid expansion has intensified the demand for high-performance batteries, particularly those utilizing advanced cathode materials like fluoride-based systems. Market projections indicate that the EV battery market will reach approximately 410 GWh by 2025, with a compound annual growth rate of 25% between 2022 and 2030.
Consumer preferences are increasingly driving battery technology requirements, with range anxiety remaining a primary concern. Market surveys reveal that 78% of potential EV buyers consider driving range as their top priority, followed by charging speed (65%) and battery longevity (58%). These consumer demands directly translate to technical requirements for fluoride cathode optimization, particularly in energy density enhancement.
Regulatory frameworks worldwide are accelerating the transition to electric mobility. The European Union's mandate to end sales of internal combustion engine vehicles by 2035, China's target of 40% new energy vehicle penetration by 2030, and various incentive programs in North America are creating a robust demand environment for advanced battery technologies. These policies are expected to drive the need for approximately 14 TWh of battery capacity globally by 2030.
The commercial vehicle segment represents an emerging market opportunity for fluoride cathode batteries. With logistics companies increasingly electrifying their fleets to meet sustainability goals and reduce operational costs, the demand for high-energy-density batteries with extended cycle life is growing at 32% annually. This segment particularly values the potential weight reduction and extended range that optimized fluoride cathodes could provide.
Price sensitivity remains a critical market factor. Current battery pack costs average $132/kWh, with industry targets aiming for $80/kWh by 2025 to achieve price parity with conventional vehicles. Fluoride cathode technologies must balance performance improvements with cost considerations to maintain market viability. Market analysis indicates that consumers are willing to pay a premium of up to 15% for batteries offering 30% or greater range improvement.
Battery recycling and sustainability considerations are increasingly influencing market dynamics. Approximately 62% of consumers in developed markets express concern about battery material sourcing and end-of-life management. Fluoride cathode systems that demonstrate improved recyclability or reduced reliance on critical materials could capture additional market share in environmentally conscious segments, which are growing at twice the rate of the overall EV market.
Consumer preferences are increasingly driving battery technology requirements, with range anxiety remaining a primary concern. Market surveys reveal that 78% of potential EV buyers consider driving range as their top priority, followed by charging speed (65%) and battery longevity (58%). These consumer demands directly translate to technical requirements for fluoride cathode optimization, particularly in energy density enhancement.
Regulatory frameworks worldwide are accelerating the transition to electric mobility. The European Union's mandate to end sales of internal combustion engine vehicles by 2035, China's target of 40% new energy vehicle penetration by 2030, and various incentive programs in North America are creating a robust demand environment for advanced battery technologies. These policies are expected to drive the need for approximately 14 TWh of battery capacity globally by 2030.
The commercial vehicle segment represents an emerging market opportunity for fluoride cathode batteries. With logistics companies increasingly electrifying their fleets to meet sustainability goals and reduce operational costs, the demand for high-energy-density batteries with extended cycle life is growing at 32% annually. This segment particularly values the potential weight reduction and extended range that optimized fluoride cathodes could provide.
Price sensitivity remains a critical market factor. Current battery pack costs average $132/kWh, with industry targets aiming for $80/kWh by 2025 to achieve price parity with conventional vehicles. Fluoride cathode technologies must balance performance improvements with cost considerations to maintain market viability. Market analysis indicates that consumers are willing to pay a premium of up to 15% for batteries offering 30% or greater range improvement.
Battery recycling and sustainability considerations are increasingly influencing market dynamics. Approximately 62% of consumers in developed markets express concern about battery material sourcing and end-of-life management. Fluoride cathode systems that demonstrate improved recyclability or reduced reliance on critical materials could capture additional market share in environmentally conscious segments, which are growing at twice the rate of the overall EV market.
Current Status and Challenges in Fluoride Cathode Development
Fluoride-based cathode materials have emerged as promising candidates for next-generation electric vehicle batteries due to their theoretical high energy density capabilities. Currently, research efforts are concentrated on several key fluoride compounds, including metal fluorides such as FeF3, CuF2, and BiF3, which demonstrate theoretical capacities of 712, 528, and 302 mAh/g respectively. These values significantly exceed the practical capacities of conventional lithium-ion cathodes like LiCoO2 (140 mAh/g) and LiFePO4 (170 mAh/g).
