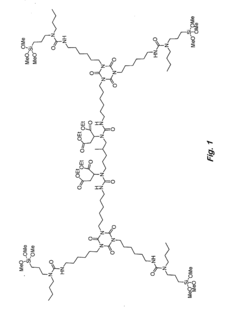
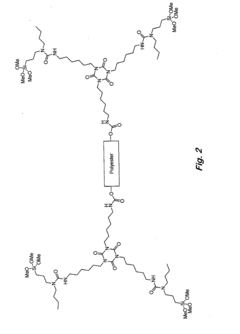
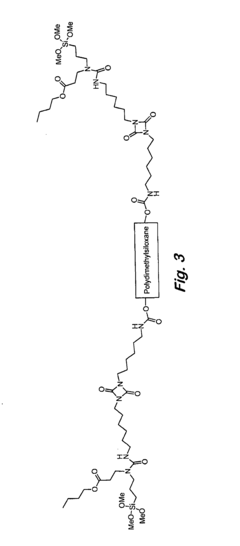
Analyzing Isocyanate Reactivity: Key Factors
JUL 10, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isocyanate Chemistry Background and Objectives
Isocyanate chemistry has been a cornerstone of polymer science and industrial applications for nearly a century. The discovery of isocyanates in the 1930s by Otto Bayer and his team at IG Farben marked the beginning of a new era in materials science. Since then, the reactivity of isocyanates has been extensively studied and exploited in various fields, particularly in the production of polyurethanes, which have become ubiquitous in modern life.
The fundamental structure of isocyanates, characterized by the highly reactive -N=C=O group, is key to their versatility. This functional group's unique electronic configuration allows for a wide range of reactions, primarily with nucleophiles such as alcohols, amines, and water. The high reactivity of isocyanates is attributed to the cumulative electron-withdrawing effect of the nitrogen and oxygen atoms on the central carbon atom, making it highly electrophilic.
Over the decades, research has focused on understanding and controlling isocyanate reactivity. This has led to the development of various types of isocyanates, including aromatic and aliphatic compounds, each with distinct reactivity profiles. Aromatic isocyanates, such as toluene diisocyanate (TDI) and methylene diphenyl diisocyanate (MDI), are generally more reactive than their aliphatic counterparts like hexamethylene diisocyanate (HDI).
The evolution of isocyanate chemistry has been driven by the need for more efficient, safer, and environmentally friendly processes. Early challenges included the high toxicity of some isocyanates and the exothermic nature of their reactions, which posed significant safety concerns. These issues have spurred ongoing research into less hazardous alternatives and improved handling techniques.
Current objectives in isocyanate chemistry research are multifaceted. There is a strong focus on developing bio-based isocyanates to reduce reliance on petroleum-derived raw materials. Additionally, researchers are exploring ways to enhance the selectivity and control of isocyanate reactions, aiming to create more sophisticated and tailored polymeric materials.
Another key goal is to understand and manipulate the factors that influence isocyanate reactivity. These factors include temperature, catalysts, steric hindrance, and the electronic effects of substituents. By gaining a deeper understanding of these influences, scientists and engineers aim to design more efficient and sustainable polymerization processes.
The environmental impact of isocyanates is also a critical consideration. Efforts are underway to develop isocyanate-free alternatives and to improve the recyclability and biodegradability of isocyanate-based products. This aligns with the broader trend towards green chemistry and sustainable materials in the chemical industry.
The fundamental structure of isocyanates, characterized by the highly reactive -N=C=O group, is key to their versatility. This functional group's unique electronic configuration allows for a wide range of reactions, primarily with nucleophiles such as alcohols, amines, and water. The high reactivity of isocyanates is attributed to the cumulative electron-withdrawing effect of the nitrogen and oxygen atoms on the central carbon atom, making it highly electrophilic.
Over the decades, research has focused on understanding and controlling isocyanate reactivity. This has led to the development of various types of isocyanates, including aromatic and aliphatic compounds, each with distinct reactivity profiles. Aromatic isocyanates, such as toluene diisocyanate (TDI) and methylene diphenyl diisocyanate (MDI), are generally more reactive than their aliphatic counterparts like hexamethylene diisocyanate (HDI).
