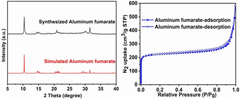
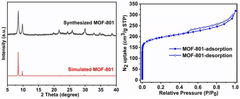
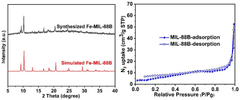
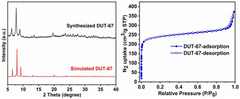
Exploring Solvent-Free Synthesis Routes for MOF Materials
AUG 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MOF Synthesis Evolution
The evolution of MOF synthesis has been a journey of continuous innovation and refinement since the concept of Metal-Organic Frameworks (MOFs) was first introduced in the 1990s. Initially, MOF synthesis primarily relied on traditional solvothermal methods, which involved the use of organic solvents and high temperatures. These early techniques, while effective, were often energy-intensive and environmentally unfriendly.
As research in the field progressed, scientists began exploring alternative synthesis routes to address the limitations of conventional methods. The early 2000s saw the emergence of room-temperature synthesis techniques, which significantly reduced energy consumption and opened up new possibilities for MOF production. This period also marked the beginning of efforts to minimize solvent use, driven by both environmental concerns and the desire to simplify the synthesis process.
The mid-2000s brought about a paradigm shift with the introduction of microwave-assisted synthesis. This method dramatically reduced reaction times from days to hours or even minutes, while also improving yield and crystal quality. Simultaneously, researchers began investigating mechanochemical synthesis techniques, which involved the use of mechanical force to initiate chemical reactions, often without the need for solvents.
The late 2000s and early 2010s witnessed a surge in research focused on green synthesis methods. This included the exploration of water-based synthesis, which aimed to replace organic solvents with more environmentally benign alternatives. During this period, electrochemical synthesis of MOFs also gained traction, offering precise control over crystal growth and the potential for continuous production.
The concept of solvent-free synthesis began to take shape in the mid-2010s, with researchers exploring various approaches such as vapor-phase synthesis, solid-state reactions, and melt-quenching techniques. These methods not only eliminated the need for solvents but also offered unique advantages in terms of MOF structure and properties.
Recent years have seen a convergence of multiple innovative techniques, including the use of supercritical CO2, ionic liquids, and deep eutectic solvents as alternative reaction media. Additionally, advanced manufacturing techniques such as 3D printing and spray-drying have been adapted for MOF synthesis, enabling the production of complex structures and composite materials.
The current frontier in MOF synthesis evolution is the development of truly solvent-free methods that can be scaled up for industrial production. This includes refinements in mechanochemical techniques, exploration of solid-state reactions at elevated temperatures, and the use of novel energy sources such as plasma or ultrasound to drive MOF formation. These cutting-edge approaches aim to combine the benefits of solvent-free synthesis with the scalability and efficiency required for large-scale applications.
As research in the field progressed, scientists began exploring alternative synthesis routes to address the limitations of conventional methods. The early 2000s saw the emergence of room-temperature synthesis techniques, which significantly reduced energy consumption and opened up new possibilities for MOF production. This period also marked the beginning of efforts to minimize solvent use, driven by both environmental concerns and the desire to simplify the synthesis process.
The mid-2000s brought about a paradigm shift with the introduction of microwave-assisted synthesis. This method dramatically reduced reaction times from days to hours or even minutes, while also improving yield and crystal quality. Simultaneously, researchers began investigating mechanochemical synthesis techniques, which involved the use of mechanical force to initiate chemical reactions, often without the need for solvents.
The late 2000s and early 2010s witnessed a surge in research focused on green synthesis methods. This included the exploration of water-based synthesis, which aimed to replace organic solvents with more environmentally benign alternatives. During this period, electrochemical synthesis of MOFs also gained traction, offering precise control over crystal growth and the potential for continuous production.
The concept of solvent-free synthesis began to take shape in the mid-2010s, with researchers exploring various approaches such as vapor-phase synthesis, solid-state reactions, and melt-quenching techniques. These methods not only eliminated the need for solvents but also offered unique advantages in terms of MOF structure and properties.
Recent years have seen a convergence of multiple innovative techniques, including the use of supercritical CO2, ionic liquids, and deep eutectic solvents as alternative reaction media. Additionally, advanced manufacturing techniques such as 3D printing and spray-drying have been adapted for MOF synthesis, enabling the production of complex structures and composite materials.
