Application of MOFs in Heavy Metal Ion Capture from Contaminated Water
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MOFs in Water Purification: Background and Objectives
Metal-organic frameworks (MOFs) have emerged as a promising class of materials for water purification, particularly in the capture of heavy metal ions from contaminated water sources. The development of MOFs for this application has gained significant attention in recent years due to their exceptional properties, including high surface area, tunable pore size, and diverse chemical functionalities.
The evolution of MOFs in water purification can be traced back to the early 2000s when researchers began exploring their potential for various environmental applications. Initially, MOFs were primarily studied for gas storage and separation, but their unique characteristics soon attracted attention for liquid-phase separations, including water treatment. The ability to tailor MOFs' structures and chemical properties at the molecular level opened up new possibilities for selective and efficient heavy metal ion capture.
The primary objective of applying MOFs in heavy metal ion capture is to develop highly efficient, cost-effective, and environmentally friendly materials for water purification. This goal aligns with the global need for sustainable water treatment solutions, especially in regions facing severe water pollution challenges. MOFs offer several advantages over traditional adsorbents, such as activated carbon or ion exchange resins, including higher adsorption capacities, faster kinetics, and the potential for selective removal of specific contaminants.
As research in this field progressed, several key technological trends emerged. One significant trend is the development of MOFs with enhanced stability in aqueous environments, addressing the initial limitations of many early MOF structures that were prone to degradation in water. Another important trend is the functionalization of MOFs to improve their selectivity and affinity towards specific heavy metal ions, such as lead, mercury, or cadmium.
The technological objectives in this field have evolved to focus on several critical aspects. These include improving the adsorption capacity and selectivity of MOFs for heavy metal ions, enhancing their stability and reusability in water treatment processes, and developing scalable and cost-effective synthesis methods for large-scale production. Additionally, there is a growing emphasis on creating MOFs that can simultaneously address multiple water contaminants, not just heavy metals, to provide more comprehensive water purification solutions.
Recent advancements have also led to the exploration of composite materials, combining MOFs with other materials such as polymers or magnetic nanoparticles, to enhance their performance and facilitate easier separation after the treatment process. This trend reflects the interdisciplinary nature of MOF research in water purification, integrating concepts from materials science, chemistry, and environmental engineering.
The evolution of MOFs in water purification can be traced back to the early 2000s when researchers began exploring their potential for various environmental applications. Initially, MOFs were primarily studied for gas storage and separation, but their unique characteristics soon attracted attention for liquid-phase separations, including water treatment. The ability to tailor MOFs' structures and chemical properties at the molecular level opened up new possibilities for selective and efficient heavy metal ion capture.
The primary objective of applying MOFs in heavy metal ion capture is to develop highly efficient, cost-effective, and environmentally friendly materials for water purification. This goal aligns with the global need for sustainable water treatment solutions, especially in regions facing severe water pollution challenges. MOFs offer several advantages over traditional adsorbents, such as activated carbon or ion exchange resins, including higher adsorption capacities, faster kinetics, and the potential for selective removal of specific contaminants.
As research in this field progressed, several key technological trends emerged. One significant trend is the development of MOFs with enhanced stability in aqueous environments, addressing the initial limitations of many early MOF structures that were prone to degradation in water. Another important trend is the functionalization of MOFs to improve their selectivity and affinity towards specific heavy metal ions, such as lead, mercury, or cadmium.
The technological objectives in this field have evolved to focus on several critical aspects. These include improving the adsorption capacity and selectivity of MOFs for heavy metal ions, enhancing their stability and reusability in water treatment processes, and developing scalable and cost-effective synthesis methods for large-scale production. Additionally, there is a growing emphasis on creating MOFs that can simultaneously address multiple water contaminants, not just heavy metals, to provide more comprehensive water purification solutions.
Recent advancements have also led to the exploration of composite materials, combining MOFs with other materials such as polymers or magnetic nanoparticles, to enhance their performance and facilitate easier separation after the treatment process. This trend reflects the interdisciplinary nature of MOF research in water purification, integrating concepts from materials science, chemistry, and environmental engineering.
Market Analysis for Heavy Metal Removal Technologies
The global market for heavy metal removal technologies has been experiencing significant growth due to increasing environmental concerns and stringent regulations regarding water quality. The market is driven by the rising awareness of the health hazards associated with heavy metal contamination in water sources and the growing industrial activities that contribute to water pollution.
