Assessing Ammonium Hydroxide in Biochemical Engineering Applications
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ammonium Hydroxide Background and Objectives
Ammonium hydroxide, a versatile compound with the chemical formula NH4OH, has played a significant role in biochemical engineering applications for decades. This aqueous solution of ammonia has been widely utilized across various industries due to its unique properties and reactivity. The historical development of ammonium hydroxide can be traced back to the early 19th century when it was first synthesized and characterized.
The evolution of ammonium hydroxide in biochemical engineering has been marked by continuous advancements in production methods, purification techniques, and application strategies. Initially used primarily in industrial cleaning and as a precursor in chemical synthesis, its potential in biochemical processes gradually became apparent. The compound's ability to regulate pH, serve as a nitrogen source, and participate in various biochemical reactions has made it an invaluable tool in the field.
In recent years, the focus on sustainable and environmentally friendly processes has led to increased interest in ammonium hydroxide as a more benign alternative to other chemical agents. This shift has driven research into optimizing its use in bioprocessing, waste treatment, and green chemistry applications. The compound's role in protein purification, enzyme stabilization, and microbial growth media formulation has also expanded, highlighting its versatility in biochemical engineering.
The objectives of assessing ammonium hydroxide in biochemical engineering applications are multifaceted. Primarily, there is a need to comprehensively evaluate its efficacy and safety across different bioprocesses. This includes investigating its impact on reaction kinetics, product yields, and overall process efficiency. Additionally, researchers aim to explore novel applications that leverage the unique properties of ammonium hydroxide, potentially leading to innovative bioengineering solutions.
Another critical objective is to address the environmental and safety concerns associated with ammonium hydroxide use. This involves developing improved handling protocols, assessing its ecological impact, and exploring methods to minimize waste and emissions. Furthermore, there is a growing interest in understanding the molecular mechanisms by which ammonium hydroxide interacts with biological systems, which could pave the way for more targeted and efficient applications.
As the field of biochemical engineering continues to evolve, the assessment of ammonium hydroxide also aims to integrate it with emerging technologies. This includes its potential role in bioelectrochemical systems, advanced bioremediation processes, and the development of smart materials for bioengineering applications. By thoroughly examining these aspects, researchers and industry professionals seek to unlock the full potential of ammonium hydroxide in advancing biochemical engineering practices and contributing to more sustainable and efficient bioprocesses.
The evolution of ammonium hydroxide in biochemical engineering has been marked by continuous advancements in production methods, purification techniques, and application strategies. Initially used primarily in industrial cleaning and as a precursor in chemical synthesis, its potential in biochemical processes gradually became apparent. The compound's ability to regulate pH, serve as a nitrogen source, and participate in various biochemical reactions has made it an invaluable tool in the field.
In recent years, the focus on sustainable and environmentally friendly processes has led to increased interest in ammonium hydroxide as a more benign alternative to other chemical agents. This shift has driven research into optimizing its use in bioprocessing, waste treatment, and green chemistry applications. The compound's role in protein purification, enzyme stabilization, and microbial growth media formulation has also expanded, highlighting its versatility in biochemical engineering.
The objectives of assessing ammonium hydroxide in biochemical engineering applications are multifaceted. Primarily, there is a need to comprehensively evaluate its efficacy and safety across different bioprocesses. This includes investigating its impact on reaction kinetics, product yields, and overall process efficiency. Additionally, researchers aim to explore novel applications that leverage the unique properties of ammonium hydroxide, potentially leading to innovative bioengineering solutions.
Another critical objective is to address the environmental and safety concerns associated with ammonium hydroxide use. This involves developing improved handling protocols, assessing its ecological impact, and exploring methods to minimize waste and emissions. Furthermore, there is a growing interest in understanding the molecular mechanisms by which ammonium hydroxide interacts with biological systems, which could pave the way for more targeted and efficient applications.
As the field of biochemical engineering continues to evolve, the assessment of ammonium hydroxide also aims to integrate it with emerging technologies. This includes its potential role in bioelectrochemical systems, advanced bioremediation processes, and the development of smart materials for bioengineering applications. By thoroughly examining these aspects, researchers and industry professionals seek to unlock the full potential of ammonium hydroxide in advancing biochemical engineering practices and contributing to more sustainable and efficient bioprocesses.
Market Analysis for Biochemical Applications
The market for ammonium hydroxide in biochemical engineering applications has been experiencing steady growth, driven by its versatile properties and increasing demand across various industries. As a key component in many biochemical processes, ammonium hydroxide plays a crucial role in pH adjustment, protein purification, and as a nitrogen source for microbial growth.
In the pharmaceutical sector, ammonium hydroxide is widely used in the production of antibiotics, vitamins, and other active pharmaceutical ingredients. The growing emphasis on healthcare and the development of novel drug formulations have contributed to the increased demand for high-purity ammonium hydroxide. Additionally, the expanding biopharmaceutical industry, particularly in emerging markets, has further bolstered the market growth for this chemical compound.
The food and beverage industry represents another significant market for ammonium hydroxide in biochemical applications. It is utilized as a leavening agent in baked goods, a pH regulator in food processing, and a cleaning agent in food production facilities. The rising consumer demand for processed and convenience foods has indirectly driven the market for ammonium hydroxide in this sector.
In the agricultural sector, ammonium hydroxide finds applications in fertilizer production and as a soil pH adjuster. The global push for increased crop yields and sustainable farming practices has led to a surge in demand for efficient fertilizers and soil treatments, thereby positively impacting the market for ammonium hydroxide.
The environmental and water treatment industries also contribute to the market growth of ammonium hydroxide. Its use in wastewater treatment processes, particularly in removing nitrogen compounds, has gained traction as environmental regulations become more stringent worldwide.
