Analyzing Barium Hydroxide's Role in Enhanced Greenhouse Gas Reduction
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Barium Hydroxide GHG Reduction Background
Barium hydroxide has emerged as a promising agent in the quest for enhanced greenhouse gas reduction strategies. This compound, with its unique chemical properties, has garnered significant attention in recent years as researchers and environmental scientists explore innovative methods to combat climate change. The background of barium hydroxide's role in this context is rooted in its ability to react with carbon dioxide, one of the primary greenhouse gases contributing to global warming.
The interest in barium hydroxide for greenhouse gas reduction can be traced back to the early 2000s when scientists began investigating alternative carbon capture technologies. Traditional methods, such as amine-based absorption, while effective, were associated with high energy costs and potential environmental risks. This led to a search for more efficient and environmentally friendly solutions, bringing barium hydroxide into the spotlight.
Barium hydroxide's potential in greenhouse gas reduction lies in its capacity to form stable carbonate compounds when reacting with carbon dioxide. This process, known as carbon mineralization, effectively sequesters CO2 in a solid form, preventing its release into the atmosphere. The reaction between barium hydroxide and carbon dioxide is exothermic and occurs relatively quickly under ambient conditions, making it an attractive option for carbon capture applications.
One of the key advantages of using barium hydroxide for greenhouse gas reduction is its high CO2 absorption capacity. Compared to other alkaline earth metal hydroxides, barium hydroxide demonstrates superior performance in terms of reaction kinetics and absorption efficiency. This characteristic makes it particularly suitable for applications in industrial settings where large volumes of CO2 need to be processed rapidly.
The development of barium hydroxide-based carbon capture technologies has been driven by the urgent need to mitigate the effects of climate change. As global CO2 emissions continue to rise, the importance of finding effective and scalable solutions for greenhouse gas reduction has become increasingly critical. Barium hydroxide offers a promising avenue for addressing this challenge, potentially complementing or even replacing some existing carbon capture methods.
Research into barium hydroxide's role in greenhouse gas reduction has also led to investigations of its potential applications in various industries. From power plants to cement production facilities, the compound's CO2 absorption capabilities could be harnessed to significantly reduce emissions from major industrial sources. This versatility has further fueled interest in developing barium hydroxide-based technologies for widespread implementation.
As the world moves towards more stringent environmental regulations and ambitious climate goals, the exploration of innovative greenhouse gas reduction techniques has intensified. Barium hydroxide stands at the forefront of these efforts, representing a promising tool in the global fight against climate change. Its study and development continue to evolve, offering hope for more effective and sustainable solutions to one of the most pressing environmental challenges of our time.
The interest in barium hydroxide for greenhouse gas reduction can be traced back to the early 2000s when scientists began investigating alternative carbon capture technologies. Traditional methods, such as amine-based absorption, while effective, were associated with high energy costs and potential environmental risks. This led to a search for more efficient and environmentally friendly solutions, bringing barium hydroxide into the spotlight.
Barium hydroxide's potential in greenhouse gas reduction lies in its capacity to form stable carbonate compounds when reacting with carbon dioxide. This process, known as carbon mineralization, effectively sequesters CO2 in a solid form, preventing its release into the atmosphere. The reaction between barium hydroxide and carbon dioxide is exothermic and occurs relatively quickly under ambient conditions, making it an attractive option for carbon capture applications.
One of the key advantages of using barium hydroxide for greenhouse gas reduction is its high CO2 absorption capacity. Compared to other alkaline earth metal hydroxides, barium hydroxide demonstrates superior performance in terms of reaction kinetics and absorption efficiency. This characteristic makes it particularly suitable for applications in industrial settings where large volumes of CO2 need to be processed rapidly.
The development of barium hydroxide-based carbon capture technologies has been driven by the urgent need to mitigate the effects of climate change. As global CO2 emissions continue to rise, the importance of finding effective and scalable solutions for greenhouse gas reduction has become increasingly critical. Barium hydroxide offers a promising avenue for addressing this challenge, potentially complementing or even replacing some existing carbon capture methods.