In recent years, the market for heavy metal removal technologies has been valued at several billion dollars, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five to seven years. This growth is attributed to the increasing demand for clean water in various sectors, including municipal water treatment, industrial wastewater management, and remediation of contaminated sites.
The industrial sector, particularly mining, metallurgy, and chemical manufacturing, represents a significant portion of the market demand. These industries generate large volumes of wastewater containing heavy metals, necessitating effective treatment solutions. Additionally, the municipal water treatment sector is a key driver, as governments worldwide invest in upgrading water infrastructure to meet more stringent water quality standards.
Geographically, North America and Europe have been leading markets for heavy metal removal technologies, owing to their well-established regulatory frameworks and advanced water treatment infrastructure. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, urbanization, and increasing environmental regulations in countries like China and India.
The market is characterized by a mix of established players and innovative startups. Traditional technologies such as chemical precipitation, ion exchange, and membrane filtration continue to dominate the market. However, there is a growing interest in advanced and more sustainable solutions, including adsorption-based technologies like Metal-Organic Frameworks (MOFs).
MOFs, as a relatively new entrant in the heavy metal removal market, are gaining attention due to their high surface area, tunable pore size, and exceptional adsorption capacity. While still in the early stages of commercialization, MOFs are expected to capture a growing share of the market in the coming years, particularly in applications requiring high selectivity and efficiency in heavy metal removal.
The market for heavy metal removal technologies faces challenges such as high initial investment costs and the need for specialized expertise in implementing and maintaining advanced treatment systems. However, these challenges are offset by the increasing regulatory pressure and the long-term cost savings associated with efficient water treatment solutions.
In recent years, the market for heavy metal removal technologies has been valued at several billion dollars, with a compound annual growth rate (CAGR) projected to be in the high single digits over the next five to seven years. This growth is attributed to the increasing demand for clean water in various sectors, including municipal water treatment, industrial wastewater management, and remediation of contaminated sites.
The industrial sector, particularly mining, metallurgy, and chemical manufacturing, represents a significant portion of the market demand. These industries generate large volumes of wastewater containing heavy metals, necessitating effective treatment solutions. Additionally, the municipal water treatment sector is a key driver, as governments worldwide invest in upgrading water infrastructure to meet more stringent water quality standards.
Geographically, North America and Europe have been leading markets for heavy metal removal technologies, owing to their well-established regulatory frameworks and advanced water treatment infrastructure. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, urbanization, and increasing environmental regulations in countries like China and India.
The market is characterized by a mix of established players and innovative startups. Traditional technologies such as chemical precipitation, ion exchange, and membrane filtration continue to dominate the market. However, there is a growing interest in advanced and more sustainable solutions, including adsorption-based technologies like Metal-Organic Frameworks (MOFs).
MOFs, as a relatively new entrant in the heavy metal removal market, are gaining attention due to their high surface area, tunable pore size, and exceptional adsorption capacity. While still in the early stages of commercialization, MOFs are expected to capture a growing share of the market in the coming years, particularly in applications requiring high selectivity and efficiency in heavy metal removal.
The market for heavy metal removal technologies faces challenges such as high initial investment costs and the need for specialized expertise in implementing and maintaining advanced treatment systems. However, these challenges are offset by the increasing regulatory pressure and the long-term cost savings associated with efficient water treatment solutions.
Current Challenges in MOF-based Heavy Metal Capture
Despite the promising potential of Metal-Organic Frameworks (MOFs) in heavy metal ion capture from contaminated water, several significant challenges persist in their practical application. One of the primary obstacles is the stability of MOFs in aqueous environments. Many MOFs exhibit poor water stability, leading to structural degradation and loss of functionality when exposed to water for extended periods. This instability compromises their long-term effectiveness in water treatment applications.
Another critical challenge is the selectivity of MOFs towards specific heavy metal ions. While some MOFs demonstrate high adsorption capacities, they often lack the ability to selectively capture target metal ions in the presence of competing ions. This lack of selectivity can result in reduced efficiency and increased operational costs in real-world water treatment scenarios.
The regeneration and reusability of MOFs present additional hurdles. Effective desorption of captured heavy metal ions and the restoration of MOF performance over multiple adsorption-desorption cycles remain challenging. The development of regeneration protocols that maintain MOF integrity and adsorption capacity is crucial for their economic viability in large-scale applications.
