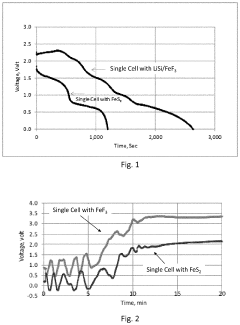
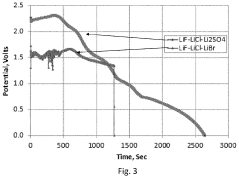
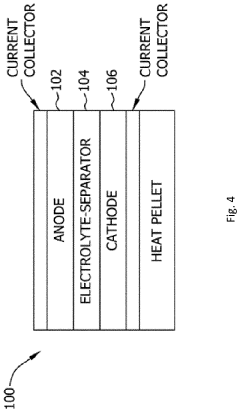
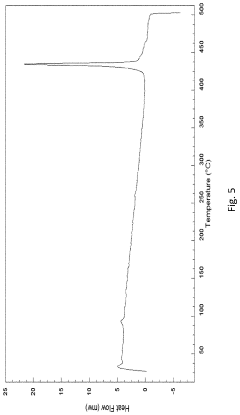
Comparison of Fluoride Cathode Conductivity with Different Electrolytes
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fluoride Cathode Technology Background and Objectives
Fluoride-based cathode materials have emerged as a promising alternative to traditional lithium-ion battery technologies due to their potential for higher energy density and improved safety characteristics. The development of fluoride cathodes can be traced back to the early 2000s when researchers began exploring multi-electron transfer reactions to overcome the capacity limitations of conventional intercalation cathodes. The evolution of this technology has been marked by significant breakthroughs in material synthesis, electrolyte compatibility, and electrode design.
The fundamental principle behind fluoride cathodes lies in their conversion reaction mechanism, which involves the formation and decomposition of metal fluorides during charge and discharge cycles. This mechanism theoretically allows for higher specific capacities compared to intercalation-based cathodes. However, the practical implementation has been hindered by several challenges, particularly the poor ionic conductivity of fluoride ions in solid-state materials.
Recent technological trends indicate a growing interest in developing advanced electrolyte systems that can enhance fluoride ion conductivity while maintaining electrochemical stability. The research community has been exploring various approaches, including solid-state electrolytes, ionic liquids, and polymer-based electrolyte systems, each offering distinct advantages and limitations for fluoride cathode applications.
The primary technical objective in this field is to systematically compare and evaluate the conductivity performance of fluoride cathodes when paired with different electrolyte systems. This comparison aims to identify optimal electrolyte compositions that can maximize fluoride ion transport while minimizing side reactions and degradation mechanisms. Additionally, the research seeks to establish correlations between electrolyte properties (such as viscosity, ionic strength, and solvation structure) and the resulting electrochemical performance of fluoride cathodes.
Another critical goal is to develop standardized testing protocols for evaluating fluoride cathode conductivity across different electrolyte systems. This standardization would facilitate more meaningful comparisons between research results and accelerate the identification of promising material combinations. Furthermore, the research aims to explore the fundamental mechanisms of fluoride ion transport at the electrode-electrolyte interface, which remains poorly understood compared to lithium-ion systems.
Looking forward, the technology roadmap for fluoride cathodes includes the development of novel electrolyte formulations specifically designed to enhance fluoride ion conductivity, the integration of these materials into practical cell configurations, and the scaling of production methods to enable commercial viability. The ultimate objective is to establish fluoride-based battery systems as a competitive alternative to current technologies, offering advantages in terms of energy density, safety, and resource sustainability.
The fundamental principle behind fluoride cathodes lies in their conversion reaction mechanism, which involves the formation and decomposition of metal fluorides during charge and discharge cycles. This mechanism theoretically allows for higher specific capacities compared to intercalation-based cathodes. However, the practical implementation has been hindered by several challenges, particularly the poor ionic conductivity of fluoride ions in solid-state materials.
Recent technological trends indicate a growing interest in developing advanced electrolyte systems that can enhance fluoride ion conductivity while maintaining electrochemical stability. The research community has been exploring various approaches, including solid-state electrolytes, ionic liquids, and polymer-based electrolyte systems, each offering distinct advantages and limitations for fluoride cathode applications.