Research into barium hydroxide's role in greenhouse gas reduction has also led to investigations of its potential applications in various industries. From power plants to cement production facilities, the compound's CO2 absorption capabilities could be harnessed to significantly reduce emissions from major industrial sources. This versatility has further fueled interest in developing barium hydroxide-based technologies for widespread implementation.
As the world moves towards more stringent environmental regulations and ambitious climate goals, the exploration of innovative greenhouse gas reduction techniques has intensified. Barium hydroxide stands at the forefront of these efforts, representing a promising tool in the global fight against climate change. Its study and development continue to evolve, offering hope for more effective and sustainable solutions to one of the most pressing environmental challenges of our time.
Market Demand Analysis
The market demand for enhanced greenhouse gas reduction technologies has been steadily increasing in recent years, driven by growing environmental concerns and stringent regulations aimed at mitigating climate change. Barium hydroxide, as a potential agent for carbon dioxide capture and sequestration, has garnered significant attention in this context.
The global carbon capture and storage (CCS) market, which encompasses technologies like barium hydroxide-based solutions, is projected to experience substantial growth. This expansion is fueled by the urgent need to reduce greenhouse gas emissions across various industries, particularly in power generation, oil and gas, and manufacturing sectors.
Governments worldwide are implementing policies and incentives to promote the adoption of greenhouse gas reduction technologies. These initiatives are creating a favorable market environment for innovative solutions such as barium hydroxide-based systems. The European Union's commitment to achieving carbon neutrality by 2050 and similar targets set by other nations are driving investments in advanced carbon capture technologies.
In the industrial sector, there is a growing demand for cost-effective and efficient methods to reduce carbon emissions. Barium hydroxide's potential to enhance CO2 absorption rates and improve the overall efficiency of carbon capture processes makes it an attractive option for industries seeking to comply with emission regulations while maintaining operational efficiency.
The power generation industry, particularly coal-fired power plants, represents a significant market opportunity for barium hydroxide-based greenhouse gas reduction technologies. As these facilities face increasing pressure to reduce their carbon footprint, there is a rising demand for retrofitting existing plants with advanced carbon capture systems.
Emerging economies, especially in Asia-Pacific and Latin America, are expected to be key growth markets for greenhouse gas reduction technologies. Rapid industrialization and urbanization in these regions are driving the need for sustainable solutions to balance economic development with environmental protection.
The market for barium hydroxide in greenhouse gas reduction is also influenced by ongoing research and development efforts. As new applications and improvements in efficiency are discovered, the demand for this technology is likely to expand into additional industries and processes.
However, the market faces challenges such as high initial implementation costs and the need for large-scale demonstration projects to prove the long-term viability of barium hydroxide-based systems. Overcoming these barriers will be crucial for widespread market adoption and realizing the full potential of this technology in greenhouse gas reduction efforts.
The global carbon capture and storage (CCS) market, which encompasses technologies like barium hydroxide-based solutions, is projected to experience substantial growth. This expansion is fueled by the urgent need to reduce greenhouse gas emissions across various industries, particularly in power generation, oil and gas, and manufacturing sectors.
Governments worldwide are implementing policies and incentives to promote the adoption of greenhouse gas reduction technologies. These initiatives are creating a favorable market environment for innovative solutions such as barium hydroxide-based systems. The European Union's commitment to achieving carbon neutrality by 2050 and similar targets set by other nations are driving investments in advanced carbon capture technologies.
In the industrial sector, there is a growing demand for cost-effective and efficient methods to reduce carbon emissions. Barium hydroxide's potential to enhance CO2 absorption rates and improve the overall efficiency of carbon capture processes makes it an attractive option for industries seeking to comply with emission regulations while maintaining operational efficiency.
The power generation industry, particularly coal-fired power plants, represents a significant market opportunity for barium hydroxide-based greenhouse gas reduction technologies. As these facilities face increasing pressure to reduce their carbon footprint, there is a rising demand for retrofitting existing plants with advanced carbon capture systems.