The current frontier in MOF synthesis evolution is the development of truly solvent-free methods that can be scaled up for industrial production. This includes refinements in mechanochemical techniques, exploration of solid-state reactions at elevated temperatures, and the use of novel energy sources such as plasma or ultrasound to drive MOF formation. These cutting-edge approaches aim to combine the benefits of solvent-free synthesis with the scalability and efficiency required for large-scale applications.
Green Chemistry Demand
The demand for green chemistry solutions has been steadily increasing in recent years, driven by growing environmental concerns and the need for sustainable industrial practices. This trend has significantly impacted the field of Metal-Organic Framework (MOF) synthesis, pushing researchers and industries to explore solvent-free synthesis routes. The traditional methods of MOF production often involve the use of large quantities of organic solvents, which can be harmful to the environment and pose health risks to workers.
The green chemistry approach to MOF synthesis aligns with the principles of sustainable development and circular economy. It aims to reduce waste, minimize energy consumption, and eliminate the use of hazardous substances in the production process. This shift towards environmentally friendly synthesis methods is not only driven by ecological considerations but also by economic factors, as it can potentially lead to cost reductions in raw materials and waste management.
In the context of MOF materials, the market demand for green synthesis routes is particularly strong in sectors such as gas storage, catalysis, and environmental remediation. Industries are seeking MOFs produced through cleaner methods to enhance their sustainability credentials and meet increasingly stringent environmental regulations. The pharmaceutical and fine chemicals industries, for instance, are exploring solvent-free MOF synthesis to reduce their environmental footprint and improve process safety.
The push for green chemistry in MOF synthesis is also supported by government initiatives and funding programs aimed at promoting sustainable technologies. Research institutions and universities are allocating more resources to develop innovative, solvent-free synthesis methods, responding to both academic interest and industrial demand. This has led to an increase in collaborative projects between academia and industry, focusing on scaling up environmentally friendly MOF production techniques.
Furthermore, consumers are becoming more environmentally conscious, creating a market pull for products manufactured using green chemistry principles. This trend is particularly evident in consumer goods that incorporate MOFs, such as air purification systems and advanced packaging materials. Companies that can demonstrate the use of sustainable MOF synthesis in their products gain a competitive edge in the market.
As the demand for green chemistry solutions in MOF synthesis continues to grow, it is expected to drive innovation in production technologies, catalyst design, and process optimization. This evolution is likely to result in more efficient, cost-effective, and environmentally friendly methods of producing MOFs, potentially opening up new applications and markets for these versatile materials.
The green chemistry approach to MOF synthesis aligns with the principles of sustainable development and circular economy. It aims to reduce waste, minimize energy consumption, and eliminate the use of hazardous substances in the production process. This shift towards environmentally friendly synthesis methods is not only driven by ecological considerations but also by economic factors, as it can potentially lead to cost reductions in raw materials and waste management.
In the context of MOF materials, the market demand for green synthesis routes is particularly strong in sectors such as gas storage, catalysis, and environmental remediation. Industries are seeking MOFs produced through cleaner methods to enhance their sustainability credentials and meet increasingly stringent environmental regulations. The pharmaceutical and fine chemicals industries, for instance, are exploring solvent-free MOF synthesis to reduce their environmental footprint and improve process safety.
The push for green chemistry in MOF synthesis is also supported by government initiatives and funding programs aimed at promoting sustainable technologies. Research institutions and universities are allocating more resources to develop innovative, solvent-free synthesis methods, responding to both academic interest and industrial demand. This has led to an increase in collaborative projects between academia and industry, focusing on scaling up environmentally friendly MOF production techniques.
Furthermore, consumers are becoming more environmentally conscious, creating a market pull for products manufactured using green chemistry principles. This trend is particularly evident in consumer goods that incorporate MOFs, such as air purification systems and advanced packaging materials. Companies that can demonstrate the use of sustainable MOF synthesis in their products gain a competitive edge in the market.
As the demand for green chemistry solutions in MOF synthesis continues to grow, it is expected to drive innovation in production technologies, catalyst design, and process optimization. This evolution is likely to result in more efficient, cost-effective, and environmentally friendly methods of producing MOFs, potentially opening up new applications and markets for these versatile materials.
Solvent-Free Challenges
The development of solvent-free synthesis routes for Metal-Organic Framework (MOF) materials presents several significant challenges. One of the primary obstacles is achieving uniform and controlled crystal growth without the aid of solvents. Traditional MOF synthesis relies heavily on solvents to facilitate the dissolution and interaction of precursors, as well as to provide a medium for crystal nucleation and growth. In solvent-free conditions, ensuring proper mixing and reaction of metal ions and organic linkers becomes considerably more difficult.