The primary technical objective in this field is to systematically compare and evaluate the conductivity performance of fluoride cathodes when paired with different electrolyte systems. This comparison aims to identify optimal electrolyte compositions that can maximize fluoride ion transport while minimizing side reactions and degradation mechanisms. Additionally, the research seeks to establish correlations between electrolyte properties (such as viscosity, ionic strength, and solvation structure) and the resulting electrochemical performance of fluoride cathodes.
Another critical goal is to develop standardized testing protocols for evaluating fluoride cathode conductivity across different electrolyte systems. This standardization would facilitate more meaningful comparisons between research results and accelerate the identification of promising material combinations. Furthermore, the research aims to explore the fundamental mechanisms of fluoride ion transport at the electrode-electrolyte interface, which remains poorly understood compared to lithium-ion systems.
Looking forward, the technology roadmap for fluoride cathodes includes the development of novel electrolyte formulations specifically designed to enhance fluoride ion conductivity, the integration of these materials into practical cell configurations, and the scaling of production methods to enable commercial viability. The ultimate objective is to establish fluoride-based battery systems as a competitive alternative to current technologies, offering advantages in terms of energy density, safety, and resource sustainability.
Market Analysis for Fluoride-Based Battery Systems
The global market for fluoride-based battery systems is experiencing significant growth, driven by increasing demand for high-energy density storage solutions across multiple sectors. Current market valuations indicate that fluoride-ion battery technology represents an emerging segment within the broader energy storage market, which is projected to reach $546 billion by 2035. While lithium-ion batteries currently dominate with over 90% market share, fluoride-based systems are positioned as potential disruptors due to their theoretical energy density advantages.
Key market segments showing interest in fluoride-based battery technologies include electric vehicles, consumer electronics, grid storage, and aerospace applications. The electric vehicle sector presents particularly strong growth potential, with manufacturers actively seeking battery technologies offering higher energy density and improved safety profiles compared to current lithium-ion solutions.
Regional analysis reveals that North America, particularly the United States, leads in fluoride battery research investment, followed by East Asia with significant contributions from Japan and China. European markets show growing interest, especially in Germany and France, where government initiatives support alternative battery technologies development.
Market adoption faces several challenges, including the current high production costs estimated at 3-4 times that of conventional lithium-ion batteries. Technical barriers related to electrolyte conductivity at practical operating temperatures remain a significant market entry obstacle. Industry analysts suggest that fluoride battery systems must achieve conductivity improvements of at least one order of magnitude to become commercially viable alternatives.
Consumer demand patterns indicate growing preference for batteries with improved safety profiles, longer lifespans, and reduced environmental impact. Fluoride-based systems potentially address these concerns, particularly regarding fire safety and resource sustainability compared to lithium-ion technologies.
Market forecasts suggest fluoride battery technologies could capture 5-8% of specialized high-energy density applications by 2030, contingent upon successful resolution of electrolyte conductivity challenges. Early market entry points likely include military applications, specialized industrial equipment, and premium consumer electronics where performance advantages outweigh cost considerations.
Investment trends show increasing venture capital interest, with approximately $420 million directed toward fluoride battery startups since 2018. Major battery manufacturers and automotive companies have established research partnerships with academic institutions to accelerate development, signaling recognition of the technology's potential market disruption capabilities.
Key market segments showing interest in fluoride-based battery technologies include electric vehicles, consumer electronics, grid storage, and aerospace applications. The electric vehicle sector presents particularly strong growth potential, with manufacturers actively seeking battery technologies offering higher energy density and improved safety profiles compared to current lithium-ion solutions.
Regional analysis reveals that North America, particularly the United States, leads in fluoride battery research investment, followed by East Asia with significant contributions from Japan and China. European markets show growing interest, especially in Germany and France, where government initiatives support alternative battery technologies development.
Market adoption faces several challenges, including the current high production costs estimated at 3-4 times that of conventional lithium-ion batteries. Technical barriers related to electrolyte conductivity at practical operating temperatures remain a significant market entry obstacle. Industry analysts suggest that fluoride battery systems must achieve conductivity improvements of at least one order of magnitude to become commercially viable alternatives.
Consumer demand patterns indicate growing preference for batteries with improved safety profiles, longer lifespans, and reduced environmental impact. Fluoride-based systems potentially address these concerns, particularly regarding fire safety and resource sustainability compared to lithium-ion technologies.