Geographically, North America and Europe have been the traditional strongholds for ammonium hydroxide consumption in biochemical applications. However, the Asia-Pacific region is emerging as a key growth market, driven by rapid industrialization, increasing investments in biotechnology, and the expansion of pharmaceutical manufacturing capabilities in countries like China and India.
Market analysts project a compound annual growth rate (CAGR) for the ammonium hydroxide market in biochemical applications to be in the range of 4-6% over the next five years. This growth is attributed to the expanding applications in existing industries and the emergence of new biotechnology-based processes that require ammonium hydroxide as a critical reagent.
In the pharmaceutical sector, ammonium hydroxide is widely used in the production of antibiotics, vitamins, and other active pharmaceutical ingredients. The growing emphasis on healthcare and the development of novel drug formulations have contributed to the increased demand for high-purity ammonium hydroxide. Additionally, the expanding biopharmaceutical industry, particularly in emerging markets, has further bolstered the market growth for this chemical compound.
The food and beverage industry represents another significant market for ammonium hydroxide in biochemical applications. It is utilized as a leavening agent in baked goods, a pH regulator in food processing, and a cleaning agent in food production facilities. The rising consumer demand for processed and convenience foods has indirectly driven the market for ammonium hydroxide in this sector.
In the agricultural sector, ammonium hydroxide finds applications in fertilizer production and as a soil pH adjuster. The global push for increased crop yields and sustainable farming practices has led to a surge in demand for efficient fertilizers and soil treatments, thereby positively impacting the market for ammonium hydroxide.
The environmental and water treatment industries also contribute to the market growth of ammonium hydroxide. Its use in wastewater treatment processes, particularly in removing nitrogen compounds, has gained traction as environmental regulations become more stringent worldwide.
Geographically, North America and Europe have been the traditional strongholds for ammonium hydroxide consumption in biochemical applications. However, the Asia-Pacific region is emerging as a key growth market, driven by rapid industrialization, increasing investments in biotechnology, and the expansion of pharmaceutical manufacturing capabilities in countries like China and India.
Market analysts project a compound annual growth rate (CAGR) for the ammonium hydroxide market in biochemical applications to be in the range of 4-6% over the next five years. This growth is attributed to the expanding applications in existing industries and the emergence of new biotechnology-based processes that require ammonium hydroxide as a critical reagent.
Technical Challenges in Ammonium Hydroxide Usage
The use of ammonium hydroxide in biochemical engineering applications presents several significant technical challenges that researchers and engineers must address. One of the primary issues is the volatility of ammonium hydroxide, which can lead to concentration fluctuations and potential safety hazards in laboratory and industrial settings. This volatility necessitates careful handling and storage procedures to maintain consistent concentrations and prevent unintended release of ammonia gas.
Another challenge lies in the pH-dependent nature of ammonium hydroxide solutions. The equilibrium between ammonia and ammonium ions is highly sensitive to pH changes, which can significantly impact biochemical processes. Maintaining precise pH control is crucial for many applications, such as enzyme reactions or cell culture media, where even slight deviations can affect product yield or cellular metabolism.
The corrosive nature of ammonium hydroxide poses additional technical difficulties, particularly in terms of material compatibility. Many common materials used in laboratory and industrial equipment can be degraded by prolonged exposure to ammonium hydroxide, leading to potential contamination of samples or products. This necessitates careful selection of corrosion-resistant materials for storage containers, pipelines, and reaction vessels.
Furthermore, the strong odor of ammonia associated with ammonium hydroxide solutions presents challenges in terms of worker safety and environmental concerns. Adequate ventilation systems and personal protective equipment are essential to prevent exposure and ensure compliance with occupational health and safety regulations. Additionally, proper waste management and disposal protocols must be implemented to mitigate environmental impact.
The reactivity of ammonium hydroxide with various compounds can also complicate its use in biochemical engineering. Unwanted side reactions or the formation of precipitates can interfere with desired processes or product purity. This requires careful consideration of reaction conditions and potential interactions with other components in the system.
Scaling up processes involving ammonium hydroxide from laboratory to industrial scale presents its own set of challenges. Maintaining uniform concentration and distribution in large-scale reactors or fermentation tanks can be difficult due to the compound's volatility and pH-dependent behavior. Engineers must develop robust mixing and control strategies to ensure consistent performance at scale.
Lastly, the regulatory landscape surrounding the use of ammonium hydroxide in biochemical applications adds another layer of complexity. Compliance with safety standards, environmental regulations, and product quality requirements necessitates rigorous documentation, monitoring, and control measures throughout the entire process chain.
Another challenge lies in the pH-dependent nature of ammonium hydroxide solutions. The equilibrium between ammonia and ammonium ions is highly sensitive to pH changes, which can significantly impact biochemical processes. Maintaining precise pH control is crucial for many applications, such as enzyme reactions or cell culture media, where even slight deviations can affect product yield or cellular metabolism.
The corrosive nature of ammonium hydroxide poses additional technical difficulties, particularly in terms of material compatibility. Many common materials used in laboratory and industrial equipment can be degraded by prolonged exposure to ammonium hydroxide, leading to potential contamination of samples or products. This necessitates careful selection of corrosion-resistant materials for storage containers, pipelines, and reaction vessels.
Furthermore, the strong odor of ammonia associated with ammonium hydroxide solutions presents challenges in terms of worker safety and environmental concerns. Adequate ventilation systems and personal protective equipment are essential to prevent exposure and ensure compliance with occupational health and safety regulations. Additionally, proper waste management and disposal protocols must be implemented to mitigate environmental impact.
The reactivity of ammonium hydroxide with various compounds can also complicate its use in biochemical engineering. Unwanted side reactions or the formation of precipitates can interfere with desired processes or product purity. This requires careful consideration of reaction conditions and potential interactions with other components in the system.