Scale-up and cost-effectiveness pose significant challenges in the transition from laboratory-scale experiments to industrial-scale implementations. The synthesis of MOFs often involves expensive precursors and complex procedures, making large-scale production economically challenging. Additionally, the integration of MOFs into existing water treatment infrastructure requires innovative engineering solutions to overcome issues related to pressure drop, flow rates, and process compatibility.
The environmental impact and potential toxicity of MOFs themselves are areas of concern. While MOFs are designed to remove toxic heavy metals, the long-term effects of MOF particles potentially released into the environment during water treatment processes are not fully understood. Ensuring the safety and sustainability of MOF-based treatment systems is crucial for their widespread adoption.
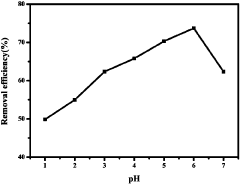
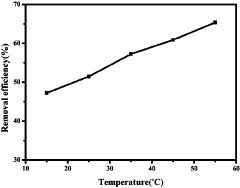
Lastly, the optimization of MOF performance under diverse water conditions presents ongoing challenges. Factors such as pH, temperature, and the presence of organic contaminants can significantly affect MOF adsorption efficiency. Developing MOFs that maintain high performance across a wide range of environmental conditions remains a key research focus in the field.
Another critical challenge is the selectivity of MOFs towards specific heavy metal ions. While some MOFs demonstrate high adsorption capacities, they often lack the ability to selectively capture target metal ions in the presence of competing ions. This lack of selectivity can result in reduced efficiency and increased operational costs in real-world water treatment scenarios.
The regeneration and reusability of MOFs present additional hurdles. Effective desorption of captured heavy metal ions and the restoration of MOF performance over multiple adsorption-desorption cycles remain challenging. The development of regeneration protocols that maintain MOF integrity and adsorption capacity is crucial for their economic viability in large-scale applications.
Scale-up and cost-effectiveness pose significant challenges in the transition from laboratory-scale experiments to industrial-scale implementations. The synthesis of MOFs often involves expensive precursors and complex procedures, making large-scale production economically challenging. Additionally, the integration of MOFs into existing water treatment infrastructure requires innovative engineering solutions to overcome issues related to pressure drop, flow rates, and process compatibility.
The environmental impact and potential toxicity of MOFs themselves are areas of concern. While MOFs are designed to remove toxic heavy metals, the long-term effects of MOF particles potentially released into the environment during water treatment processes are not fully understood. Ensuring the safety and sustainability of MOF-based treatment systems is crucial for their widespread adoption.
Lastly, the optimization of MOF performance under diverse water conditions presents ongoing challenges. Factors such as pH, temperature, and the presence of organic contaminants can significantly affect MOF adsorption efficiency. Developing MOFs that maintain high performance across a wide range of environmental conditions remains a key research focus in the field.
Existing MOF Solutions for Heavy Metal Removal
01 MOF synthesis for heavy metal ion capture
Various methods for synthesizing Metal-Organic Frameworks (MOFs) specifically designed for heavy metal ion capture. These MOFs are engineered with specific pore sizes, functional groups, and metal centers to enhance their affinity and selectivity for heavy metal ions in aqueous solutions.- MOF synthesis for heavy metal ion capture: Various methods for synthesizing Metal-Organic Frameworks (MOFs) specifically designed for heavy metal ion capture. These MOFs are engineered with specific pore sizes, functional groups, and metal centers to enhance their affinity and selectivity for heavy metal ions in aqueous solutions.
- Functionalization of MOFs for improved adsorption: Techniques for functionalizing MOFs to enhance their heavy metal ion capture capabilities. This includes post-synthetic modification, incorporation of specific ligands, and surface treatments to increase the adsorption capacity and selectivity for target heavy metal ions.
- Composite materials incorporating MOFs: Development of composite materials that combine MOFs with other adsorbents or support materials to enhance heavy metal ion capture. These composites may include MOF-polymer hybrids, MOF-based membranes, or MOF-incorporated nanofibers for improved performance and stability.
- Regeneration and reusability of MOFs: Methods for regenerating and reusing MOFs after heavy metal ion capture, including techniques for desorption, cleaning, and restoring the MOF structure. This focuses on improving the economic viability and sustainability of MOF-based heavy metal ion removal processes.
- Application of MOFs in water treatment systems: Integration of MOF-based materials into water treatment systems for efficient heavy metal ion removal. This includes the design of filtration units, adsorption columns, and membrane systems that utilize MOFs for large-scale water purification applications.