Market forecasts suggest fluoride battery technologies could capture 5-8% of specialized high-energy density applications by 2030, contingent upon successful resolution of electrolyte conductivity challenges. Early market entry points likely include military applications, specialized industrial equipment, and premium consumer electronics where performance advantages outweigh cost considerations.
Investment trends show increasing venture capital interest, with approximately $420 million directed toward fluoride battery startups since 2018. Major battery manufacturers and automotive companies have established research partnerships with academic institutions to accelerate development, signaling recognition of the technology's potential market disruption capabilities.
Current Challenges in Fluoride Cathode Conductivity
Despite significant advancements in fluoride-ion battery (FIB) technology, fluoride cathode conductivity remains a critical bottleneck limiting widespread commercial adoption. The fundamental challenge stems from the inherent poor ionic conductivity of fluoride ions, which are significantly larger than lithium ions and possess stronger electrostatic interactions with the host lattice. This results in sluggish ion transport kinetics, particularly at room temperature, severely hampering battery performance metrics.
Current electrolyte systems demonstrate substantial limitations when paired with fluoride cathodes. Solid-state electrolytes, while offering improved safety profiles, typically exhibit conductivities in the range of 10^-6 to 10^-4 S/cm at room temperature—orders of magnitude lower than required for practical applications. Liquid electrolytes based on ionic liquids show marginally better performance but suffer from narrow electrochemical stability windows and problematic interfacial resistance issues at the cathode-electrolyte interface.
The interface between fluoride cathodes and electrolytes presents particularly challenging problems. Formation of insulating layers due to chemical reactions between cathode materials and electrolyte components creates high impedance barriers for fluoride ion transport. This interfacial resistance increases with cycling, leading to capacity fade and shortened battery lifespan. Experimental data indicates that interface resistance can account for up to 70% of the total cell resistance in some fluoride cathode systems.
Temperature dependence represents another significant hurdle. Most fluoride cathode systems demonstrate acceptable conductivity only at elevated temperatures (>150°C), which introduces additional engineering challenges related to thermal management and safety. The activation energy for fluoride ion conduction typically ranges between 0.7-1.2 eV, considerably higher than the 0.2-0.4 eV observed in lithium-ion systems.
Material stability during cycling presents ongoing challenges. Many promising fluoride cathode materials undergo significant volume changes during fluorination/defluorination cycles, leading to mechanical degradation and particle isolation. This structural instability directly impacts electronic conductivity pathways within the cathode matrix, further exacerbating conductivity issues.
Electrolyte decomposition at operating potentials remains problematic across different electrolyte chemistries. Fluoride anions are highly reactive, particularly at high potentials, leading to parasitic side reactions that consume active fluoride ions and form resistive surface films. These reactions are particularly pronounced with organic solvent-based electrolytes, where C-F bond formation occurs readily at cathode surfaces.
Current electrolyte systems demonstrate substantial limitations when paired with fluoride cathodes. Solid-state electrolytes, while offering improved safety profiles, typically exhibit conductivities in the range of 10^-6 to 10^-4 S/cm at room temperature—orders of magnitude lower than required for practical applications. Liquid electrolytes based on ionic liquids show marginally better performance but suffer from narrow electrochemical stability windows and problematic interfacial resistance issues at the cathode-electrolyte interface.
The interface between fluoride cathodes and electrolytes presents particularly challenging problems. Formation of insulating layers due to chemical reactions between cathode materials and electrolyte components creates high impedance barriers for fluoride ion transport. This interfacial resistance increases with cycling, leading to capacity fade and shortened battery lifespan. Experimental data indicates that interface resistance can account for up to 70% of the total cell resistance in some fluoride cathode systems.
Temperature dependence represents another significant hurdle. Most fluoride cathode systems demonstrate acceptable conductivity only at elevated temperatures (>150°C), which introduces additional engineering challenges related to thermal management and safety. The activation energy for fluoride ion conduction typically ranges between 0.7-1.2 eV, considerably higher than the 0.2-0.4 eV observed in lithium-ion systems.
Material stability during cycling presents ongoing challenges. Many promising fluoride cathode materials undergo significant volume changes during fluorination/defluorination cycles, leading to mechanical degradation and particle isolation. This structural instability directly impacts electronic conductivity pathways within the cathode matrix, further exacerbating conductivity issues.