Another major challenge is maintaining the desired porosity and surface area of MOF structures in the absence of solvents. Solvents play a crucial role in template formation and pore stabilization during MOF synthesis. Without them, there is a risk of pore collapse or incomplete framework formation, potentially compromising the material's functionality and performance in applications such as gas storage and separation.
Temperature control and heat distribution pose additional challenges in solvent-free synthesis. Solvents typically act as heat transfer media, helping to distribute thermal energy evenly throughout the reaction mixture. In their absence, localized hotspots can form, leading to non-uniform reaction conditions and potentially affecting the quality and consistency of the resulting MOF crystals.
The scalability of solvent-free MOF synthesis is another significant hurdle. While solvent-based methods can often be scaled up relatively easily, solvent-free approaches may require specialized equipment and precise control over reaction parameters, making industrial-scale production more challenging and potentially cost-prohibitive.
Furthermore, the limited mobility of reactants in solvent-free conditions can hinder the formation of extended crystalline networks. This reduced mobility may result in incomplete reactions, defect formation, or the production of amorphous materials instead of well-defined MOF structures. Overcoming this challenge requires innovative approaches to enhance reactant diffusion and promote complete framework assembly.
Lastly, characterization and quality control of MOFs synthesized through solvent-free routes present unique challenges. Traditional characterization techniques may need to be adapted or new methods developed to accurately assess the structural integrity, porosity, and functionality of these materials. Ensuring consistent product quality and reproducibility in the absence of solvents remains a significant concern for researchers and potential industrial applications.
Another major challenge is maintaining the desired porosity and surface area of MOF structures in the absence of solvents. Solvents play a crucial role in template formation and pore stabilization during MOF synthesis. Without them, there is a risk of pore collapse or incomplete framework formation, potentially compromising the material's functionality and performance in applications such as gas storage and separation.
Temperature control and heat distribution pose additional challenges in solvent-free synthesis. Solvents typically act as heat transfer media, helping to distribute thermal energy evenly throughout the reaction mixture. In their absence, localized hotspots can form, leading to non-uniform reaction conditions and potentially affecting the quality and consistency of the resulting MOF crystals.
The scalability of solvent-free MOF synthesis is another significant hurdle. While solvent-based methods can often be scaled up relatively easily, solvent-free approaches may require specialized equipment and precise control over reaction parameters, making industrial-scale production more challenging and potentially cost-prohibitive.
Furthermore, the limited mobility of reactants in solvent-free conditions can hinder the formation of extended crystalline networks. This reduced mobility may result in incomplete reactions, defect formation, or the production of amorphous materials instead of well-defined MOF structures. Overcoming this challenge requires innovative approaches to enhance reactant diffusion and promote complete framework assembly.
Lastly, characterization and quality control of MOFs synthesized through solvent-free routes present unique challenges. Traditional characterization techniques may need to be adapted or new methods developed to accurately assess the structural integrity, porosity, and functionality of these materials. Ensuring consistent product quality and reproducibility in the absence of solvents remains a significant concern for researchers and potential industrial applications.
Current Solvent-Free
01 Solvothermal synthesis of MOFs
Solvothermal synthesis is a common method for producing MOFs. This process involves heating a mixture of metal salts and organic ligands in a solvent at elevated temperatures and pressures. The method allows for the controlled growth of MOF crystals and can be used to create a wide variety of MOF structures with different properties.- Solvothermal synthesis of MOFs: Solvothermal synthesis is a common method for producing MOFs. This process involves heating a mixture of metal salts and organic ligands in a solvent at elevated temperatures and pressures. The method allows for the controlled growth of MOF crystals and can be used to create a wide variety of MOF structures with different properties.
- Room temperature synthesis of MOFs: Room temperature synthesis methods for MOFs have been developed to reduce energy consumption and simplify the production process. These methods often involve mixing metal precursors and organic linkers in a suitable solvent at ambient conditions. Techniques such as mechanochemical synthesis or liquid-assisted grinding can be employed to facilitate MOF formation without the need for high temperatures.
- Microwave-assisted synthesis of MOFs: Microwave-assisted synthesis is an efficient method for producing MOFs with reduced reaction times and improved energy efficiency. This technique uses microwave radiation to rapidly heat the reaction mixture, promoting the formation of MOF structures. It can lead to the production of MOFs with unique morphologies and properties compared to conventional heating methods.