The evolution of isocyanate chemistry has been driven by the need for more efficient, safer, and environmentally friendly processes. Early challenges included the high toxicity of some isocyanates and the exothermic nature of their reactions, which posed significant safety concerns. These issues have spurred ongoing research into less hazardous alternatives and improved handling techniques.
Current objectives in isocyanate chemistry research are multifaceted. There is a strong focus on developing bio-based isocyanates to reduce reliance on petroleum-derived raw materials. Additionally, researchers are exploring ways to enhance the selectivity and control of isocyanate reactions, aiming to create more sophisticated and tailored polymeric materials.
Another key goal is to understand and manipulate the factors that influence isocyanate reactivity. These factors include temperature, catalysts, steric hindrance, and the electronic effects of substituents. By gaining a deeper understanding of these influences, scientists and engineers aim to design more efficient and sustainable polymerization processes.
The environmental impact of isocyanates is also a critical consideration. Efforts are underway to develop isocyanate-free alternatives and to improve the recyclability and biodegradability of isocyanate-based products. This aligns with the broader trend towards green chemistry and sustainable materials in the chemical industry.
Industrial Applications and Market Demand
Isocyanates play a crucial role in various industrial applications, driving significant market demand across multiple sectors. The polyurethane industry, in particular, heavily relies on isocyanate reactivity for producing a wide range of products, including foams, coatings, adhesives, and elastomers. The global polyurethane market has been experiencing steady growth, with increasing demand from construction, automotive, and furniture industries.
In the construction sector, isocyanate-based products are extensively used for insulation, sealants, and coatings. The growing emphasis on energy efficiency in buildings has led to a surge in demand for polyurethane insulation materials, which offer excellent thermal properties. This trend is expected to continue as countries worldwide implement stricter energy conservation regulations.
The automotive industry represents another major market for isocyanate-based products. Polyurethane components are used in vehicle interiors, seating, and exterior parts due to their lightweight properties and durability. As the automotive sector shifts towards electric vehicles and focuses on reducing vehicle weight to improve fuel efficiency, the demand for isocyanate-based materials is projected to increase further.
The furniture and bedding industry also contributes significantly to the market demand for isocyanate-based products. Flexible polyurethane foams are widely used in mattresses, upholstery, and cushioning applications. The growing consumer preference for comfortable and durable furniture is driving the demand for high-quality polyurethane materials.
In the coatings industry, isocyanate-based products are valued for their excellent chemical resistance, durability, and aesthetic properties. Industrial coatings, automotive refinish coatings, and protective coatings for infrastructure all rely on isocyanate chemistry. The increasing focus on sustainable and environmentally friendly coatings has led to the development of water-based and low-VOC isocyanate formulations, opening up new market opportunities.
The adhesives sector is another area where isocyanate reactivity finds extensive application. Polyurethane adhesives are used in various industries, including packaging, footwear, and woodworking. The superior bonding strength and versatility of these adhesives have led to their increased adoption in demanding applications.
As environmental concerns and sustainability become more prominent, there is a growing interest in bio-based isocyanates and alternative chemistries. This trend is driving research and development efforts to create more sustainable and eco-friendly isocyanate-based products, potentially expanding market opportunities in the future.
In the construction sector, isocyanate-based products are extensively used for insulation, sealants, and coatings. The growing emphasis on energy efficiency in buildings has led to a surge in demand for polyurethane insulation materials, which offer excellent thermal properties. This trend is expected to continue as countries worldwide implement stricter energy conservation regulations.
The automotive industry represents another major market for isocyanate-based products. Polyurethane components are used in vehicle interiors, seating, and exterior parts due to their lightweight properties and durability. As the automotive sector shifts towards electric vehicles and focuses on reducing vehicle weight to improve fuel efficiency, the demand for isocyanate-based materials is projected to increase further.