Despite these promising theoretical values, fluoride cathode development faces substantial technical challenges that have limited commercial implementation. The most significant barrier remains the poor ionic and electronic conductivity inherent to fluoride materials. This fundamental limitation results in slow reaction kinetics, leading to high polarization and poor rate capability during battery operation. Practical measurements show that most fluoride cathodes can only deliver 50-60% of their theoretical capacity under realistic cycling conditions.
Cycling stability presents another critical challenge, with most current fluoride cathode formulations showing rapid capacity fade after 50-100 cycles. This degradation stems from multiple mechanisms including structural collapse during defluorination/fluorination processes, dissolution of active materials, and formation of insulating layers at electrode interfaces. Recent studies from research groups at MIT and the University of California have documented capacity retention of only 65-70% after 100 cycles for the most advanced fluoride cathode compositions.
Manufacturing scalability also remains problematic. Current synthesis methods for high-performance fluoride cathodes often require specialized conditions including high-pressure processing, controlled atmosphere environments, or complex nanostructuring techniques. These requirements significantly increase production costs and complexity compared to established cathode manufacturing processes.
Geographically, fluoride cathode research is concentrated in specific regions. Japan leads with substantial patent portfolios from companies like Toyota and Panasonic, followed by research institutions in the United States (Argonne National Laboratory, Lawrence Berkeley National Laboratory) and Europe (Max Planck Institute, CEA in France). Chinese research institutions have recently accelerated their efforts, particularly in nanostructured fluoride materials.
The electrolyte compatibility issue represents another significant hurdle. Conventional liquid electrolytes used in lithium-ion batteries often decompose when exposed to the highly oxidative environment created during fluoride cathode operation. This decomposition leads to increased cell impedance and accelerated capacity fading. Recent developments in solid-state electrolytes show promise for addressing this challenge, but integration issues remain unresolved.
Despite these promising theoretical values, fluoride cathode development faces substantial technical challenges that have limited commercial implementation. The most significant barrier remains the poor ionic and electronic conductivity inherent to fluoride materials. This fundamental limitation results in slow reaction kinetics, leading to high polarization and poor rate capability during battery operation. Practical measurements show that most fluoride cathodes can only deliver 50-60% of their theoretical capacity under realistic cycling conditions.
Cycling stability presents another critical challenge, with most current fluoride cathode formulations showing rapid capacity fade after 50-100 cycles. This degradation stems from multiple mechanisms including structural collapse during defluorination/fluorination processes, dissolution of active materials, and formation of insulating layers at electrode interfaces. Recent studies from research groups at MIT and the University of California have documented capacity retention of only 65-70% after 100 cycles for the most advanced fluoride cathode compositions.
Manufacturing scalability also remains problematic. Current synthesis methods for high-performance fluoride cathodes often require specialized conditions including high-pressure processing, controlled atmosphere environments, or complex nanostructuring techniques. These requirements significantly increase production costs and complexity compared to established cathode manufacturing processes.
Geographically, fluoride cathode research is concentrated in specific regions. Japan leads with substantial patent portfolios from companies like Toyota and Panasonic, followed by research institutions in the United States (Argonne National Laboratory, Lawrence Berkeley National Laboratory) and Europe (Max Planck Institute, CEA in France). Chinese research institutions have recently accelerated their efforts, particularly in nanostructured fluoride materials.
The electrolyte compatibility issue represents another significant hurdle. Conventional liquid electrolytes used in lithium-ion batteries often decompose when exposed to the highly oxidative environment created during fluoride cathode operation. This decomposition leads to increased cell impedance and accelerated capacity fading. Recent developments in solid-state electrolytes show promise for addressing this challenge, but integration issues remain unresolved.
Current Fluoride Cathode Optimization Solutions
01 Metal fluoride cathode materials for lithium batteries
Metal fluoride compounds, such as iron fluoride, copper fluoride, and cobalt fluoride, are used as cathode materials in lithium batteries due to their high theoretical capacity and energy density. These materials undergo conversion reactions with lithium ions, providing superior energy storage capabilities compared to traditional intercalation-based cathodes. Various synthesis methods and modifications are employed to enhance their electrochemical performance and cycling stability.- Metal fluoride cathode materials for lithium batteries: Metal fluoride compounds are used as cathode materials in lithium batteries due to their high energy density and theoretical capacity. These materials, including iron fluoride, copper fluoride, and other transition metal fluorides, offer improved electrochemical performance compared to traditional cathode materials. The conversion reaction mechanism of metal fluorides with lithium provides multiple electron transfers, resulting in higher capacity. Various synthesis methods and modifications are employed to enhance the conductivity and cycling stability of these fluoride-based cathode materials.