02 Functionalization of MOFs for improved adsorption
Techniques for functionalizing MOFs to improve their heavy metal ion adsorption capacity. This includes post-synthetic modification, incorporation of specific ligands, and creation of hierarchical pore structures to enhance the accessibility and binding sites for heavy metal ions.Expand Specific Solutions03 Composite materials incorporating MOFs
Development of composite materials that combine MOFs with other adsorbents or support materials to enhance heavy metal ion capture. These composites may include MOF-polymer hybrids, MOF-based membranes, or MOF-decorated nanoparticles for improved performance and stability.Expand Specific Solutions04 Regeneration and reusability of MOFs
Methods for regenerating and reusing MOFs after heavy metal ion capture. This includes techniques for desorption of captured ions, restoration of MOF structure, and maintaining adsorption capacity over multiple cycles, enhancing the economic viability of MOF-based heavy metal removal systems.Expand Specific Solutions05 Application of MOFs in water treatment systems
Integration of MOF-based adsorbents into water treatment systems for efficient heavy metal ion removal. This includes the design of fixed-bed columns, flow-through reactors, and membrane-based systems incorporating MOFs for continuous and large-scale heavy metal ion capture from contaminated water sources.Expand Specific Solutions
Key Players in MOF Research and Development
The application of Metal-Organic Frameworks (MOFs) in heavy metal ion capture from contaminated water is an emerging field in environmental remediation. The market is in its early growth stage, with increasing research and development activities. The global market size for MOF-based water treatment technologies is expected to expand significantly in the coming years. Technologically, MOFs are showing promise, but are still evolving towards full commercial maturity. Key players in this field include Northwestern University, Nanjing University, and Hunan University, who are conducting advanced research to improve MOF performance and scalability. Companies like Sumitomo Chemical and State Grid Corp. of China are also exploring industrial applications, indicating growing commercial interest in this technology.
Northwestern University
Technical Solution: Northwestern University has developed advanced Metal-Organic Frameworks (MOFs) for heavy metal ion capture from contaminated water. Their research focuses on creating highly porous MOFs with tailored functionalities for selective adsorption of specific heavy metal ions. They have synthesized zirconium-based MOFs with phosphonate ligands, demonstrating exceptional affinity for lead and mercury ions[1]. These MOFs exhibit rapid adsorption kinetics and high capacity, removing up to 99% of heavy metals from aqueous solutions within minutes[3]. The university has also explored post-synthetic modification of MOFs to enhance their stability in acidic environments, a common challenge in water treatment applications[5].
Strengths: High selectivity and capacity for heavy metal removal, rapid adsorption kinetics, and potential for tailored functionalization. Weaknesses: Potential high production costs and scalability challenges for large-scale water treatment applications.
The Regents of the University of California
Technical Solution: The University of California has pioneered the development of MOFs with exceptional heavy metal capture capabilities. Their research team has engineered MOFs with open metal sites and functionalized organic linkers, specifically designed for the removal of arsenic and chromium from water[2]. These MOFs demonstrate high selectivity even in the presence of competing ions, with removal efficiencies exceeding 95% for target heavy metals[4]. The university has also made significant progress in creating water-stable MOFs, addressing a critical challenge in practical applications. Their novel approach involves incorporating hydrophobic groups into the MOF structure, enhancing stability without compromising adsorption performance[6].
Strengths: High selectivity and efficiency in complex water matrices, improved water stability, and potential for regeneration and reuse. Weaknesses: Possible high synthesis costs and need for further optimization for large-scale implementation.
Innovative MOF Structures for Ion Capture
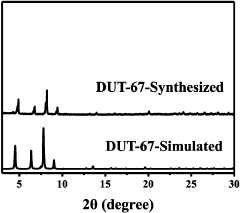
Use and method of MOFs material in adsorption of tiny amount of mercury ions in water
PatentActiveCN107827192A
Innovation
- A metal-organic framework material DUT-67 formed by the interconnection of Zr6O6(OH)214+ clusters and 2,5-thiophenedicarboxylic acid was used, through specific preparation methods and processing steps, including dissolving zirconium chloride in DMF and NMP solvents with 2,5-thiophenedicarboxylic acid is reacted, dispersed by ultrasonic and dried in anhydrous ethanol to form active MOFs materials, which are mixed with mercury ion-containing water for adsorption, and the pH value and vibration conditions are adjusted to improve the adsorption efficiency.