Electrolyte decomposition at operating potentials remains problematic across different electrolyte chemistries. Fluoride anions are highly reactive, particularly at high potentials, leading to parasitic side reactions that consume active fluoride ions and form resistive surface films. These reactions are particularly pronounced with organic solvent-based electrolytes, where C-F bond formation occurs readily at cathode surfaces.
Comparative Analysis of Electrolyte Solutions
01 Conductive additives for fluoride cathodes
Various conductive additives can be incorporated into fluoride cathode materials to enhance their electronic conductivity. These additives include carbon-based materials such as carbon black, graphene, and carbon nanotubes, as well as conductive polymers. The addition of these materials creates conductive networks within the cathode structure, facilitating electron transport and improving overall battery performance.- Conductive additives for fluoride cathodes: Various conductive additives can be incorporated into fluoride cathode materials to enhance their electronic conductivity. These additives include carbon-based materials such as carbon black, graphene, and carbon nanotubes, as well as metallic conductors. The addition of these conductive materials creates electron pathways throughout the cathode structure, significantly improving the overall conductivity and electrochemical performance of fluoride-based battery systems.
- Metal fluoride composite cathodes: Metal fluoride composite cathodes combine fluoride compounds with other materials to enhance conductivity and electrochemical properties. These composites typically incorporate transition metal fluorides (such as FeF3, CoF2, or CuF2) with conductive matrices. The resulting composite structures offer improved ion transport, better electronic conductivity, and enhanced cycling stability compared to pure metal fluoride cathodes, making them promising for high-energy density battery applications.
- Polymer electrolyte interfaces for fluoride cathodes: Polymer electrolyte interfaces can be designed to improve the ionic conductivity between fluoride cathodes and electrolytes. These interfaces facilitate fluoride ion transport while providing a stable barrier against unwanted side reactions. By incorporating specialized polymers with high fluoride ion conductivity, researchers can enhance the overall performance of fluoride-based battery systems, reduce interfacial resistance, and improve cycling stability at the cathode-electrolyte interface.
- Doping strategies for fluoride cathode materials: Doping fluoride cathode materials with various elements can significantly enhance their electronic and ionic conductivity. Common dopants include transition metals, rare earth elements, and alkali metals that can modify the crystal structure and electronic properties of the host fluoride material. These doping strategies create defects or additional charge carriers in the fluoride lattice, facilitating improved ion mobility and electronic conductivity, which leads to better electrochemical performance in battery applications.
- Nanostructured fluoride cathode designs: Nanostructuring fluoride cathode materials offers a promising approach to overcome their inherent conductivity limitations. By reducing particle size to nanoscale dimensions and creating specialized architectures such as nanoparticles, nanowires, or porous nanostructures, the ion diffusion paths are shortened and electronic contact is improved. These nanostructured designs provide larger electrode-electrolyte contact areas, enhance reaction kinetics, and improve the overall conductivity and capacity utilization of fluoride-based cathode materials.
02 Metal fluoride composite cathodes
Composite cathodes combining metal fluorides with other materials can significantly improve conductivity. These composites often incorporate transition metal fluorides (such as FeF3, CoF2, or CuF2) with conductive matrices. The synergistic effect between the metal fluoride active material and the conductive component enhances electron transport while maintaining high energy density, addressing the inherent conductivity limitations of pure metal fluoride cathodes.Expand Specific Solutions03 Nanostructured fluoride cathode materials
Nanostructuring of fluoride cathode materials can significantly improve their conductivity properties. By reducing particle size to nanoscale dimensions, the electron and ion transport paths are shortened, enhancing overall conductivity. Various nanostructures including nanoparticles, nanowires, and nanocomposites can be employed to improve the electrochemical performance of fluoride-based cathodes, resulting in better rate capability and cycling stability.Expand Specific Solutions04 Solid electrolyte interfaces for fluoride cathodes
The development of specialized solid electrolyte interfaces (SEI) for fluoride cathodes can enhance ionic conductivity at the electrode-electrolyte interface. These interfaces can be engineered through surface modifications, coatings, or additives that facilitate fluoride ion transport while preventing unwanted side reactions. Properly designed SEI layers improve the overall conductivity of the cathode system while enhancing stability and cycle life of fluoride-based batteries.Expand Specific Solutions05 Doping strategies for fluoride cathodes
Doping fluoride cathode materials with various elements can significantly enhance their electronic and ionic conductivity. Introduction of aliovalent dopants can create defects in the crystal structure that serve as charge carriers, improving overall conductivity. Common dopants include transition metals, rare earth elements, and non-metals. The strategic selection of dopant type and concentration can be tailored to optimize the conductivity properties while maintaining structural stability of the fluoride cathode materials.Expand Specific Solutions
Leading Research Groups and Industrial Players
The fluoride cathode conductivity market is in an early growth phase, characterized by significant research activity but limited commercial deployment. Market size remains modest but is expanding as energy storage demands increase, particularly in high-density applications. Technical maturity varies across electrolyte formulations, with leading research institutions (California Institute of Technology, CNRS, University of Maryland) establishing fundamental science while commercial entities (Wildcat Discovery Technologies, Panasonic, Toyota) focus on practical applications. Companies like Contour Energy Systems and Seeo are developing specialized fluoride-based battery technologies, while established manufacturers (SANYO, Daikin) are exploring integration into existing product lines. The competitive landscape shows a balance between academic innovation and industrial commercialization efforts, with Asian companies (particularly Japanese and Chinese) demonstrating growing interest in this technology.