Scaling up processes involving ammonium hydroxide from laboratory to industrial scale presents its own set of challenges. Maintaining uniform concentration and distribution in large-scale reactors or fermentation tanks can be difficult due to the compound's volatility and pH-dependent behavior. Engineers must develop robust mixing and control strategies to ensure consistent performance at scale.
Lastly, the regulatory landscape surrounding the use of ammonium hydroxide in biochemical applications adds another layer of complexity. Compliance with safety standards, environmental regulations, and product quality requirements necessitates rigorous documentation, monitoring, and control measures throughout the entire process chain.
Current Ammonium Hydroxide Solutions
01 Use in chemical processes
Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it suitable for neutralizing acids and controlling pH levels in different applications.- Use in chemical processes: Ammonium hydroxide is widely used in various chemical processes as a reactant, catalyst, or pH regulator. It plays a crucial role in the synthesis of organic compounds, production of fertilizers, and treatment of industrial waste. Its alkaline properties make it suitable for neutralizing acids and controlling pH levels in different applications.
- Application in cleaning and surface treatment: Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. Its ability to dissolve certain metals and oxides makes it valuable in metal surface treatment and electroplating applications.
- Role in textile and leather processing: In the textile and leather industries, ammonium hydroxide serves multiple purposes. It is used in dyeing processes to adjust pH levels and improve color fastness. In leather tanning, it helps in dehairing and softening the hides. Additionally, it can be used to neutralize acidic residues in fabrics and leather goods, enhancing their quality and durability.
- Environmental and agricultural applications: Ammonium hydroxide finds applications in environmental remediation and agriculture. It is used in flue gas treatment to reduce nitrogen oxide emissions from power plants. In agriculture, it serves as a source of nitrogen for fertilizers and can be used to adjust soil pH. Its ability to capture and neutralize certain pollutants makes it valuable in wastewater treatment processes.
- Use in personal care and food products: Ammonium hydroxide is employed in the production of certain personal care and food products. In cosmetics, it can be used as a pH adjuster in hair dyes and perming solutions. In the food industry, it serves as a leavening agent in baked goods and helps regulate acidity in some processed foods. However, its use in these applications is subject to strict regulations and safety guidelines.
02 Application in cleaning and surface treatment
Ammonium hydroxide is utilized in cleaning formulations and surface treatment processes. It is effective in removing grease, oils, and other contaminants from various surfaces. In the semiconductor industry, it is used for etching and cleaning silicon wafers. Its ability to dissolve certain metals and oxides makes it valuable in metal surface treatment and electroplating applications.Expand Specific Solutions03 Role in textile and leather processing
Ammonium hydroxide finds applications in the textile and leather industries. It is used in dyeing processes to adjust pH levels and improve color fastness. In leather processing, it helps in dehairing and softening hides. Its alkaline nature aids in breaking down proteins and fats, facilitating various stages of textile and leather manufacturing.Expand Specific Solutions04 Environmental and agricultural uses
Ammonium hydroxide has important environmental and agricultural applications. It is used in air pollution control systems to neutralize acidic gases. In agriculture, it serves as a source of nitrogen for fertilizers and can be directly applied to soil to increase nitrogen content. It also plays a role in composting processes and treatment of agricultural waste.Expand Specific Solutions05 Pharmaceutical and personal care applications
Ammonium hydroxide is utilized in the pharmaceutical and personal care industries. It is used in the production of certain medications and as a pH adjuster in various formulations. In personal care products, it can be found in hair dyes, skin care products, and some cosmetic preparations. Its ability to neutralize acids and maintain pH balance makes it valuable in these applications.Expand Specific Solutions
Key Industry Players and Competitors
The competitive landscape for assessing ammonium hydroxide in biochemical engineering applications is in a mature stage, with a substantial market size due to its widespread use in various industries. The technology is well-established, with major players like DuPont de Nemours, Amgen, and Baker Hughes Co. leading research and development efforts. Academic institutions such as Zhejiang University and Massachusetts Institute of Technology contribute significantly to advancing the field. The market is characterized by a mix of large chemical companies, specialized biotech firms, and research institutions, indicating a diverse and competitive environment with ongoing innovation in application-specific solutions.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed advanced ammonium hydroxide-based solutions for biochemical engineering applications. Their approach involves using precisely controlled concentrations of ammonium hydroxide in bioreactors to optimize pH levels and nitrogen availability for microbial fermentation processes[1]. This technology enables enhanced production of high-value biochemicals and biofuels. DuPont has also pioneered the use of ammonium hydroxide as a key component in their proprietary enzyme stabilization formulations, significantly extending the shelf-life and activity of industrial enzymes used in various bioprocessing applications[2].
Strengths: Extensive experience in industrial-scale biochemical processes, strong R&D capabilities. Weaknesses: Potential environmental concerns related to ammonia emissions.
Amgen, Inc.
Technical Solution: Amgen has developed a novel approach to using ammonium hydroxide in their biopharmaceutical manufacturing processes. They employ a controlled-release ammonium hydroxide system in cell culture media to maintain optimal pH and provide a steady source of nitrogen for recombinant protein production[3]. This technology has been shown to increase product yield and quality in the production of monoclonal antibodies and other therapeutic proteins. Additionally, Amgen has implemented advanced ammonia recycling systems in their bioprocessing facilities, significantly reducing waste and improving overall process efficiency[4].
Strengths: Cutting-edge bioprocessing technology, focus on sustainability. Weaknesses: Limited application outside of biopharmaceutical industry.