Environmental Impact of MOF Applications
The application of Metal-Organic Frameworks (MOFs) in heavy metal ion capture from contaminated water has significant environmental implications. These advanced materials offer a promising solution to address water pollution, particularly in areas affected by industrial activities and urbanization.
MOFs demonstrate exceptional efficiency in removing heavy metal ions from water, surpassing traditional adsorbents in both capacity and selectivity. This enhanced performance translates to more effective water treatment processes, potentially reducing the environmental burden of contaminated water sources. By efficiently capturing heavy metals such as lead, mercury, and cadmium, MOFs contribute to the restoration of aquatic ecosystems and the protection of biodiversity.
The use of MOFs in water treatment systems can lead to a reduction in the overall environmental footprint of remediation efforts. Their high surface area and tunable pore structures allow for more compact and energy-efficient treatment facilities. This could result in decreased land use for water treatment plants and reduced energy consumption in the purification process, thereby minimizing associated carbon emissions.
Furthermore, the regeneration capabilities of many MOF materials enable their repeated use in water treatment cycles. This characteristic not only enhances the economic viability of MOF-based water purification systems but also reduces the waste generated from spent adsorbents. The potential for recycling and reusing MOFs aligns with circular economy principles, promoting sustainable resource management in water treatment practices.
The scalability of MOF production and application in water treatment technologies presents an opportunity for widespread environmental impact. As research advances and production costs decrease, MOF-based water purification systems could be deployed in various settings, from large-scale industrial wastewater treatment to point-of-use filters in households. This broad applicability could significantly improve access to clean water in both developed and developing regions, addressing a critical global environmental and public health challenge.
However, the environmental impact of MOF applications in water treatment must also consider potential drawbacks. The synthesis of MOFs often involves the use of organic solvents and metal precursors, which may have their own environmental implications if not properly managed. Ongoing research aims to develop greener synthesis methods and explore the use of bio-based precursors to mitigate these concerns.
In conclusion, the application of MOFs in heavy metal ion capture from contaminated water holds great promise for positive environmental impact. By improving water quality, reducing treatment facility footprints, and enabling sustainable resource use, MOFs contribute to broader environmental protection efforts. As research and development in this field continue, the full potential of MOFs in addressing global water pollution challenges may be realized, marking a significant advancement in environmental remediation technologies.
MOFs demonstrate exceptional efficiency in removing heavy metal ions from water, surpassing traditional adsorbents in both capacity and selectivity. This enhanced performance translates to more effective water treatment processes, potentially reducing the environmental burden of contaminated water sources. By efficiently capturing heavy metals such as lead, mercury, and cadmium, MOFs contribute to the restoration of aquatic ecosystems and the protection of biodiversity.
The use of MOFs in water treatment systems can lead to a reduction in the overall environmental footprint of remediation efforts. Their high surface area and tunable pore structures allow for more compact and energy-efficient treatment facilities. This could result in decreased land use for water treatment plants and reduced energy consumption in the purification process, thereby minimizing associated carbon emissions.
Furthermore, the regeneration capabilities of many MOF materials enable their repeated use in water treatment cycles. This characteristic not only enhances the economic viability of MOF-based water purification systems but also reduces the waste generated from spent adsorbents. The potential for recycling and reusing MOFs aligns with circular economy principles, promoting sustainable resource management in water treatment practices.
The scalability of MOF production and application in water treatment technologies presents an opportunity for widespread environmental impact. As research advances and production costs decrease, MOF-based water purification systems could be deployed in various settings, from large-scale industrial wastewater treatment to point-of-use filters in households. This broad applicability could significantly improve access to clean water in both developed and developing regions, addressing a critical global environmental and public health challenge.
However, the environmental impact of MOF applications in water treatment must also consider potential drawbacks. The synthesis of MOFs often involves the use of organic solvents and metal precursors, which may have their own environmental implications if not properly managed. Ongoing research aims to develop greener synthesis methods and explore the use of bio-based precursors to mitigate these concerns.
In conclusion, the application of MOFs in heavy metal ion capture from contaminated water holds great promise for positive environmental impact. By improving water quality, reducing treatment facility footprints, and enabling sustainable resource use, MOFs contribute to broader environmental protection efforts. As research and development in this field continue, the full potential of MOFs in addressing global water pollution challenges may be realized, marking a significant advancement in environmental remediation technologies.