California Institute of Technology
Technical Solution: California Institute of Technology (Caltech) has developed advanced fluoride-ion battery technologies with improved cathode conductivity. Their research focuses on room-temperature fluoride-ion batteries using liquid electrolytes based on fluorinated solvents and fluoride salts. Caltech researchers have demonstrated that by using specifically designed organic liquid electrolytes containing fluorinated ethers and fluoride salts, they can significantly enhance the ionic conductivity of fluoride cathodes. Their approach involves creating stable fluoride-conducting interfaces between the cathode material and electrolyte, which facilitates faster ion transport. The team has also explored doping strategies for cathode materials to increase their intrinsic conductivity when paired with these specialized electrolytes. Their work has shown that proper electrolyte formulation can reduce the activation energy for fluoride ion migration within cathode materials, leading to improved rate capabilities and cycling performance in fluoride-based battery systems.
Strengths: Pioneering work in room-temperature fluoride-ion batteries with innovative electrolyte formulations that significantly enhance cathode conductivity. Their approach addresses fundamental challenges in fluoride ion mobility. Weaknesses: The specialized fluorinated electrolytes may face challenges in terms of cost-effectiveness for large-scale production, and long-term stability issues under various operating conditions remain to be fully resolved.
Contour Energy Systems, Inc.
Technical Solution: Contour Energy Systems has developed an innovative approach to enhancing fluoride cathode conductivity through their advanced electrolyte technology. Their proprietary system utilizes fluorinated ionic liquids combined with specifically engineered additives to create high-performance electrolyte formulations for fluoride-based battery systems. Contour's research has demonstrated that their electrolytes can significantly improve the effective ionic conductivity of metal fluoride cathodes, particularly for materials like FeF3, CuF2, and BiF3. Their technology addresses one of the fundamental challenges in fluoride battery systems - the poor ionic conductivity of fluoride ions in conventional electrolytes. By utilizing room-temperature ionic liquids with fluorinated anions and carefully selected fluoride salts, they've achieved ionic conductivities exceeding 5 mS/cm at room temperature, representing a substantial improvement over earlier systems. Contour has also developed specialized electrolyte additives that form beneficial interface layers on cathode surfaces, reducing interfacial resistance and enhancing overall performance. Their comprehensive approach includes tailoring the electrolyte composition to specific cathode materials, optimizing factors such as salt concentration, solvent ratios, and additive packages to maximize conductivity while maintaining electrochemical stability.
Strengths: Their ionic liquid-based electrolytes offer excellent fluoride ion conductivity at room temperature while providing good electrochemical stability and safety characteristics. The system demonstrates compatibility with multiple cathode chemistries. Weaknesses: Ionic liquid-based electrolytes may face challenges related to cost and scalability for mass production. Some formulations may exhibit increased viscosity at lower temperatures, potentially limiting low-temperature performance.
Key Patents and Literature on Fluoride Conductivity
Cathode plate, secondary battery, battery module, battery pack, and electric device
PatentPendingUS20240079600A1
Innovation
- A cathode plate design featuring a conductive coating with a conductive agent content of 20-50% and a binder content of 50-80%, which alleviates damage to the oxide layer on the aluminum foil, enhances tensile strength, and improves electronic conduction and peel strength, thereby stabilizing the cathode active layer and enhancing cycle performance.