The furniture and bedding industry also contributes significantly to the market demand for isocyanate-based products. Flexible polyurethane foams are widely used in mattresses, upholstery, and cushioning applications. The growing consumer preference for comfortable and durable furniture is driving the demand for high-quality polyurethane materials.
In the coatings industry, isocyanate-based products are valued for their excellent chemical resistance, durability, and aesthetic properties. Industrial coatings, automotive refinish coatings, and protective coatings for infrastructure all rely on isocyanate chemistry. The increasing focus on sustainable and environmentally friendly coatings has led to the development of water-based and low-VOC isocyanate formulations, opening up new market opportunities.
The adhesives sector is another area where isocyanate reactivity finds extensive application. Polyurethane adhesives are used in various industries, including packaging, footwear, and woodworking. The superior bonding strength and versatility of these adhesives have led to their increased adoption in demanding applications.
As environmental concerns and sustainability become more prominent, there is a growing interest in bio-based isocyanates and alternative chemistries. This trend is driving research and development efforts to create more sustainable and eco-friendly isocyanate-based products, potentially expanding market opportunities in the future.
Current Challenges in Isocyanate Reactivity Analysis
The analysis of isocyanate reactivity presents several significant challenges in current research and industrial applications. One of the primary difficulties lies in the complex nature of isocyanate reactions, which are influenced by a multitude of factors. These factors include temperature, catalyst type and concentration, solvent effects, and the presence of impurities or competing reactants. The interplay between these variables creates a highly dynamic system that is challenging to predict and control with precision.
Another major hurdle is the development of accurate and reliable analytical methods for measuring isocyanate reactivity in real-time. Traditional techniques often struggle to capture the rapid kinetics of isocyanate reactions, particularly in industrial settings where reaction conditions can change quickly. This limitation hampers the ability to optimize processes and ensure consistent product quality, especially in the production of polyurethanes and other isocyanate-based materials.
The reactivity of isocyanates is also highly sensitive to environmental conditions, such as humidity and atmospheric contaminants. This sensitivity poses significant challenges in maintaining consistent reaction rates and product properties across different production batches or geographical locations. Researchers and manufacturers must contend with the need for stringent environmental controls and the development of robust formulations that can withstand variations in ambient conditions.
Furthermore, the health and safety concerns associated with isocyanates present additional challenges in their handling and analysis. The high reactivity of isocyanates, particularly their propensity to form harmful vapors, necessitates specialized safety protocols and equipment. This requirement can limit the types of analytical techniques that can be employed and may introduce complexities in data interpretation due to the need for protective measures.
The diversity of isocyanate compounds and their applications also contributes to the challenges in reactivity analysis. Different isocyanates exhibit varying degrees of reactivity and selectivity towards different nucleophiles. This diversity makes it difficult to develop universal analytical methods or predictive models that can be applied across the entire spectrum of isocyanate chemistry. Researchers must often tailor their approaches to specific isocyanate systems, which can be time-consuming and resource-intensive.
Lastly, the increasing demand for sustainable and environmentally friendly materials has introduced new challenges in isocyanate reactivity analysis. The push towards bio-based isocyanates and alternative curing systems requires the development of new analytical techniques and a deeper understanding of how these novel compounds behave under various conditions. This evolving landscape necessitates continuous adaptation of analytical methods and theoretical models to keep pace with innovations in isocyanate chemistry.
Another major hurdle is the development of accurate and reliable analytical methods for measuring isocyanate reactivity in real-time. Traditional techniques often struggle to capture the rapid kinetics of isocyanate reactions, particularly in industrial settings where reaction conditions can change quickly. This limitation hampers the ability to optimize processes and ensure consistent product quality, especially in the production of polyurethanes and other isocyanate-based materials.
The reactivity of isocyanates is also highly sensitive to environmental conditions, such as humidity and atmospheric contaminants. This sensitivity poses significant challenges in maintaining consistent reaction rates and product properties across different production batches or geographical locations. Researchers and manufacturers must contend with the need for stringent environmental controls and the development of robust formulations that can withstand variations in ambient conditions.