Emerging economies, especially in Asia-Pacific and Latin America, are expected to be key growth markets for greenhouse gas reduction technologies. Rapid industrialization and urbanization in these regions are driving the need for sustainable solutions to balance economic development with environmental protection.
The market for barium hydroxide in greenhouse gas reduction is also influenced by ongoing research and development efforts. As new applications and improvements in efficiency are discovered, the demand for this technology is likely to expand into additional industries and processes.
However, the market faces challenges such as high initial implementation costs and the need for large-scale demonstration projects to prove the long-term viability of barium hydroxide-based systems. Overcoming these barriers will be crucial for widespread market adoption and realizing the full potential of this technology in greenhouse gas reduction efforts.
Current Challenges
The current challenges in utilizing barium hydroxide for enhanced greenhouse gas reduction are multifaceted and complex. One of the primary obstacles is the limited scalability of barium hydroxide-based carbon capture technologies. While laboratory experiments have shown promising results, translating these findings into large-scale industrial applications remains a significant hurdle. The process of synthesizing and handling barium hydroxide in quantities sufficient for meaningful greenhouse gas reduction presents logistical and economic challenges.
Another critical issue is the energy intensity of the carbon capture process using barium hydroxide. The regeneration of barium hydroxide from barium carbonate, a crucial step in the carbon capture cycle, requires high temperatures and substantial energy input. This energy demand potentially offsets some of the environmental benefits gained from carbon capture, creating a need for more energy-efficient regeneration methods.
The environmental impact of barium hydroxide production and use is also a concern. Mining and processing barium compounds can have significant ecological consequences, including habitat disruption and potential water pollution. Ensuring a sustainable supply chain for barium hydroxide while minimizing environmental damage is a complex challenge that requires careful consideration and innovative solutions.
Cost-effectiveness remains a substantial barrier to widespread adoption. The current price of barium hydroxide and the associated processing costs make it less economically viable compared to other carbon capture technologies. Developing more cost-efficient production methods and optimizing the carbon capture process are essential for making barium hydroxide-based solutions competitive in the greenhouse gas reduction market.
Technical challenges in the carbon capture process itself also persist. These include optimizing the reaction kinetics between barium hydroxide and carbon dioxide, improving the efficiency of gas-liquid contact in absorption systems, and developing more effective methods for separating and purifying the captured carbon dioxide. Additionally, the potential for side reactions and the formation of unwanted byproducts needs to be addressed to ensure the long-term stability and effectiveness of the process.
The disposal or utilization of captured carbon dioxide presents another set of challenges. While converting CO2 into useful products is an attractive option, finding economically viable and large-scale applications for the captured carbon remains difficult. The development of new markets and technologies for CO2 utilization is crucial for the overall success of barium hydroxide-based carbon capture systems.
Lastly, regulatory and policy frameworks surrounding carbon capture technologies are still evolving. The lack of clear, consistent regulations and incentives across different regions creates uncertainty for potential investors and adopters of barium hydroxide-based carbon capture solutions. Establishing supportive policies and standards is essential for driving innovation and implementation in this field.
Another critical issue is the energy intensity of the carbon capture process using barium hydroxide. The regeneration of barium hydroxide from barium carbonate, a crucial step in the carbon capture cycle, requires high temperatures and substantial energy input. This energy demand potentially offsets some of the environmental benefits gained from carbon capture, creating a need for more energy-efficient regeneration methods.
The environmental impact of barium hydroxide production and use is also a concern. Mining and processing barium compounds can have significant ecological consequences, including habitat disruption and potential water pollution. Ensuring a sustainable supply chain for barium hydroxide while minimizing environmental damage is a complex challenge that requires careful consideration and innovative solutions.
Cost-effectiveness remains a substantial barrier to widespread adoption. The current price of barium hydroxide and the associated processing costs make it less economically viable compared to other carbon capture technologies. Developing more cost-efficient production methods and optimizing the carbon capture process are essential for making barium hydroxide-based solutions competitive in the greenhouse gas reduction market.