Innovative Research in Ammonium Hydroxide
Formulations for the synthesis of paramagnetic particles and methods that utilize the particles for biochemical applications
PatentInactiveUS20150140595A1
Innovation
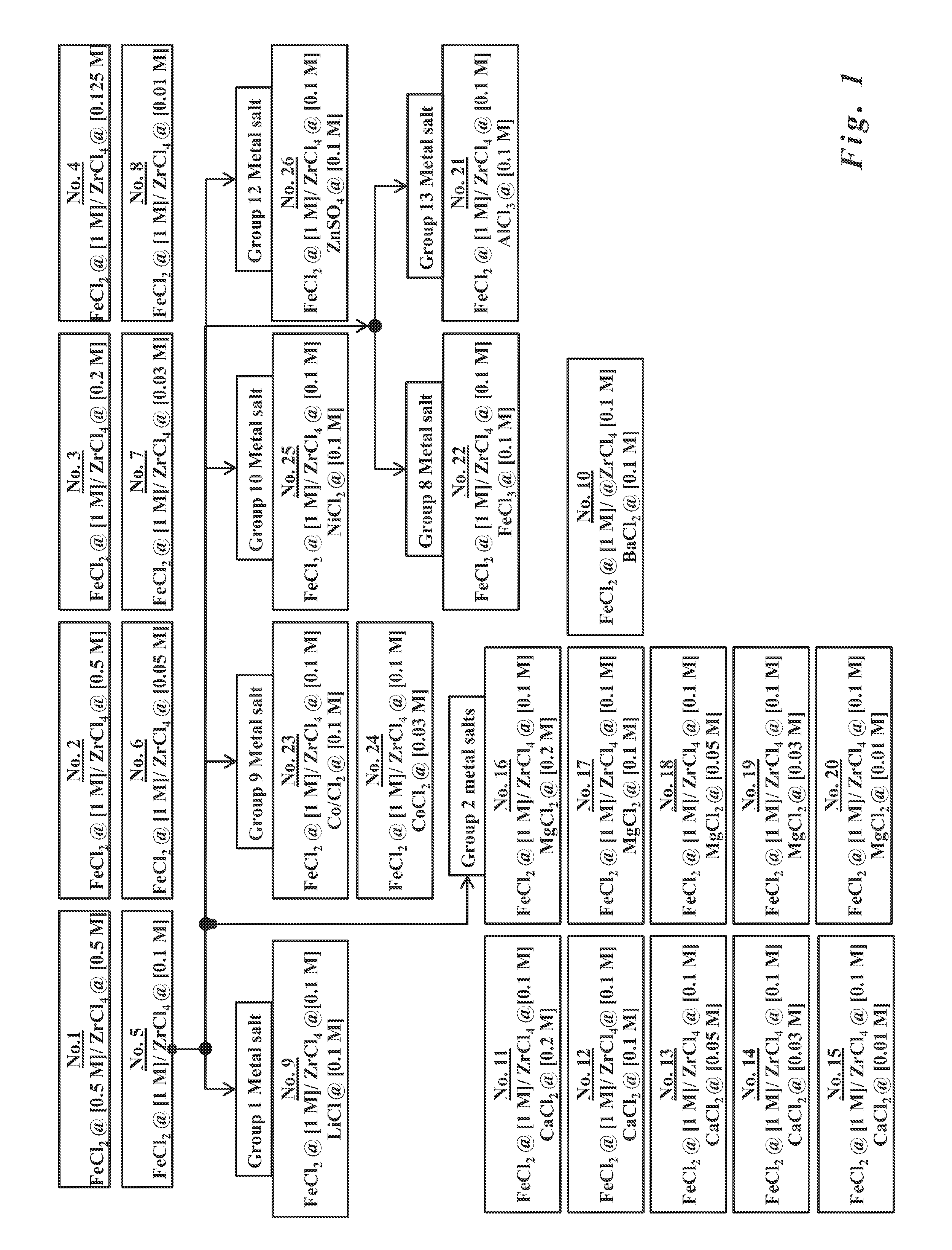
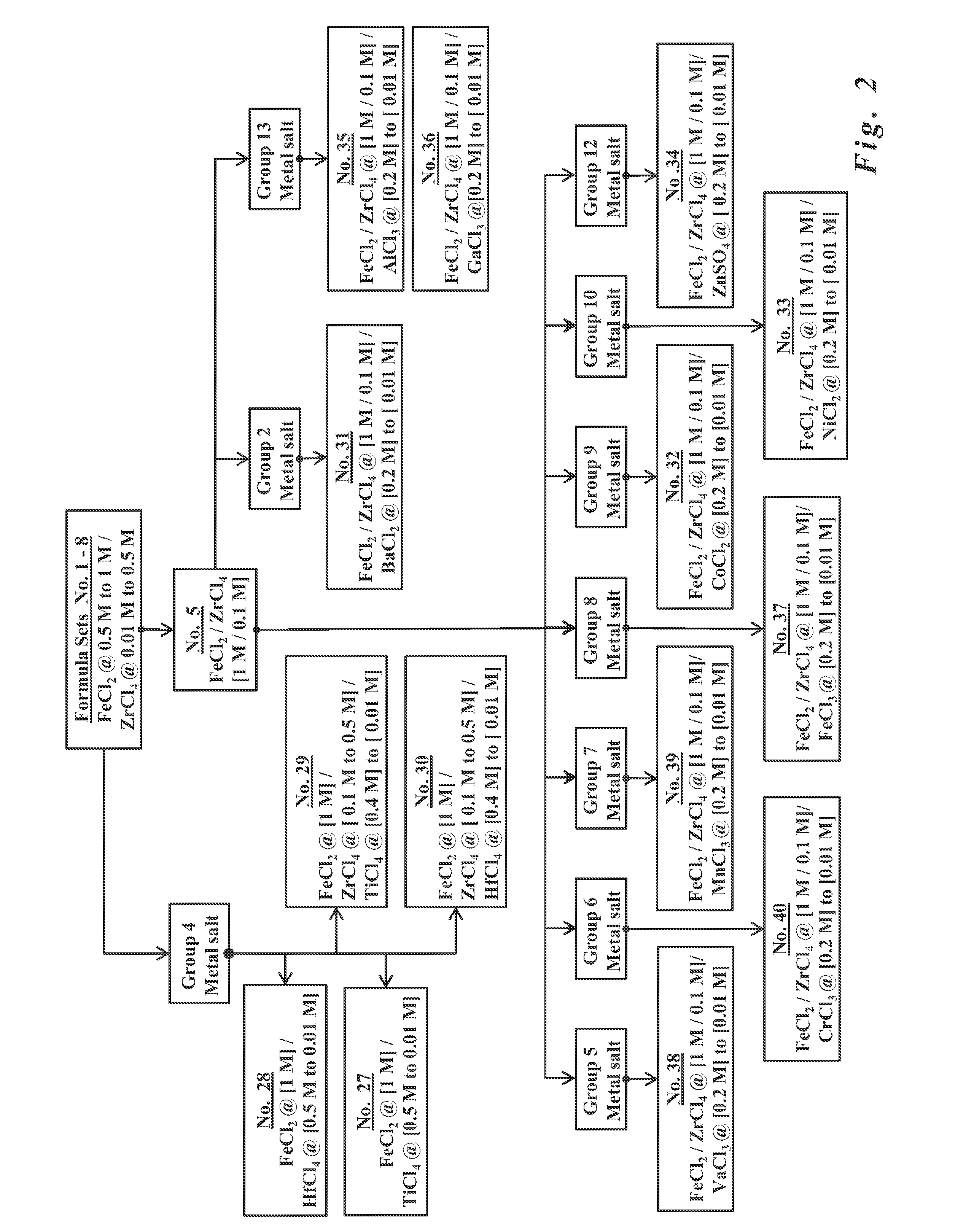
- The development of paramagnetic particles synthesized via co-precipitation methods using metal salt solutions with ferrous salts and tetravalent Group IV salts, such as zirconium, titanium, or hafnium, which provide strong paramagnetic properties and stability in water, allowing for effective magnetic separation and biochemical compatibility without the need for additional coatings.
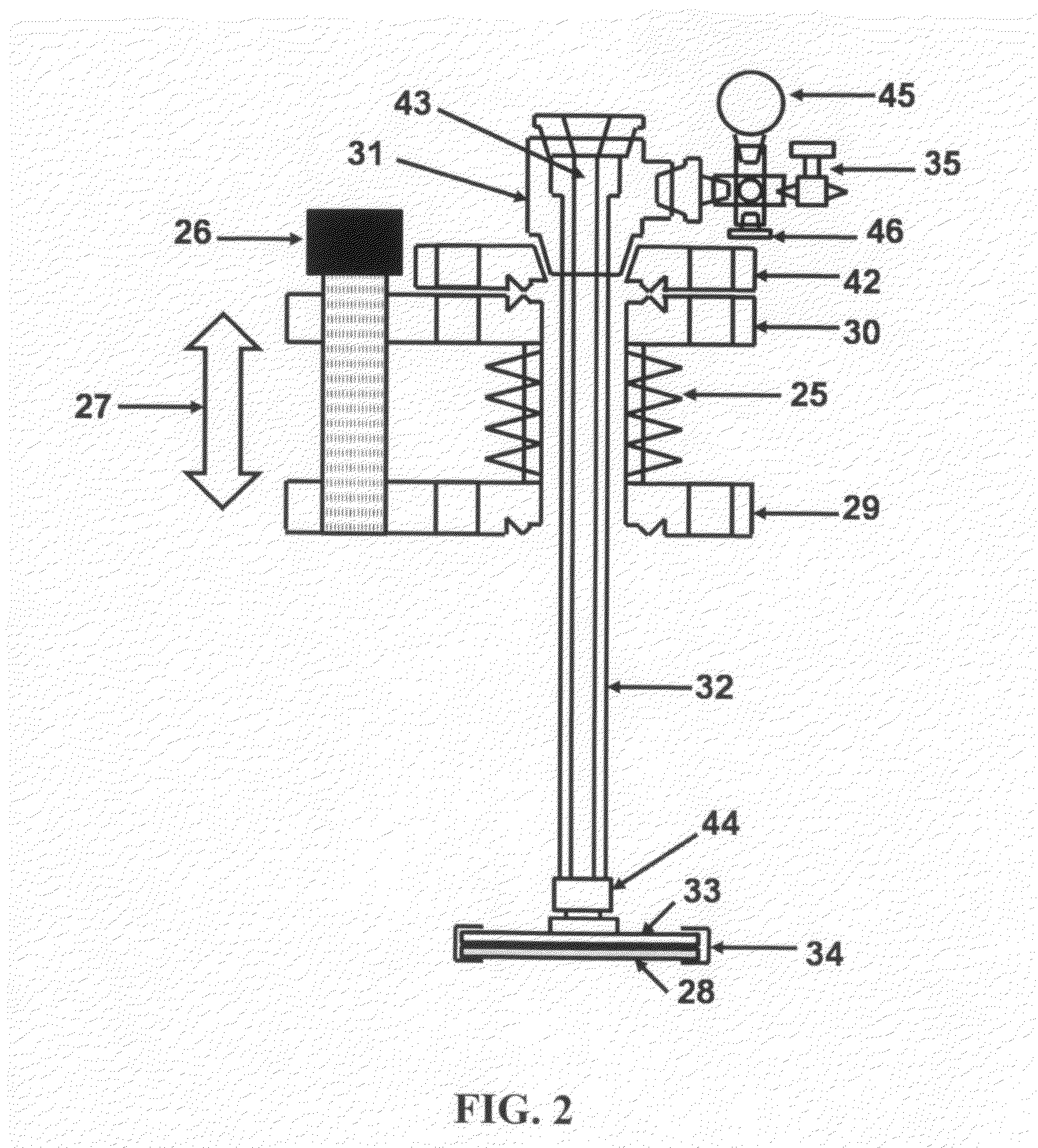
High purity, environmentally clean method and apparatus, for high rate, liquid anisotropic etching of single crystal silicon or etching of polycrystalline silicon, using an overpressure of ammonia gas above aqueous ammonium hydroxide
PatentInactiveUS8790531B2
Innovation
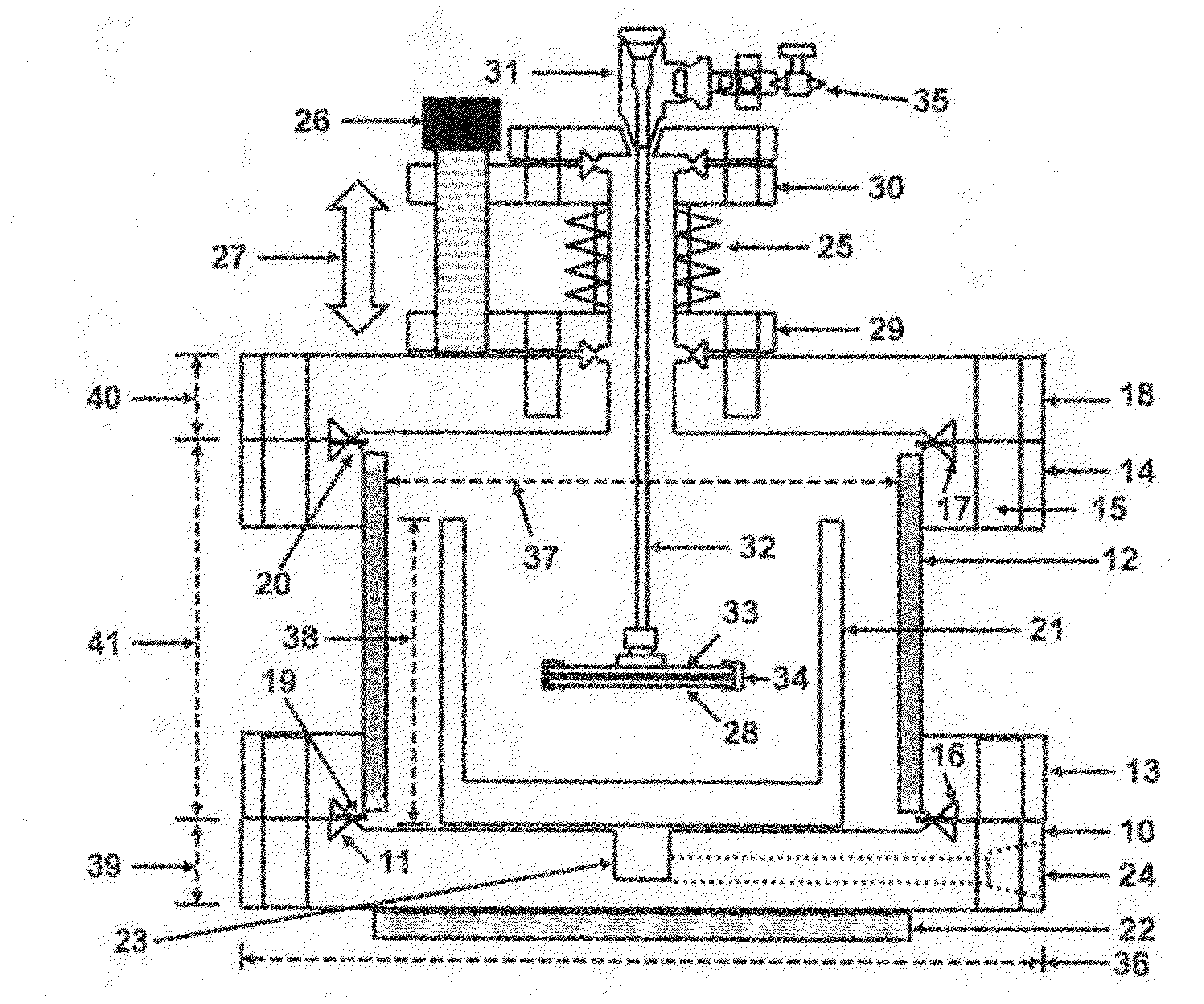
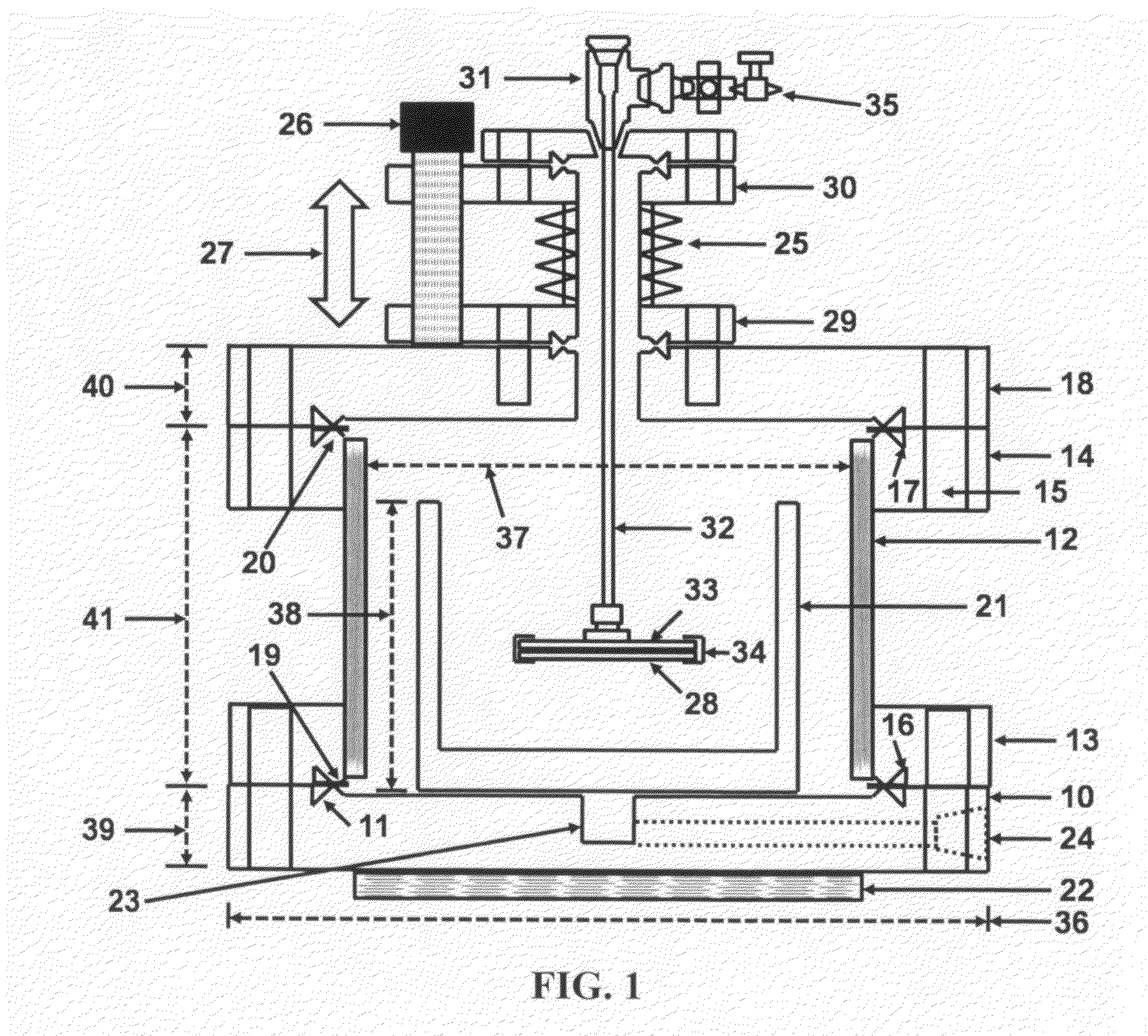