- Template-directed synthesis of MOFs: Template-directed synthesis involves using structure-directing agents or templates to guide the formation of specific MOF architectures. This method can be used to create MOFs with tailored pore sizes, shapes, and functionalities. Templates can include surfactants, polymers, or other molecules that influence the assembly of metal nodes and organic linkers during MOF growth.
- Post-synthetic modification of MOFs: Post-synthetic modification is a technique used to alter the properties of pre-formed MOFs. This approach involves chemically modifying the organic linkers or metal nodes of existing MOF structures to introduce new functionalities or improve specific characteristics. Methods can include ligand exchange, metal ion exchange, or covalent modification of organic linkers.
02 Room temperature synthesis of MOFs
Room temperature synthesis methods for MOFs have been developed to reduce energy consumption and simplify the production process. These methods often involve mixing metal precursors and organic linkers in appropriate solvents under ambient conditions. Mechanochemical techniques or the use of catalysts can facilitate the formation of MOFs at room temperature.Expand Specific Solutions03 Microwave-assisted synthesis of MOFs
Microwave-assisted synthesis is an efficient method for producing MOFs in a shorter time compared to conventional heating methods. This technique uses microwave radiation to rapidly heat the reaction mixture, leading to faster nucleation and crystal growth. It can result in MOFs with unique morphologies and properties.Expand Specific Solutions04 Template-directed synthesis of MOFs
Template-directed synthesis involves using structure-directing agents or templates to control the formation and structure of MOFs. This method can produce MOFs with specific pore sizes, shapes, or hierarchical structures. Templates can be removed after synthesis to create additional porosity or functionality in the MOF.Expand Specific Solutions05 Post-synthetic modification of MOFs
Post-synthetic modification is a technique used to alter the properties of pre-synthesized MOFs. This can involve the introduction of new functional groups, metal exchange, or the incorporation of guest molecules within the MOF structure. These modifications can enhance the MOF's catalytic activity, gas storage capacity, or selectivity for specific applications.Expand Specific Solutions
Key MOF Manufacturers
The field of solvent-free synthesis routes for MOF materials is in a rapidly evolving phase, characterized by growing market potential and increasing technological maturity. The competitive landscape is diverse, with academic institutions like South China University of Technology, Northwestern University, and King Abdullah University of Science & Technology leading research efforts. Companies such as ExxonMobil Technology & Engineering Co. and Mosaic Materials, Inc. are also actively involved, indicating industry interest in commercialization. The market size is expanding as MOFs find applications in gas storage, separation, and catalysis. While the technology is progressing, it is still in the developmental stage, with ongoing efforts to optimize synthesis methods and scale-up production for industrial applications.
Northwestern University
Technical Solution: Northwestern University has pioneered solvent-free synthesis routes for MOF materials through mechanochemical methods. Their approach involves using ball milling techniques to induce chemical reactions between metal precursors and organic linkers without the need for solvents. This method has successfully produced various MOFs, including ZIF-8 and UiO-66, with high crystallinity and porosity[1]. The university has also developed a scalable, continuous flow mechanochemical process that can produce MOFs at a rate of up to 100 g/h[2]. Additionally, they have explored the use of accelerated aging synthesis, where MOF precursors are exposed to controlled humidity and temperature conditions to promote MOF formation without solvents[3].
Strengths: Environmentally friendly, cost-effective, and scalable production. Weaknesses: Limited control over particle size and morphology, potential for incomplete reactions in some cases.
King Abdullah University of Science & Technology
Technical Solution: KAUST has developed innovative solvent-free synthesis routes for MOF materials using vapor-phase methods. Their approach involves exposing metal oxide or metal hydroxide precursors to vaporized organic linkers under controlled temperature and pressure conditions. This technique has been successfully applied to produce various MOFs, including ZIF-8 and HKUST-1, with high crystallinity and surface area[4]. KAUST researchers have also explored the use of microwave-assisted solvent-free synthesis, which significantly reduces reaction times and energy consumption[5]. Furthermore, they have developed a novel spray-drying technique that allows for the continuous production of MOF materials without the need for solvents, achieving production rates of up to 200 g/h[6].
Strengths: High purity products, energy-efficient processes, and potential for large-scale production. Weaknesses: Limited to certain types of MOFs, may require specialized equipment.