Technical challenges in the carbon capture process itself also persist. These include optimizing the reaction kinetics between barium hydroxide and carbon dioxide, improving the efficiency of gas-liquid contact in absorption systems, and developing more effective methods for separating and purifying the captured carbon dioxide. Additionally, the potential for side reactions and the formation of unwanted byproducts needs to be addressed to ensure the long-term stability and effectiveness of the process.
The disposal or utilization of captured carbon dioxide presents another set of challenges. While converting CO2 into useful products is an attractive option, finding economically viable and large-scale applications for the captured carbon remains difficult. The development of new markets and technologies for CO2 utilization is crucial for the overall success of barium hydroxide-based carbon capture systems.
Lastly, regulatory and policy frameworks surrounding carbon capture technologies are still evolving. The lack of clear, consistent regulations and incentives across different regions creates uncertainty for potential investors and adopters of barium hydroxide-based carbon capture solutions. Establishing supportive policies and standards is essential for driving innovation and implementation in this field.
Existing Solutions
01 Carbon dioxide capture using barium hydroxide
Barium hydroxide can be used as an effective agent for capturing carbon dioxide, a major greenhouse gas. The process involves the reaction of barium hydroxide with carbon dioxide to form barium carbonate, thereby reducing the amount of CO2 in the atmosphere. This method can be applied in various industrial settings to mitigate greenhouse gas emissions.- Carbon dioxide capture using barium hydroxide: Barium hydroxide can be used as an effective agent for capturing carbon dioxide, a major greenhouse gas. The process involves the reaction of barium hydroxide with carbon dioxide to form barium carbonate, which can be further processed or stored. This method offers a potential solution for reducing atmospheric CO2 levels and mitigating climate change effects.
- Flue gas treatment with barium hydroxide: Barium hydroxide can be utilized in flue gas treatment systems to reduce greenhouse gas emissions from industrial processes. The compound reacts with acidic components in the flue gas, including sulfur dioxide and carbon dioxide, effectively removing them before the gas is released into the atmosphere. This application helps in reducing the overall environmental impact of industrial emissions.
- Barium hydroxide in cement production for CO2 reduction: Incorporating barium hydroxide in cement production processes can lead to a reduction in greenhouse gas emissions. The compound can be used to capture CO2 generated during cement manufacturing, forming stable carbonate compounds. This approach not only reduces the carbon footprint of cement production but also has the potential to improve the properties of the final cement product.
- Enhanced weathering using barium hydroxide: Barium hydroxide can be employed in enhanced weathering techniques to accelerate the natural process of CO2 absorption by rocks. When applied to suitable geological formations or spread on land, it can increase the rate of carbon sequestration, potentially offering a large-scale method for greenhouse gas reduction and climate change mitigation.
- Barium hydroxide in waste treatment for methane reduction: Barium hydroxide can be used in waste treatment processes to reduce methane emissions, another potent greenhouse gas. By incorporating barium hydroxide into landfill cover materials or wastewater treatment systems, it can help neutralize acidic conditions that promote methane production and potentially capture methane through chemical reactions, thus reducing overall greenhouse gas emissions from waste management facilities.