Furthermore, the health and safety concerns associated with isocyanates present additional challenges in their handling and analysis. The high reactivity of isocyanates, particularly their propensity to form harmful vapors, necessitates specialized safety protocols and equipment. This requirement can limit the types of analytical techniques that can be employed and may introduce complexities in data interpretation due to the need for protective measures.
The diversity of isocyanate compounds and their applications also contributes to the challenges in reactivity analysis. Different isocyanates exhibit varying degrees of reactivity and selectivity towards different nucleophiles. This diversity makes it difficult to develop universal analytical methods or predictive models that can be applied across the entire spectrum of isocyanate chemistry. Researchers must often tailor their approaches to specific isocyanate systems, which can be time-consuming and resource-intensive.
Lastly, the increasing demand for sustainable and environmentally friendly materials has introduced new challenges in isocyanate reactivity analysis. The push towards bio-based isocyanates and alternative curing systems requires the development of new analytical techniques and a deeper understanding of how these novel compounds behave under various conditions. This evolving landscape necessitates continuous adaptation of analytical methods and theoretical models to keep pace with innovations in isocyanate chemistry.
Analytical Methods for Isocyanate Reactivity
01 Reactivity with hydroxyl groups
Isocyanates are highly reactive with hydroxyl groups, forming urethane linkages. This reaction is fundamental in polyurethane chemistry and is utilized in various applications such as coatings, adhesives, and foams. The reactivity can be influenced by factors like temperature, catalysts, and the structure of the isocyanate.- Reactivity with hydroxyl groups: Isocyanates are highly reactive with hydroxyl groups, forming urethane linkages. This reaction is fundamental in polyurethane chemistry and is used in various applications such as coatings, adhesives, and foams. The reactivity can be influenced by factors like temperature, catalysts, and the structure of the isocyanate.
- Moisture sensitivity and side reactions: Isocyanates are sensitive to moisture, reacting to form carbon dioxide and amines. This property is utilized in some applications but can also lead to unwanted side reactions in others. Understanding and controlling this reactivity is crucial in formulating isocyanate-based products and managing their storage and handling.
- Catalysis of isocyanate reactions: Various catalysts can be used to control and enhance isocyanate reactivity. These include metal-based catalysts, tertiary amines, and organometallic compounds. The choice of catalyst can significantly affect reaction rates, selectivity, and the properties of the final product.
- Blocked isocyanates for controlled reactivity: Blocked isocyanates are used to control reactivity in certain applications. These compounds have the isocyanate group temporarily deactivated by a blocking agent, which can be removed under specific conditions to restore reactivity. This approach allows for improved stability during storage and processing.
- Structure-reactivity relationships: The reactivity of isocyanates is influenced by their molecular structure. Aromatic isocyanates generally show higher reactivity than aliphatic ones. Steric hindrance and electronic effects can also play a role in determining reactivity. Understanding these relationships is crucial for designing isocyanates with specific properties.
02 Moisture sensitivity and curing
Isocyanates are sensitive to moisture, reacting with water to form carbon dioxide and amines. This property is exploited in moisture-curing systems for sealants and coatings. The reaction with moisture can also be a challenge in storage and handling of isocyanate-containing products.Expand Specific Solutions03 Catalysis of isocyanate reactions
Various catalysts can be used to accelerate isocyanate reactions, including tertiary amines and organometallic compounds. These catalysts play a crucial role in controlling reaction rates and selectivity in polyurethane production and other isocyanate-based processes.Expand Specific Solutions04 Blocked isocyanates for controlled reactivity
Blocked isocyanates are used to control reactivity in certain applications. These compounds have the isocyanate group temporarily deactivated by a blocking agent, which can be removed under specific conditions to restore reactivity. This approach is useful in coatings and adhesives where delayed curing is desired.Expand Specific Solutions05 Isocyanate reactivity in polymer synthesis
The high reactivity of isocyanates is utilized in the synthesis of various polymers and copolymers. This includes not only polyurethanes but also polyureas, polyamides, and other specialty polymers. The versatility of isocyanate chemistry allows for the creation of materials with tailored properties for specific applications.Expand Specific Solutions
Major Players in Isocyanate Industry
The isocyanate reactivity analysis market is in a mature stage, with established players and well-developed technologies. The global market size for isocyanates is substantial, driven by demand in polyurethane production across various industries. Key players like Dow, BASF, Covestro, and Wanhua Chemical dominate the market, leveraging their extensive R&D capabilities and global presence. These companies have achieved high levels of technical maturity in isocyanate chemistry, focusing on improving reactivity, selectivity, and environmental performance. Emerging players and research institutions are also contributing to advancements in this field, exploring novel catalysts and reaction mechanisms to enhance isocyanate reactivity and expand applications.