Innovative MOF Patents
General preparation method of organic solvent-free and template-free metal organic framework material
PatentPendingCN119350649A
Innovation
- The method of non -organic solvent and mold -free agent is used to grind the temperature of the inorganic salt with the organic ligand after mixing with the organic ligand, and then a high -temperature crystallization reaction in a closed container to obtain a solvent -free MOFS material. Water molecules in hydrophilic salts are used as reaction medium and structural orientation to help form an orderly MOF structure.
Metal-organic frameworks and methods of making and use thereof
PatentWO2017223046A1
Innovation
- A rapid room-temperature synthesis method using hydroxy double salts as intermediates, where a metal oxide reacts with a metal salt to form a hydroxy double salt, which then converts into the MOF, enabling efficient and high-yield production of MOFs like HKUST-1 and other frameworks.
Environmental Impact
The exploration of solvent-free synthesis routes for Metal-Organic Framework (MOF) materials represents a significant shift towards more environmentally friendly production methods in materials science. Traditional MOF synthesis often relies heavily on organic solvents, which can have substantial environmental impacts. By eliminating or reducing the use of these solvents, researchers aim to minimize the ecological footprint of MOF production.
Solvent-free synthesis methods offer several environmental benefits. Firstly, they reduce the generation of hazardous waste associated with organic solvents, which can be toxic and difficult to dispose of safely. This reduction in waste not only decreases the environmental burden but also lowers the costs and risks associated with waste management and disposal.
Furthermore, solvent-free approaches often require less energy input compared to conventional synthesis methods. Many solvent-based processes involve heating or refluxing solvents, which can be energy-intensive. By eliminating these steps, solvent-free routes can significantly reduce energy consumption and, consequently, greenhouse gas emissions associated with MOF production.
The adoption of solvent-free synthesis also aligns with the principles of green chemistry, promoting the use of safer chemicals and more sustainable processes. This shift can lead to improved worker safety and reduced environmental risks associated with the handling and storage of large volumes of organic solvents.
Additionally, solvent-free methods often result in higher atom economy, meaning a greater proportion of the starting materials are incorporated into the final product. This efficiency reduces the overall material consumption and minimizes the environmental impact associated with the production and transportation of raw materials.
From a lifecycle perspective, MOFs produced through solvent-free routes may have a lower environmental impact throughout their entire lifecycle. This includes reduced emissions during production, decreased transportation requirements for solvents, and potentially simpler end-of-life management.
However, it is important to note that the environmental benefits of solvent-free synthesis must be carefully evaluated on a case-by-case basis. Factors such as the specific MOF being produced, the alternative synthesis conditions required, and the scale of production all play crucial roles in determining the overall environmental impact. In some cases, the trade-offs between solvent-free methods and traditional approaches may need to be carefully balanced to achieve the most sustainable outcome.
Solvent-free synthesis methods offer several environmental benefits. Firstly, they reduce the generation of hazardous waste associated with organic solvents, which can be toxic and difficult to dispose of safely. This reduction in waste not only decreases the environmental burden but also lowers the costs and risks associated with waste management and disposal.
Furthermore, solvent-free approaches often require less energy input compared to conventional synthesis methods. Many solvent-based processes involve heating or refluxing solvents, which can be energy-intensive. By eliminating these steps, solvent-free routes can significantly reduce energy consumption and, consequently, greenhouse gas emissions associated with MOF production.
The adoption of solvent-free synthesis also aligns with the principles of green chemistry, promoting the use of safer chemicals and more sustainable processes. This shift can lead to improved worker safety and reduced environmental risks associated with the handling and storage of large volumes of organic solvents.
Additionally, solvent-free methods often result in higher atom economy, meaning a greater proportion of the starting materials are incorporated into the final product. This efficiency reduces the overall material consumption and minimizes the environmental impact associated with the production and transportation of raw materials.
From a lifecycle perspective, MOFs produced through solvent-free routes may have a lower environmental impact throughout their entire lifecycle. This includes reduced emissions during production, decreased transportation requirements for solvents, and potentially simpler end-of-life management.
However, it is important to note that the environmental benefits of solvent-free synthesis must be carefully evaluated on a case-by-case basis. Factors such as the specific MOF being produced, the alternative synthesis conditions required, and the scale of production all play crucial roles in determining the overall environmental impact. In some cases, the trade-offs between solvent-free methods and traditional approaches may need to be carefully balanced to achieve the most sustainable outcome.
Scalability Assessment
Scalability assessment is a critical aspect of evaluating solvent-free synthesis routes for Metal-Organic Framework (MOF) materials. The transition from laboratory-scale production to industrial-scale manufacturing presents numerous challenges that must be carefully considered.