- The use of high purity aqueous ammonium hydroxide (NH4OH) solution generated at the point of use from semiconductor-grade ammonia gas dissolved in deionized water, maintained in a hermetically enclosed chamber with an overpressure of ammonia, preventing evaporation and allowing high anisotropic etching rates between 70-90°C, within a corrosion-resistant nickel alloy apparatus.
Safety and Environmental Considerations
The use of ammonium hydroxide in biochemical engineering applications necessitates careful consideration of safety and environmental factors. Ammonium hydroxide, a solution of ammonia in water, poses significant risks due to its corrosive and toxic nature. In laboratory and industrial settings, proper handling procedures are crucial to prevent accidents and protect personnel. This includes the use of appropriate personal protective equipment (PPE) such as chemical-resistant gloves, safety goggles, and respiratory protection when working with concentrated solutions.
Ventilation is a critical safety measure when handling ammonium hydroxide. Adequate fume hoods or local exhaust ventilation systems must be in place to prevent the accumulation of ammonia vapors, which can cause respiratory irritation and, in high concentrations, lead to severe health effects. Emergency eyewash stations and safety showers should be readily accessible in areas where ammonium hydroxide is used or stored.
Storage considerations are equally important. Ammonium hydroxide should be kept in tightly sealed containers in well-ventilated areas, away from heat sources and incompatible materials. Proper labeling and segregation from acids and oxidizing agents are essential to prevent accidental mixing and potentially dangerous reactions.
From an environmental perspective, the release of ammonium hydroxide can have significant impacts on aquatic ecosystems. It can lead to increased pH levels in water bodies, potentially harming aquatic life and disrupting ecological balance. Proper disposal methods must be implemented to prevent environmental contamination. This may include neutralization procedures or specialized waste treatment facilities capable of handling alkaline solutions.
Spill response protocols are crucial for minimizing environmental impact and ensuring worker safety. Facilities using ammonium hydroxide should have well-defined spill containment and cleanup procedures, including the use of appropriate absorbents and neutralizing agents. Staff should be trained in these procedures and equipped with the necessary materials to respond quickly and effectively to spills.
Regulatory compliance is another critical aspect of safety and environmental considerations. Organizations must adhere to local, national, and international regulations governing the use, storage, and disposal of ammonium hydroxide. This may include obtaining necessary permits, conducting regular safety audits, and maintaining detailed records of chemical usage and disposal.
In biochemical engineering applications, the potential for ammonia emissions during processes must be carefully managed. This may involve the implementation of closed-loop systems, scrubbers, or other emission control technologies to minimize the release of ammonia into the atmosphere. Regular monitoring of air quality in and around facilities using ammonium hydroxide is essential to ensure compliance with environmental standards and protect community health.
Ventilation is a critical safety measure when handling ammonium hydroxide. Adequate fume hoods or local exhaust ventilation systems must be in place to prevent the accumulation of ammonia vapors, which can cause respiratory irritation and, in high concentrations, lead to severe health effects. Emergency eyewash stations and safety showers should be readily accessible in areas where ammonium hydroxide is used or stored.