Dow Global Technologies LLC
Technical Solution: Dow Global Technologies has developed advanced methods for analyzing isocyanate reactivity, focusing on key factors such as temperature, catalysts, and functional group interactions. Their approach involves using real-time FTIR spectroscopy to monitor reaction kinetics[1], allowing for precise measurement of isocyanate consumption rates. They have also implemented computational modeling techniques to predict reactivity based on molecular structure and electronic properties[3]. Additionally, Dow has explored the use of novel catalysts to control isocyanate reactions, particularly in polyurethane foam applications, where they've achieved significant improvements in reaction speed and selectivity[5].
Strengths: Comprehensive analytical techniques, advanced modeling capabilities, and expertise in catalyst development. Weaknesses: May be limited to specific application areas within their product portfolio.
Wanhua Chemical Group Co., Ltd.
Technical Solution: Wanhua Chemical has developed a multi-faceted approach to analyzing isocyanate reactivity, focusing on structure-property relationships and reaction kinetics. They utilize advanced spectroscopic techniques, including in-situ NMR and mass spectrometry, to monitor reaction progress and identify intermediates[2]. Wanhua has also invested in high-throughput screening methods to rapidly assess the reactivity of various isocyanate formulations under different conditions[4]. Their research extends to studying the impact of different polyols and chain extenders on isocyanate reactivity, particularly in the context of high-performance polyurethane materials[6].
Strengths: Extensive experience with various isocyanate types, strong focus on industrial applications. Weaknesses: May have less emphasis on fundamental research compared to academic institutions.
Key Innovations in Isocyanate Reaction Mechanisms
Measurement of total reactive isocyanate groups in samples using bifunctional nucleophiles such as 1,8-diaminonaphthalene (DAN)
PatentInactiveEP1579207A2
Innovation
- A method using 1,8-diaminonaphthalene (DAN) as a bifunctional nucleophilic isocyanate derivatizing agent that reacts with isocyanates to form a cyclic reaction product, allowing for the detection and quantification of total isocyanate groups regardless of the specific species present, using a two-step process of derivatization and cyclization.
Siloxane-based coatings containing polymers with urea linkages and terminal alkoxysilanes
PatentActiveUS20170183534A1
Innovation
- Development of flexible, two-component siloxane-based coatings using alkoxysilane-terminated urea polymers with urea linkages and moisture-curable alkoxysilane groups, which reduce crosslink density for improved flexibility while maintaining durability and solvent resistance, eliminating the need for isocyanates.
Environmental and Safety Considerations
Isocyanates, widely used in the production of polyurethanes, pose significant environmental and safety risks that require careful consideration and management. These highly reactive compounds can have severe impacts on human health and the environment if not handled properly.
From an environmental perspective, isocyanates can contribute to air pollution and water contamination. When released into the atmosphere, they can react with other pollutants to form harmful secondary compounds. In aquatic environments, isocyanates can hydrolyze to form toxic amines, potentially harming aquatic life and ecosystems. Proper containment and disposal methods are crucial to prevent environmental contamination.