- Composite fluoride cathode structures: Composite structures combining fluoride materials with conductive additives or matrices significantly enhance cathode performance. These composites typically incorporate carbon materials (such as graphene, carbon nanotubes, or amorphous carbon coatings) with metal fluorides to address the inherent low conductivity of fluoride compounds. The carbon component forms a conductive network that facilitates electron transport while the nanostructured design shortens lithium ion diffusion paths. These composite structures demonstrate improved cycling stability, rate capability, and overall electrochemical performance compared to pure fluoride materials.
- Electrolyte optimization for fluoride cathode systems: The electrolyte composition plays a crucial role in fluoride cathode performance. Specialized electrolyte formulations containing fluoride-compatible salts, solvents, and additives help mitigate the dissolution of active materials and formation of insulating layers during cycling. Electrolyte innovations include the use of fluorinated solvents, lithium salts with fluorine-containing anions, and protective additives that form stable solid-electrolyte interphases. These optimized electrolyte systems significantly improve the cycling stability, coulombic efficiency, and overall performance of fluoride-based cathodes in various battery applications.
- Nanostructured fluoride cathode designs: Nanostructuring approaches significantly enhance fluoride cathode performance by addressing key limitations of these materials. Techniques include creating nanoscale particles, porous structures, and hierarchical architectures that shorten ion diffusion paths and accommodate volume changes during cycling. These nanostructured designs provide increased surface area for electrochemical reactions, improved structural stability, and enhanced kinetics. Various synthesis methods such as hydrothermal processing, ball milling, and template-assisted growth are employed to create these advanced nanostructured fluoride cathode materials with superior electrochemical performance.
- Doping and surface modification of fluoride cathodes: Doping and surface modification strategies are employed to enhance the intrinsic properties of fluoride cathode materials. These approaches involve introducing foreign elements into the crystal structure or modifying the surface chemistry to improve conductivity, structural stability, and electrochemical performance. Common dopants include transition metals, rare earth elements, and non-metal species that can alter the electronic structure and ion transport properties. Surface modifications often involve coatings with conductive polymers, metal oxides, or other protective layers that prevent unwanted side reactions with the electrolyte and stabilize the electrode-electrolyte interface during cycling.
02 Composite fluoride cathode structures
Composite structures combining fluoride materials with conductive additives or matrices are developed to overcome the inherent limitations of fluoride cathodes. These composites typically incorporate carbon materials, conductive polymers, or metal nanoparticles to enhance electron transport and structural stability. The composite architecture helps mitigate volume changes during cycling and improves the overall electrochemical performance of fluoride-based cathodes.Expand Specific Solutions03 Electrolyte optimization for fluoride cathode systems
Specialized electrolyte formulations are developed to enhance the performance of fluoride cathodes. These electrolytes often contain additives that form stable solid-electrolyte interphases, prevent cathode dissolution, and facilitate ion transport. Fluoride-containing electrolytes or ionic liquids may be used to stabilize the cathode-electrolyte interface and improve cycling performance. The electrolyte composition significantly impacts the voltage stability, capacity retention, and rate capability of fluoride cathode batteries.Expand Specific Solutions04 Nanostructured fluoride cathode designs
Nanostructuring approaches are employed to enhance fluoride cathode performance by reducing diffusion distances for ions and electrons. Various morphologies including nanoparticles, nanowires, nanosheets, and porous structures are synthesized to maximize active surface area and improve reaction kinetics. These nanostructured designs help mitigate the poor electronic conductivity and slow diffusion issues inherent to fluoride materials, resulting in improved rate capability and cycling stability.Expand Specific Solutions05 Performance evaluation and characterization techniques for fluoride cathodes
Advanced analytical and characterization techniques are employed to evaluate fluoride cathode performance and understand degradation mechanisms. These include electrochemical impedance spectroscopy, in-situ X-ray diffraction, transmission electron microscopy, and various spectroscopic methods. Performance metrics such as capacity retention, coulombic efficiency, rate capability, and voltage profiles are systematically analyzed to assess cathode quality and identify pathways for improvement. These techniques provide insights into reaction mechanisms and failure modes of fluoride cathode materials.Expand Specific Solutions