Fluorine-based cathode materials for thermal batteries
PatentInactiveUS20200083543A1
Innovation
- The development of thermal batteries with a lithium alloy anode and a metal-fluoride cathode, specifically using NiF2, NiF2/V2O5, NiF2/LiVO3, or their mixtures, along with a conductive carbon material, to achieve higher specific energy and power density, while being environmentally friendly and potentially inexpensive for large-scale production.
Safety and Stability Considerations
Safety considerations are paramount when evaluating fluoride cathode systems with different electrolytes. The high reactivity of fluoride ions presents significant challenges, particularly regarding the potential formation of hydrogen fluoride (HF) gas when exposed to moisture. This toxic and corrosive gas poses serious health risks and requires stringent containment protocols during both research and potential commercial applications. Laboratory testing must incorporate specialized ventilation systems and personal protective equipment to mitigate these hazards.
Thermal stability represents another critical concern in fluoride cathode systems. Many fluoride-based electrolytes exhibit narrow electrochemical stability windows, leading to potential decomposition at elevated temperatures or during high-rate charging. This decomposition can trigger exothermic reactions, resulting in thermal runaway scenarios that compromise both performance and safety. Recent studies indicate that solid-state fluoride electrolytes generally offer superior thermal stability compared to their liquid counterparts, though often at the expense of ionic conductivity.
Long-term cycling stability presents unique challenges in fluoride systems. The repeated insertion and extraction of fluoride ions can induce significant structural changes in cathode materials, leading to capacity fading and potential mechanical failure. Electrolyte compatibility plays a decisive role in this aspect, as certain electrolyte formulations may accelerate cathode degradation through parasitic side reactions. Particularly noteworthy is the tendency of some liquid electrolytes to promote dissolution of active cathode materials during extended cycling.
Environmental considerations must also factor into safety assessments. The synthesis and disposal of fluoride-containing materials require specialized protocols to prevent environmental contamination. Solid electrolytes generally present fewer environmental concerns during operation but may pose greater challenges during end-of-life recycling processes. Conversely, liquid electrolytes typically offer simpler recycling pathways but present higher risks of leakage during operation.
Material compatibility between cathode materials, electrolytes, and cell components represents another critical stability consideration. Certain fluoride electrolytes demonstrate aggressive corrosion behavior toward conventional current collectors and cell casings, necessitating specialized materials that add complexity and cost. Recent research has focused on developing protective interface layers to mitigate these corrosion effects while maintaining efficient fluoride ion transport.
Pressure effects must also be considered, particularly for solid-state fluoride electrolyte systems. The mechanical stress generated during cycling can compromise interfacial contact between cathode and electrolyte, leading to increased impedance and potential safety hazards. Composite electrolyte systems incorporating both solid and gel components have shown promise in addressing these mechanical stability challenges while maintaining acceptable conductivity levels.
Thermal stability represents another critical concern in fluoride cathode systems. Many fluoride-based electrolytes exhibit narrow electrochemical stability windows, leading to potential decomposition at elevated temperatures or during high-rate charging. This decomposition can trigger exothermic reactions, resulting in thermal runaway scenarios that compromise both performance and safety. Recent studies indicate that solid-state fluoride electrolytes generally offer superior thermal stability compared to their liquid counterparts, though often at the expense of ionic conductivity.
Long-term cycling stability presents unique challenges in fluoride systems. The repeated insertion and extraction of fluoride ions can induce significant structural changes in cathode materials, leading to capacity fading and potential mechanical failure. Electrolyte compatibility plays a decisive role in this aspect, as certain electrolyte formulations may accelerate cathode degradation through parasitic side reactions. Particularly noteworthy is the tendency of some liquid electrolytes to promote dissolution of active cathode materials during extended cycling.
Environmental considerations must also factor into safety assessments. The synthesis and disposal of fluoride-containing materials require specialized protocols to prevent environmental contamination. Solid electrolytes generally present fewer environmental concerns during operation but may pose greater challenges during end-of-life recycling processes. Conversely, liquid electrolytes typically offer simpler recycling pathways but present higher risks of leakage during operation.