Worker safety is a primary concern when dealing with isocyanates. Exposure can occur through inhalation, skin contact, or ingestion, leading to various health issues. Respiratory sensitization is a significant risk, potentially causing occupational asthma with prolonged exposure. Skin contact can result in dermatitis and other allergic reactions. To mitigate these risks, comprehensive safety protocols must be implemented, including proper ventilation systems, personal protective equipment (PPE), and regular health monitoring for workers.
Regulatory compliance is another critical aspect of isocyanate handling. Many countries have strict regulations governing the use, storage, and disposal of isocyanates. Compliance with these regulations is essential not only for legal reasons but also to ensure the safety of workers and the environment. This includes proper labeling, storage in appropriate containers, and adherence to exposure limits set by occupational safety authorities.
Emergency response planning is vital when working with isocyanates. Facilities must have well-defined procedures for spills, leaks, or accidental exposures. This includes having appropriate spill containment materials, decontamination equipment, and trained personnel ready to respond to incidents. Regular drills and training sessions should be conducted to ensure all staff are prepared for potential emergencies.
The development of safer alternatives and greener chemistry approaches is an ongoing area of research in the field of isocyanate chemistry. Efforts are being made to find less hazardous substitutes or to modify production processes to reduce risks. These innovations aim to maintain the beneficial properties of isocyanate-based products while minimizing environmental and safety concerns.
In conclusion, addressing the environmental and safety considerations of isocyanate reactivity requires a multifaceted approach. This includes rigorous safety protocols, environmental protection measures, regulatory compliance, emergency preparedness, and ongoing research into safer alternatives. By prioritizing these aspects, industries can harness the benefits of isocyanates while minimizing their potential negative impacts on human health and the environment.
From an environmental perspective, isocyanates can contribute to air pollution and water contamination. When released into the atmosphere, they can react with other pollutants to form harmful secondary compounds. In aquatic environments, isocyanates can hydrolyze to form toxic amines, potentially harming aquatic life and ecosystems. Proper containment and disposal methods are crucial to prevent environmental contamination.
Worker safety is a primary concern when dealing with isocyanates. Exposure can occur through inhalation, skin contact, or ingestion, leading to various health issues. Respiratory sensitization is a significant risk, potentially causing occupational asthma with prolonged exposure. Skin contact can result in dermatitis and other allergic reactions. To mitigate these risks, comprehensive safety protocols must be implemented, including proper ventilation systems, personal protective equipment (PPE), and regular health monitoring for workers.
Regulatory compliance is another critical aspect of isocyanate handling. Many countries have strict regulations governing the use, storage, and disposal of isocyanates. Compliance with these regulations is essential not only for legal reasons but also to ensure the safety of workers and the environment. This includes proper labeling, storage in appropriate containers, and adherence to exposure limits set by occupational safety authorities.
Emergency response planning is vital when working with isocyanates. Facilities must have well-defined procedures for spills, leaks, or accidental exposures. This includes having appropriate spill containment materials, decontamination equipment, and trained personnel ready to respond to incidents. Regular drills and training sessions should be conducted to ensure all staff are prepared for potential emergencies.
The development of safer alternatives and greener chemistry approaches is an ongoing area of research in the field of isocyanate chemistry. Efforts are being made to find less hazardous substitutes or to modify production processes to reduce risks. These innovations aim to maintain the beneficial properties of isocyanate-based products while minimizing environmental and safety concerns.
In conclusion, addressing the environmental and safety considerations of isocyanate reactivity requires a multifaceted approach. This includes rigorous safety protocols, environmental protection measures, regulatory compliance, emergency preparedness, and ongoing research into safer alternatives. By prioritizing these aspects, industries can harness the benefits of isocyanates while minimizing their potential negative impacts on human health and the environment.
Regulatory Framework for Isocyanate Usage
The regulatory framework for isocyanate usage has become increasingly stringent due to the potential health and environmental risks associated with these compounds. Governments and international organizations have implemented comprehensive regulations to ensure the safe handling, storage, and use of isocyanates across various industries.