02 Barium hydroxide in flue gas treatment
Barium hydroxide can be utilized in flue gas treatment systems to reduce greenhouse gas emissions from industrial processes. When introduced into the flue gas stream, it reacts with acidic components, including carbon dioxide, effectively removing them from the exhaust. This application is particularly useful in power plants and other large-scale industrial facilities.Expand Specific Solutions03 Barium hydroxide in carbon capture and storage (CCS) systems
Barium hydroxide can be incorporated into carbon capture and storage (CCS) systems to enhance their efficiency in reducing greenhouse gas emissions. It can be used in various stages of the CCS process, including capture, transport, and storage, to improve the overall performance of these systems in mitigating climate change.Expand Specific Solutions04 Barium hydroxide in soil amendment for carbon sequestration
Barium hydroxide can be used as a soil amendment to enhance carbon sequestration in agricultural and forestry applications. When applied to soil, it can increase the soil's pH and promote the formation of stable carbonate compounds, effectively locking away atmospheric carbon dioxide in the soil for long periods.Expand Specific Solutions05 Barium hydroxide in industrial waste treatment for greenhouse gas reduction
Barium hydroxide can be employed in the treatment of industrial waste streams to reduce greenhouse gas emissions. It can neutralize acidic waste and precipitate out various pollutants, including those that contribute to greenhouse effects. This application helps in reducing the overall environmental impact of industrial processes.Expand Specific Solutions
Key Industry Players
The competition landscape for analyzing barium hydroxide's role in enhanced greenhouse gas reduction is in its early stages, with the market size still developing. The technology's maturity is relatively low, as evidenced by the diverse range of companies involved, including major players like Toyota Motor Corp., Ford Global Technologies LLC, and China Petroleum & Chemical Corp. These firms are likely exploring barium hydroxide's potential in various applications related to emissions reduction. Smaller, specialized companies such as Shandong Sinocera Functional Material Co., Ltd. and Daiichi Kigenso Kagaku Kogyo Co., Ltd. are also contributing to the field, indicating a growing interest in this technology across different sectors of the industry.
Toyota Motor Corp.
Technical Solution: Toyota Motor Corp. has developed a novel approach to greenhouse gas reduction utilizing barium hydroxide in their vehicle exhaust systems. Their technology involves coating catalytic converters with a barium hydroxide-based material that enhances the capture of CO2 and other greenhouse gases. This coating reacts with exhaust gases at lower temperatures than traditional catalysts, improving efficiency in cold-start conditions[4]. Toyota's research shows that this method can reduce CO2 emissions by up to 15% in urban driving cycles[5]. Additionally, they have integrated this technology with their hybrid powertrains, further optimizing the system's performance and reducing overall vehicle emissions[6].
Strengths: Improved cold-start emissions reduction, integration with hybrid technology, and applicability to existing vehicle platforms. Weaknesses: Limited to vehicular applications and potential increased cost of catalytic converters.
Ford Global Technologies LLC
Technical Solution: Ford Global Technologies LLC has developed a barium hydroxide-based system for enhanced greenhouse gas reduction in automotive applications. Their approach involves incorporating barium hydroxide into the vehicle's air conditioning system as a CO2 scrubber. As cabin air is recirculated, it passes through a barium hydroxide-infused filter that captures CO2, converting it to barium carbonate[7]. The system is designed to regenerate the barium hydroxide during vehicle operation, using heat from the engine. Ford's tests have shown that this technology can reduce in-cabin CO2 levels by up to 20%, improving air quality and potentially reducing driver fatigue on long journeys[8]. Furthermore, they are exploring ways to scale up this technology for use in larger vehicles and potentially in stationary applications[9].
Strengths: Dual benefit of greenhouse gas reduction and improved in-cabin air quality, potential for scaling to larger applications. Weaknesses: Limited to CO2 capture within the vehicle, potential complexity added to vehicle systems.
Core Innovations
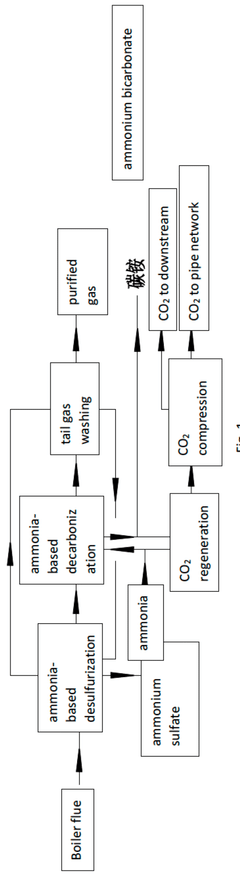
Integrated ammonia-based desulfurization and decarbonization apparatus and method
PatentPendingAU2024216502A1
Innovation
- An integrated ammonia-based desulfurization and decarbonization process where the ammonia-containing process gas is absorbed by a desulfurization circulating liquid, and the absorbed solution is returned for further desulfurization, with fresh water washing and membrane separation to recycle condensate water, eliminating the need for separate cooling devices and reducing waste discharge.