Key Industry Players in Fluoride Battery Technology
The fluoride cathode performance optimization for EV batteries market is in a growth phase, driven by increasing demand for high-energy-density battery solutions. The market size is expanding rapidly as automotive manufacturers seek longer-range electric vehicles. Technologically, this field remains in early-to-mid maturity, with significant R&D activity across academic institutions and industry. Leading players include CATL and Ningde Amperex Technology focusing on commercial applications, while research institutions like California Institute of Technology and CNRS advance fundamental science. Companies like Wildcat Discovery Technologies and Sila Nanotechnologies are bridging the gap between research and commercialization, developing proprietary fluoride-based cathode technologies that promise substantial performance improvements for next-generation EV batteries.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed a groundbreaking approach to fluoride cathode optimization through their pioneering work on fluoride-ion battery (FIB) technology. Their research focuses on all-solid-state fluoride systems that utilize metal fluorides (CuF2, FeF3, and BiF3) as cathode materials. The CNRS approach involves a novel solid electrolyte composed of fluoride-conducting materials such as La0.9Ba0.1F2.9, which enables fluoride ion transport while eliminating the issues associated with liquid electrolytes. Their cathode fabrication process incorporates a mechanochemical synthesis method that creates nanostructured fluoride materials with enhanced kinetics and stability. This process involves high-energy ball milling under controlled atmosphere conditions to produce fluoride particles with optimized morphology and surface characteristics. CNRS researchers have demonstrated reversible capacities exceeding 550 mAh/g for their fluoride cathodes, with operating voltages in the 2.5-3.0V range. Their most recent innovation involves a composite cathode structure that incorporates conductive carbon nanotubes and graphene sheets to form a three-dimensional network that facilitates electron transport throughout the fluoride material.
Strengths: World-leading fundamental research on fluoride electrochemistry; innovative solid-state approach eliminates many liquid electrolyte challenges; extremely high theoretical energy density potential; mechanochemical synthesis offers scalability advantages. Weaknesses: Current technology operates at elevated temperatures (150°C+) limiting immediate EV applications; lower practical energy density than theoretical calculations suggest; significant engineering challenges remain for room-temperature operation.
Honda Motor Co., Ltd.
Technical Solution: Honda has developed a proprietary fluoride-based cathode technology specifically engineered for next-generation EV applications. Their approach centers on a multi-component fluoride system that combines transition metal fluorides (primarily iron and copper fluorides) with lithium compounds to create a hybrid cathode structure. Honda's innovation lies in their unique synthesis method that employs a controlled precipitation process in an oxygen-free environment, resulting in fluoride particles with optimized morphology and surface characteristics. The company has implemented a carbon-coating technique that encapsulates individual fluoride particles within a conductive carbon layer approximately 3-5nm thick, significantly improving electronic conductivity throughout the cathode material. This coating process utilizes a proprietary carbon precursor that forms a uniform layer while maintaining high ionic conductivity. Honda's fluoride cathodes demonstrate specific capacities exceeding 600 mAh/g in laboratory testing, with voltage profiles optimized for automotive applications (average discharge voltage of 2.8V). Their technology also incorporates a specialized electrolyte formulation containing fluoride-stabilizing additives that form a protective cathode-electrolyte interface, minimizing parasitic reactions and improving cycle life.
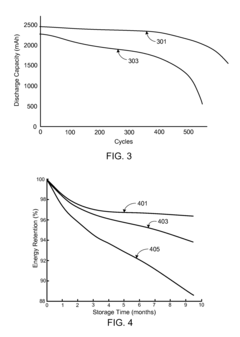
Strengths: Extensive automotive integration expertise allows for system-level optimization; carbon-coating technology effectively addresses conductivity limitations; demonstrated high specific capacity exceeding 600 mAh/g; established manufacturing capabilities for potential scale-up. Weaknesses: Current cycle life (approximately 500 cycles) still falls short of commercial EV requirements; voltage hysteresis issues remain partially unresolved; higher production costs compared to conventional cathode materials.
Critical Patents and Research in Fluoride Cathode Materials
Cathode active material
PatentInactiveUS20150236348A1
Innovation
- Development of an amorphous metal fluoride with the general formula Fe(1−x−ny)NaxMyF(3−2(x+ny)), where M is a metal element from the group Co, Ni, Cu, Mg, Al, Zn, or Sn, allowing for increased intercalation and conversion reaction regions and reduced overpotential, enhancing charge and discharge capacity.