In the United States, the Occupational Safety and Health Administration (OSHA) has established specific standards for isocyanate exposure in the workplace. These standards set permissible exposure limits (PELs) and require employers to implement engineering controls, work practices, and personal protective equipment to minimize worker exposure. Additionally, the Environmental Protection Agency (EPA) regulates isocyanates under the Toxic Substances Control Act (TSCA), which mandates reporting, record-keeping, and testing requirements.
The European Union has adopted the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to isocyanates and their derivatives. Under REACH, manufacturers and importers must register substances and provide safety data, including information on reactivity and potential hazards. The Classification, Labeling, and Packaging (CLP) Regulation further ensures that the hazards of isocyanates are clearly communicated to workers and consumers.
In Asia, countries like Japan and South Korea have implemented their own chemical control laws, which include specific provisions for isocyanates. These regulations often align with international standards but may have additional requirements tailored to local conditions and industrial practices.
Global harmonization efforts, such as the Globally Harmonized System of Classification and Labeling of Chemicals (GHS), have played a crucial role in standardizing the communication of isocyanate hazards across borders. This system provides a common framework for hazard classification, labeling, and safety data sheets, facilitating international trade while maintaining safety standards.
Industry-specific regulations also govern isocyanate usage in sectors such as automotive manufacturing, construction, and furniture production. These regulations often focus on product safety, emissions control, and waste management. For instance, the California Air Resources Board (CARB) has established strict formaldehyde emission standards for composite wood products, indirectly affecting the use of isocyanate-based adhesives in these materials.
As research continues to unveil the long-term effects of isocyanate exposure, regulatory bodies are adapting their frameworks to address emerging concerns. This includes the development of more sensitive analytical methods for detecting isocyanates in various matrices and the establishment of biomonitoring programs to assess occupational exposure.
The evolving regulatory landscape necessitates ongoing compliance efforts from industry stakeholders. Companies must stay informed about regulatory changes, invest in research and development to find safer alternatives or improved handling methods, and implement robust safety management systems to ensure compliance and protect workers and the environment.
In the United States, the Occupational Safety and Health Administration (OSHA) has established specific standards for isocyanate exposure in the workplace. These standards set permissible exposure limits (PELs) and require employers to implement engineering controls, work practices, and personal protective equipment to minimize worker exposure. Additionally, the Environmental Protection Agency (EPA) regulates isocyanates under the Toxic Substances Control Act (TSCA), which mandates reporting, record-keeping, and testing requirements.
The European Union has adopted the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation, which applies to isocyanates and their derivatives. Under REACH, manufacturers and importers must register substances and provide safety data, including information on reactivity and potential hazards. The Classification, Labeling, and Packaging (CLP) Regulation further ensures that the hazards of isocyanates are clearly communicated to workers and consumers.
In Asia, countries like Japan and South Korea have implemented their own chemical control laws, which include specific provisions for isocyanates. These regulations often align with international standards but may have additional requirements tailored to local conditions and industrial practices.
Global harmonization efforts, such as the Globally Harmonized System of Classification and Labeling of Chemicals (GHS), have played a crucial role in standardizing the communication of isocyanate hazards across borders. This system provides a common framework for hazard classification, labeling, and safety data sheets, facilitating international trade while maintaining safety standards.
Industry-specific regulations also govern isocyanate usage in sectors such as automotive manufacturing, construction, and furniture production. These regulations often focus on product safety, emissions control, and waste management. For instance, the California Air Resources Board (CARB) has established strict formaldehyde emission standards for composite wood products, indirectly affecting the use of isocyanate-based adhesives in these materials.
As research continues to unveil the long-term effects of isocyanate exposure, regulatory bodies are adapting their frameworks to address emerging concerns. This includes the development of more sensitive analytical methods for detecting isocyanates in various matrices and the establishment of biomonitoring programs to assess occupational exposure.
The evolving regulatory landscape necessitates ongoing compliance efforts from industry stakeholders. Companies must stay informed about regulatory changes, invest in research and development to find safer alternatives or improved handling methods, and implement robust safety management systems to ensure compliance and protect workers and the environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!