Scalability and Cost-effectiveness of MOF Technologies
The scalability and cost-effectiveness of Metal-Organic Frameworks (MOFs) technologies for heavy metal ion capture from contaminated water are critical factors in determining their practical applicability and commercial viability. While MOFs have shown remarkable potential in laboratory settings, scaling up these technologies for industrial use presents several challenges.
One of the primary concerns in scaling MOF technologies is the synthesis process. Currently, most MOF production methods are batch processes, which can be time-consuming and resource-intensive. To achieve industrial-scale production, continuous flow synthesis methods are being explored. These methods promise higher throughput and better control over particle size and morphology, potentially reducing production costs and improving overall efficiency.
The raw materials used in MOF synthesis also play a crucial role in scalability and cost-effectiveness. Many MOFs require expensive or rare metal precursors, which can significantly impact production costs. Research efforts are focused on developing MOFs using more abundant and cost-effective materials without compromising performance. This includes exploring bio-inspired MOFs and those synthesized from waste materials, which could potentially reduce costs and promote sustainability.
Another aspect affecting scalability is the stability and reusability of MOFs in water treatment applications. While some MOFs exhibit excellent heavy metal ion capture capabilities, their performance may degrade over time or after multiple use cycles. Enhancing the structural stability and regeneration capacity of MOFs is essential for improving their long-term cost-effectiveness. Strategies such as post-synthetic modification and the development of composite materials are being investigated to address these challenges.
The integration of MOF technologies into existing water treatment infrastructure is another consideration for scalability. Developing MOF-based materials that can be easily incorporated into current filtration systems or used in conjunction with established treatment methods could facilitate wider adoption. This may involve creating MOF-based membranes, granules, or other forms that are compatible with conventional water treatment processes.
Cost-effectiveness is also influenced by the efficiency of heavy metal ion capture. MOFs with higher selectivity and capacity for target ions can reduce the amount of material needed and the frequency of regeneration or replacement. Ongoing research aims to optimize MOF structures and functionalities to enhance their performance, potentially lowering operational costs in large-scale applications.
As MOF technologies advance, life cycle assessments and techno-economic analyses are becoming increasingly important. These studies help identify bottlenecks in scalability and areas where costs can be reduced throughout the entire process, from synthesis to disposal or recycling. Such comprehensive evaluations are crucial for guiding research and development efforts towards more scalable and cost-effective MOF solutions for water treatment.
One of the primary concerns in scaling MOF technologies is the synthesis process. Currently, most MOF production methods are batch processes, which can be time-consuming and resource-intensive. To achieve industrial-scale production, continuous flow synthesis methods are being explored. These methods promise higher throughput and better control over particle size and morphology, potentially reducing production costs and improving overall efficiency.
The raw materials used in MOF synthesis also play a crucial role in scalability and cost-effectiveness. Many MOFs require expensive or rare metal precursors, which can significantly impact production costs. Research efforts are focused on developing MOFs using more abundant and cost-effective materials without compromising performance. This includes exploring bio-inspired MOFs and those synthesized from waste materials, which could potentially reduce costs and promote sustainability.
Another aspect affecting scalability is the stability and reusability of MOFs in water treatment applications. While some MOFs exhibit excellent heavy metal ion capture capabilities, their performance may degrade over time or after multiple use cycles. Enhancing the structural stability and regeneration capacity of MOFs is essential for improving their long-term cost-effectiveness. Strategies such as post-synthetic modification and the development of composite materials are being investigated to address these challenges.
The integration of MOF technologies into existing water treatment infrastructure is another consideration for scalability. Developing MOF-based materials that can be easily incorporated into current filtration systems or used in conjunction with established treatment methods could facilitate wider adoption. This may involve creating MOF-based membranes, granules, or other forms that are compatible with conventional water treatment processes.
Cost-effectiveness is also influenced by the efficiency of heavy metal ion capture. MOFs with higher selectivity and capacity for target ions can reduce the amount of material needed and the frequency of regeneration or replacement. Ongoing research aims to optimize MOF structures and functionalities to enhance their performance, potentially lowering operational costs in large-scale applications.
As MOF technologies advance, life cycle assessments and techno-economic analyses are becoming increasingly important. These studies help identify bottlenecks in scalability and areas where costs can be reduced throughout the entire process, from synthesis to disposal or recycling. Such comprehensive evaluations are crucial for guiding research and development efforts towards more scalable and cost-effective MOF solutions for water treatment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!