Storage considerations are equally important. Ammonium hydroxide should be kept in tightly sealed containers in well-ventilated areas, away from heat sources and incompatible materials. Proper labeling and segregation from acids and oxidizing agents are essential to prevent accidental mixing and potentially dangerous reactions.
From an environmental perspective, the release of ammonium hydroxide can have significant impacts on aquatic ecosystems. It can lead to increased pH levels in water bodies, potentially harming aquatic life and disrupting ecological balance. Proper disposal methods must be implemented to prevent environmental contamination. This may include neutralization procedures or specialized waste treatment facilities capable of handling alkaline solutions.
Spill response protocols are crucial for minimizing environmental impact and ensuring worker safety. Facilities using ammonium hydroxide should have well-defined spill containment and cleanup procedures, including the use of appropriate absorbents and neutralizing agents. Staff should be trained in these procedures and equipped with the necessary materials to respond quickly and effectively to spills.
Regulatory compliance is another critical aspect of safety and environmental considerations. Organizations must adhere to local, national, and international regulations governing the use, storage, and disposal of ammonium hydroxide. This may include obtaining necessary permits, conducting regular safety audits, and maintaining detailed records of chemical usage and disposal.
In biochemical engineering applications, the potential for ammonia emissions during processes must be carefully managed. This may involve the implementation of closed-loop systems, scrubbers, or other emission control technologies to minimize the release of ammonia into the atmosphere. Regular monitoring of air quality in and around facilities using ammonium hydroxide is essential to ensure compliance with environmental standards and protect community health.
Regulatory Framework for Ammonium Hydroxide
The regulatory framework for ammonium hydroxide in biochemical engineering applications is complex and multifaceted, involving various governmental agencies and international bodies. In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating the use and handling of ammonium hydroxide under the Toxic Substances Control Act (TSCA). The EPA sets guidelines for storage, transportation, and disposal of this chemical, as well as monitoring its environmental impact.
The Occupational Safety and Health Administration (OSHA) is responsible for ensuring workplace safety related to ammonium hydroxide usage. OSHA has established permissible exposure limits (PELs) and requires proper labeling, safety data sheets, and employee training for handling this substance. Additionally, the Department of Transportation (DOT) regulates the transportation of ammonium hydroxide, classifying it as a hazardous material and mandating specific packaging and shipping requirements.
On the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to chemical hazard communication. Many countries have adopted GHS guidelines, which include specific criteria for classifying and labeling ammonium hydroxide based on its concentration and potential health and environmental hazards.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation governs the use of ammonium hydroxide in biochemical engineering applications. REACH requires manufacturers and importers to register chemicals and provide safety information, ensuring a high level of protection for human health and the environment.
The Food and Drug Administration (FDA) in the United States regulates the use of ammonium hydroxide in food processing and pharmaceutical applications. The FDA has established guidelines for its use as a processing aid and sets limits on residual amounts in final products. Similarly, the European Food Safety Authority (EFSA) provides regulations for the use of ammonium hydroxide in food production within the EU.
For biochemical engineering applications specifically, regulatory bodies often require detailed risk assessments and safety protocols. These may include environmental impact studies, worker exposure monitoring, and the implementation of best practices for handling and disposal. Many countries also mandate regular inspections and audits of facilities using ammonium hydroxide to ensure compliance with safety and environmental regulations.
As the field of biochemical engineering continues to evolve, regulatory frameworks are adapting to address new applications and potential risks. This includes ongoing research into the long-term effects of ammonium hydroxide exposure and its environmental impact, which may lead to future adjustments in regulations. Companies operating in this sector must stay informed about these evolving regulations and be prepared to adapt their processes accordingly to maintain compliance and ensure the safe use of ammonium hydroxide in their applications.
The Occupational Safety and Health Administration (OSHA) is responsible for ensuring workplace safety related to ammonium hydroxide usage. OSHA has established permissible exposure limits (PELs) and requires proper labeling, safety data sheets, and employee training for handling this substance. Additionally, the Department of Transportation (DOT) regulates the transportation of ammonium hydroxide, classifying it as a hazardous material and mandating specific packaging and shipping requirements.
On the international level, the United Nations' Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides a standardized approach to chemical hazard communication. Many countries have adopted GHS guidelines, which include specific criteria for classifying and labeling ammonium hydroxide based on its concentration and potential health and environmental hazards.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation governs the use of ammonium hydroxide in biochemical engineering applications. REACH requires manufacturers and importers to register chemicals and provide safety information, ensuring a high level of protection for human health and the environment.
The Food and Drug Administration (FDA) in the United States regulates the use of ammonium hydroxide in food processing and pharmaceutical applications. The FDA has established guidelines for its use as a processing aid and sets limits on residual amounts in final products. Similarly, the European Food Safety Authority (EFSA) provides regulations for the use of ammonium hydroxide in food production within the EU.
For biochemical engineering applications specifically, regulatory bodies often require detailed risk assessments and safety protocols. These may include environmental impact studies, worker exposure monitoring, and the implementation of best practices for handling and disposal. Many countries also mandate regular inspections and audits of facilities using ammonium hydroxide to ensure compliance with safety and environmental regulations.
As the field of biochemical engineering continues to evolve, regulatory frameworks are adapting to address new applications and potential risks. This includes ongoing research into the long-term effects of ammonium hydroxide exposure and its environmental impact, which may lead to future adjustments in regulations. Companies operating in this sector must stay informed about these evolving regulations and be prepared to adapt their processes accordingly to maintain compliance and ensure the safe use of ammonium hydroxide in their applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!