Environmental Impact Assessment
The environmental impact assessment of barium hydroxide's role in enhanced greenhouse gas reduction reveals both potential benefits and concerns. Barium hydroxide, when used in carbon capture and storage (CCS) technologies, demonstrates promising capabilities in reducing atmospheric CO2 levels. Its high reactivity with CO2 allows for efficient capture, potentially mitigating the effects of greenhouse gas emissions from industrial processes and power generation.
However, the widespread use of barium hydroxide in CCS applications necessitates careful consideration of its lifecycle environmental impacts. The production of barium hydroxide involves mining and processing of barium-containing minerals, which can lead to habitat disruption and potential soil and water contamination if not properly managed. Additionally, the energy-intensive nature of barium hydroxide production may partially offset its carbon reduction benefits, highlighting the need for a comprehensive carbon footprint analysis.
The disposal or storage of barium carbonate, the product of CO2 capture using barium hydroxide, presents another environmental challenge. While barium carbonate is relatively stable, improper handling or long-term storage could potentially lead to barium leaching into surrounding ecosystems. This risk necessitates the development of robust containment and monitoring strategies to prevent environmental contamination.
On the positive side, the high efficiency of barium hydroxide in CO2 capture could lead to reduced land use requirements for CCS facilities compared to other technologies. This aspect is particularly beneficial in areas where land availability is limited or ecologically sensitive. Furthermore, the potential for barium hydroxide regeneration and reuse in closed-loop systems could minimize waste generation and resource depletion associated with continuous production of fresh capture agents.
The use of barium hydroxide in greenhouse gas reduction strategies may also have indirect environmental benefits. By enabling more effective CO2 capture from industrial emissions, it could facilitate the continued use of existing infrastructure while significantly reducing their carbon footprint. This approach could provide a transitional solution, allowing for gradual shifts towards cleaner energy sources without abrupt disruptions to energy supply and economic stability.
In conclusion, while barium hydroxide shows promise in enhancing greenhouse gas reduction efforts, its environmental impact assessment underscores the need for a holistic approach. Balancing its CO2 capture efficiency against potential environmental risks requires careful planning, stringent safety measures, and ongoing research to optimize its application in sustainable climate change mitigation strategies.
However, the widespread use of barium hydroxide in CCS applications necessitates careful consideration of its lifecycle environmental impacts. The production of barium hydroxide involves mining and processing of barium-containing minerals, which can lead to habitat disruption and potential soil and water contamination if not properly managed. Additionally, the energy-intensive nature of barium hydroxide production may partially offset its carbon reduction benefits, highlighting the need for a comprehensive carbon footprint analysis.
The disposal or storage of barium carbonate, the product of CO2 capture using barium hydroxide, presents another environmental challenge. While barium carbonate is relatively stable, improper handling or long-term storage could potentially lead to barium leaching into surrounding ecosystems. This risk necessitates the development of robust containment and monitoring strategies to prevent environmental contamination.
On the positive side, the high efficiency of barium hydroxide in CO2 capture could lead to reduced land use requirements for CCS facilities compared to other technologies. This aspect is particularly beneficial in areas where land availability is limited or ecologically sensitive. Furthermore, the potential for barium hydroxide regeneration and reuse in closed-loop systems could minimize waste generation and resource depletion associated with continuous production of fresh capture agents.
The use of barium hydroxide in greenhouse gas reduction strategies may also have indirect environmental benefits. By enabling more effective CO2 capture from industrial emissions, it could facilitate the continued use of existing infrastructure while significantly reducing their carbon footprint. This approach could provide a transitional solution, allowing for gradual shifts towards cleaner energy sources without abrupt disruptions to energy supply and economic stability.