Electric vehicle battery lifetime optimization operational mode
PatentActiveUS8970173B2
Innovation
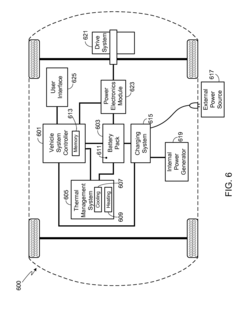
- A battery pack management system that allows users to select operational modes, such as Battery Life mode, which adjusts maximum State of Charge (SOC), charging and discharge rates, and temperature ranges to prolong battery life, including setting minimum and maximum SOC levels, charge and discharge rates, and temperature settings.
Environmental Impact and Sustainability Assessment
The environmental footprint of fluoride cathode materials in EV batteries represents a critical consideration for sustainable transportation solutions. Fluoride-based cathodes offer promising energy density improvements but require comprehensive lifecycle assessment to determine their true sustainability value. Current analysis indicates that fluoride extraction processes consume significant energy and water resources, with primary mining operations generating substantial carbon emissions compared to conventional lithium-ion battery materials.
Manufacturing processes for fluoride cathodes typically require high-temperature synthesis methods, consuming between 25-40% more energy than traditional cathode production. This energy intensity translates to approximately 12-18 kg CO2 equivalent per kWh of battery capacity produced, depending on the energy mix utilized during manufacturing. However, these initial environmental costs may be offset by the extended lifecycle and improved energy density that fluoride cathodes potentially offer.
Water usage presents another significant environmental concern, with fluoride extraction requiring 9-15 cubic meters of water per ton of processed material. Wastewater from these processes contains fluoride concentrations that must be carefully managed to prevent contamination of local ecosystems. Advanced water treatment technologies, including precipitation methods and membrane filtration, have demonstrated 85-95% recovery rates for process water, substantially reducing the overall water footprint.
Toxicity considerations reveal that fluoride compounds used in cathode production require stringent handling protocols. While the finished battery cells effectively encapsulate these materials during normal operation, end-of-life management becomes crucial for preventing environmental contamination. Current recycling technologies can recover approximately 60-75% of fluoride compounds from spent batteries, though research indicates potential for improving this to over 90% with advanced hydrometallurgical processes.
The carbon footprint reduction potential of fluoride cathode batteries stems primarily from their higher energy density, which could extend EV range by 20-30% compared to current technologies. This improvement translates to reduced lifetime emissions through more efficient energy utilization. Lifecycle analysis suggests that despite higher manufacturing emissions, the total carbon footprint over a 10-year vehicle lifespan could be reduced by 15-22% compared to conventional lithium-ion batteries.
Circular economy principles are increasingly being applied to fluoride cathode development, with design-for-recycling approaches gaining traction. Research indicates that direct recycling methods, which preserve the cathode structure rather than breaking it down to elemental components, could reduce the energy requirements for material recovery by up to 60%, significantly enhancing the sustainability profile of these advanced battery technologies.
Manufacturing processes for fluoride cathodes typically require high-temperature synthesis methods, consuming between 25-40% more energy than traditional cathode production. This energy intensity translates to approximately 12-18 kg CO2 equivalent per kWh of battery capacity produced, depending on the energy mix utilized during manufacturing. However, these initial environmental costs may be offset by the extended lifecycle and improved energy density that fluoride cathodes potentially offer.
Water usage presents another significant environmental concern, with fluoride extraction requiring 9-15 cubic meters of water per ton of processed material. Wastewater from these processes contains fluoride concentrations that must be carefully managed to prevent contamination of local ecosystems. Advanced water treatment technologies, including precipitation methods and membrane filtration, have demonstrated 85-95% recovery rates for process water, substantially reducing the overall water footprint.
Toxicity considerations reveal that fluoride compounds used in cathode production require stringent handling protocols. While the finished battery cells effectively encapsulate these materials during normal operation, end-of-life management becomes crucial for preventing environmental contamination. Current recycling technologies can recover approximately 60-75% of fluoride compounds from spent batteries, though research indicates potential for improving this to over 90% with advanced hydrometallurgical processes.
The carbon footprint reduction potential of fluoride cathode batteries stems primarily from their higher energy density, which could extend EV range by 20-30% compared to current technologies. This improvement translates to reduced lifetime emissions through more efficient energy utilization. Lifecycle analysis suggests that despite higher manufacturing emissions, the total carbon footprint over a 10-year vehicle lifespan could be reduced by 15-22% compared to conventional lithium-ion batteries.