Material compatibility between cathode materials, electrolytes, and cell components represents another critical stability consideration. Certain fluoride electrolytes demonstrate aggressive corrosion behavior toward conventional current collectors and cell casings, necessitating specialized materials that add complexity and cost. Recent research has focused on developing protective interface layers to mitigate these corrosion effects while maintaining efficient fluoride ion transport.
Pressure effects must also be considered, particularly for solid-state fluoride electrolyte systems. The mechanical stress generated during cycling can compromise interfacial contact between cathode and electrolyte, leading to increased impedance and potential safety hazards. Composite electrolyte systems incorporating both solid and gel components have shown promise in addressing these mechanical stability challenges while maintaining acceptable conductivity levels.
Environmental Impact Assessment
The environmental impact of fluoride cathode technologies varies significantly depending on the electrolyte systems employed. Traditional liquid electrolytes containing fluoride often pose substantial environmental concerns due to their toxicity, volatility, and potential for groundwater contamination. These electrolytes typically contain hydrogen fluoride or other fluoride compounds that require careful handling and disposal protocols to prevent environmental release.
Solid-state electrolytes represent a more environmentally favorable alternative, significantly reducing leakage risks and eliminating volatile organic compound emissions associated with liquid systems. However, the manufacturing processes for solid electrolytes often require higher energy inputs and may involve rare earth elements with their own extraction-related environmental footprints.
Life cycle assessments of fluoride cathode batteries with different electrolytes reveal varying carbon footprints. Polymer-based electrolyte systems generally demonstrate lower greenhouse gas emissions during production compared to ceramic or glass-based solid electrolytes, though their performance characteristics differ substantially. The end-of-life management presents another critical environmental consideration, with solid electrolyte systems typically offering better recyclability potential.
Water consumption represents a significant environmental factor in electrolyte production. Ionic liquid electrolytes for fluoride cathodes often require extensive purification processes that consume substantial water resources, while ceramic electrolytes may require less water but more energy-intensive sintering processes. This trade-off necessitates regional environmental impact considerations based on local water scarcity and energy grid composition.
The chemical stability of different electrolytes also influences their long-term environmental impact. More stable electrolytes reduce the frequency of battery replacement, thereby decreasing the cumulative environmental burden of manufacturing and disposal. Research indicates that multi-component electrolytes optimized for fluoride cathode interfaces often achieve superior stability but may introduce additional complexity to recycling processes.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of battery technologies, with particular attention to fluoride-containing systems. The European Union's Battery Directive and similar regulations in North America and Asia are evolving to incorporate specific provisions for advanced battery chemistries, potentially influencing the commercial viability of different fluoride cathode-electrolyte combinations based on their environmental performance profiles.
Solid-state electrolytes represent a more environmentally favorable alternative, significantly reducing leakage risks and eliminating volatile organic compound emissions associated with liquid systems. However, the manufacturing processes for solid electrolytes often require higher energy inputs and may involve rare earth elements with their own extraction-related environmental footprints.
Life cycle assessments of fluoride cathode batteries with different electrolytes reveal varying carbon footprints. Polymer-based electrolyte systems generally demonstrate lower greenhouse gas emissions during production compared to ceramic or glass-based solid electrolytes, though their performance characteristics differ substantially. The end-of-life management presents another critical environmental consideration, with solid electrolyte systems typically offering better recyclability potential.
Water consumption represents a significant environmental factor in electrolyte production. Ionic liquid electrolytes for fluoride cathodes often require extensive purification processes that consume substantial water resources, while ceramic electrolytes may require less water but more energy-intensive sintering processes. This trade-off necessitates regional environmental impact considerations based on local water scarcity and energy grid composition.
The chemical stability of different electrolytes also influences their long-term environmental impact. More stable electrolytes reduce the frequency of battery replacement, thereby decreasing the cumulative environmental burden of manufacturing and disposal. Research indicates that multi-component electrolytes optimized for fluoride cathode interfaces often achieve superior stability but may introduce additional complexity to recycling processes.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of battery technologies, with particular attention to fluoride-containing systems. The European Union's Battery Directive and similar regulations in North America and Asia are evolving to incorporate specific provisions for advanced battery chemistries, potentially influencing the commercial viability of different fluoride cathode-electrolyte combinations based on their environmental performance profiles.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!