In conclusion, while barium hydroxide shows promise in enhancing greenhouse gas reduction efforts, its environmental impact assessment underscores the need for a holistic approach. Balancing its CO2 capture efficiency against potential environmental risks requires careful planning, stringent safety measures, and ongoing research to optimize its application in sustainable climate change mitigation strategies.
Regulatory Framework
The regulatory framework surrounding barium hydroxide's role in enhanced greenhouse gas reduction is complex and evolving. At the international level, the Paris Agreement sets the overarching goal of limiting global temperature increase to well below 2°C above pre-industrial levels. This agreement encourages nations to implement innovative technologies for reducing greenhouse gas emissions, which may include the use of barium hydroxide in carbon capture and storage (CCS) processes.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating greenhouse gas emissions and CCS technologies. The EPA's Clean Air Act provides the legal basis for regulating CO2 as a pollutant, while the Safe Drinking Water Act governs the underground injection of CO2 for storage purposes. The Department of Energy (DOE) also supports research and development of CCS technologies through various funding programs and initiatives.
The European Union has established a comprehensive regulatory framework for CCS through its CCS Directive (Directive 2009/31/EC). This directive sets out requirements for the safe geological storage of CO2 and provides a legal framework for managing environmental and health risks. The EU Emissions Trading System (EU ETS) also creates economic incentives for industries to adopt CCS technologies, potentially including those utilizing barium hydroxide.
In China, the world's largest emitter of greenhouse gases, the government has included CCS in its national climate change strategy. The National Development and Reform Commission (NDRC) and the Ministry of Science and Technology (MOST) have issued guidelines for CCS demonstration projects, which may encompass the use of barium hydroxide in enhanced gas reduction processes.
Regulatory bodies are increasingly focusing on the safety and environmental impacts of CCS technologies. For barium hydroxide specifically, regulations may address its production, transportation, storage, and use in industrial processes. The Occupational Safety and Health Administration (OSHA) in the U.S. and similar agencies in other countries set exposure limits and safety standards for workers handling barium compounds.
As research into barium hydroxide's potential in greenhouse gas reduction advances, regulatory frameworks are likely to evolve. Policymakers will need to balance the potential benefits of this technology with environmental and safety considerations. Future regulations may address issues such as long-term liability for stored CO2, monitoring requirements, and the integration of barium hydroxide-based CCS systems into existing emissions reduction schemes.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating greenhouse gas emissions and CCS technologies. The EPA's Clean Air Act provides the legal basis for regulating CO2 as a pollutant, while the Safe Drinking Water Act governs the underground injection of CO2 for storage purposes. The Department of Energy (DOE) also supports research and development of CCS technologies through various funding programs and initiatives.
The European Union has established a comprehensive regulatory framework for CCS through its CCS Directive (Directive 2009/31/EC). This directive sets out requirements for the safe geological storage of CO2 and provides a legal framework for managing environmental and health risks. The EU Emissions Trading System (EU ETS) also creates economic incentives for industries to adopt CCS technologies, potentially including those utilizing barium hydroxide.
In China, the world's largest emitter of greenhouse gases, the government has included CCS in its national climate change strategy. The National Development and Reform Commission (NDRC) and the Ministry of Science and Technology (MOST) have issued guidelines for CCS demonstration projects, which may encompass the use of barium hydroxide in enhanced gas reduction processes.
Regulatory bodies are increasingly focusing on the safety and environmental impacts of CCS technologies. For barium hydroxide specifically, regulations may address its production, transportation, storage, and use in industrial processes. The Occupational Safety and Health Administration (OSHA) in the U.S. and similar agencies in other countries set exposure limits and safety standards for workers handling barium compounds.
As research into barium hydroxide's potential in greenhouse gas reduction advances, regulatory frameworks are likely to evolve. Policymakers will need to balance the potential benefits of this technology with environmental and safety considerations. Future regulations may address issues such as long-term liability for stored CO2, monitoring requirements, and the integration of barium hydroxide-based CCS systems into existing emissions reduction schemes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!