One of the primary factors in assessing scalability is the ability to maintain consistent product quality across different production scales. Solvent-free synthesis methods, such as mechanochemical approaches, often rely on precise control of reaction conditions. As the batch size increases, ensuring uniform energy distribution and mixing becomes increasingly complex. This can lead to variations in particle size, crystallinity, and porosity of the MOF products.
The choice of equipment for large-scale production is another crucial consideration. While ball mills or planetary mills are commonly used for small-scale mechanochemical synthesis, their direct scale-up may not be feasible or economically viable. Industrial-scale equipment, such as continuous flow reactors or twin-screw extruders, may need to be adapted or developed specifically for solvent-free MOF synthesis.
Energy efficiency is a key parameter in scalability assessment. Solvent-free methods often require significant mechanical or thermal energy input. As production scales increase, optimizing energy consumption becomes essential for economic viability and environmental sustainability. This may involve developing more efficient milling processes or exploring alternative energy sources, such as microwave or ultrasonic irradiation.
Raw material handling and product separation are additional challenges in scaling up solvent-free MOF synthesis. The absence of solvents can lead to issues with material flow, mixing, and heat transfer in large-scale reactors. Furthermore, efficient separation and purification of the final product without the use of solvents may require the development of novel downstream processing techniques.
The environmental impact of scaled-up solvent-free synthesis must also be evaluated. While these methods eliminate the need for large volumes of organic solvents, they may generate fine particulate matter or require energy-intensive processes. Life cycle assessments comparing solvent-free routes with traditional solvent-based methods at industrial scales are necessary to determine the overall environmental benefits.
Regulatory compliance and safety considerations play a crucial role in scalability assessment. As production volumes increase, the potential hazards associated with handling large quantities of reactive precursors and fine MOF powders must be addressed. This may involve implementing advanced dust control systems, explosion prevention measures, and worker protection protocols.
In conclusion, the scalability assessment of solvent-free synthesis routes for MOF materials involves a multifaceted analysis of technical, economic, and environmental factors. While these methods offer promising advantages in terms of sustainability and process simplification, their successful implementation at industrial scales requires overcoming significant engineering challenges and optimizing process parameters to ensure consistent product quality and economic viability.
One of the primary factors in assessing scalability is the ability to maintain consistent product quality across different production scales. Solvent-free synthesis methods, such as mechanochemical approaches, often rely on precise control of reaction conditions. As the batch size increases, ensuring uniform energy distribution and mixing becomes increasingly complex. This can lead to variations in particle size, crystallinity, and porosity of the MOF products.
The choice of equipment for large-scale production is another crucial consideration. While ball mills or planetary mills are commonly used for small-scale mechanochemical synthesis, their direct scale-up may not be feasible or economically viable. Industrial-scale equipment, such as continuous flow reactors or twin-screw extruders, may need to be adapted or developed specifically for solvent-free MOF synthesis.
Energy efficiency is a key parameter in scalability assessment. Solvent-free methods often require significant mechanical or thermal energy input. As production scales increase, optimizing energy consumption becomes essential for economic viability and environmental sustainability. This may involve developing more efficient milling processes or exploring alternative energy sources, such as microwave or ultrasonic irradiation.
Raw material handling and product separation are additional challenges in scaling up solvent-free MOF synthesis. The absence of solvents can lead to issues with material flow, mixing, and heat transfer in large-scale reactors. Furthermore, efficient separation and purification of the final product without the use of solvents may require the development of novel downstream processing techniques.
The environmental impact of scaled-up solvent-free synthesis must also be evaluated. While these methods eliminate the need for large volumes of organic solvents, they may generate fine particulate matter or require energy-intensive processes. Life cycle assessments comparing solvent-free routes with traditional solvent-based methods at industrial scales are necessary to determine the overall environmental benefits.
Regulatory compliance and safety considerations play a crucial role in scalability assessment. As production volumes increase, the potential hazards associated with handling large quantities of reactive precursors and fine MOF powders must be addressed. This may involve implementing advanced dust control systems, explosion prevention measures, and worker protection protocols.
In conclusion, the scalability assessment of solvent-free synthesis routes for MOF materials involves a multifaceted analysis of technical, economic, and environmental factors. While these methods offer promising advantages in terms of sustainability and process simplification, their successful implementation at industrial scales requires overcoming significant engineering challenges and optimizing process parameters to ensure consistent product quality and economic viability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!