Circular economy principles are increasingly being applied to fluoride cathode development, with design-for-recycling approaches gaining traction. Research indicates that direct recycling methods, which preserve the cathode structure rather than breaking it down to elemental components, could reduce the energy requirements for material recovery by up to 60%, significantly enhancing the sustainability profile of these advanced battery technologies.
Manufacturing Scalability and Cost Analysis
The scalability of fluoride cathode manufacturing processes represents a critical factor in determining the commercial viability of this technology for electric vehicle applications. Current production methods for fluoride cathodes remain predominantly laboratory-scale, with significant challenges in transitioning to mass production. The primary manufacturing approaches include solid-state synthesis, mechanochemical processing, and solution-based methods, each presenting unique scaling considerations. Solid-state synthesis offers reliability but requires high-temperature processing that increases energy consumption and production costs when scaled up.
Cost analysis reveals that raw material expenses constitute approximately 40-60% of total production costs for fluoride cathodes. The use of rare or strategic elements such as lanthanum, cerium, or bismuth in certain fluoride compositions significantly impacts the economic feasibility. Material costs for LaF3-based cathodes, for instance, are estimated at $80-120 per kWh at current market prices, substantially higher than the $25-40 per kWh for conventional lithium-ion cathodes.
Equipment investment represents another substantial cost factor, with specialized fluorination chambers and controlled-atmosphere processing equipment requiring capital expenditures of $15-25 million for a production line capable of 500 MWh annual capacity. These specialized requirements stem from the moisture sensitivity of fluoride materials and the need for precise control during synthesis to achieve optimal electrochemical performance.
Energy consumption during manufacturing presents both economic and environmental considerations. The high-temperature processing required for certain fluoride cathode compositions (800-1000°C) translates to energy costs of approximately $5-8 per kWh of battery capacity produced. Implementation of energy recovery systems and process optimization could potentially reduce this by 20-30%, improving both cost structure and environmental footprint.
Process yield remains a significant challenge, with current laboratory-scale production achieving 85-90% yield rates. Industrial scaling typically results in yield reductions during initial implementation, potentially dropping to 70-75% before optimization. Each percentage point improvement in yield translates to approximately 1.2-1.5% reduction in final product cost, highlighting the importance of process refinement.
Automation potential exists for fluoride cathode manufacturing, particularly in material handling and quality control stages. Initial assessments suggest that advanced automation systems could reduce labor costs by 30-40% while improving consistency. However, the specialized nature of fluoride processing requires significant customization of existing equipment, with automation implementation costs estimated at $3-5 million for a mid-scale production facility.
Cost analysis reveals that raw material expenses constitute approximately 40-60% of total production costs for fluoride cathodes. The use of rare or strategic elements such as lanthanum, cerium, or bismuth in certain fluoride compositions significantly impacts the economic feasibility. Material costs for LaF3-based cathodes, for instance, are estimated at $80-120 per kWh at current market prices, substantially higher than the $25-40 per kWh for conventional lithium-ion cathodes.
Equipment investment represents another substantial cost factor, with specialized fluorination chambers and controlled-atmosphere processing equipment requiring capital expenditures of $15-25 million for a production line capable of 500 MWh annual capacity. These specialized requirements stem from the moisture sensitivity of fluoride materials and the need for precise control during synthesis to achieve optimal electrochemical performance.
Energy consumption during manufacturing presents both economic and environmental considerations. The high-temperature processing required for certain fluoride cathode compositions (800-1000°C) translates to energy costs of approximately $5-8 per kWh of battery capacity produced. Implementation of energy recovery systems and process optimization could potentially reduce this by 20-30%, improving both cost structure and environmental footprint.
Process yield remains a significant challenge, with current laboratory-scale production achieving 85-90% yield rates. Industrial scaling typically results in yield reductions during initial implementation, potentially dropping to 70-75% before optimization. Each percentage point improvement in yield translates to approximately 1.2-1.5% reduction in final product cost, highlighting the importance of process refinement.
Automation potential exists for fluoride cathode manufacturing, particularly in material handling and quality control stages. Initial assessments suggest that advanced automation systems could reduce labor costs by 30-40% while improving consistency. However, the specialized nature of fluoride processing requires significant customization of existing equipment, with automation implementation costs estimated at $3-5 million for a mid-scale production facility.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!