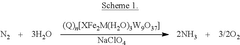Benchmarking Catalysts for Ammonia Selectivity under Ambient Conditions in Electrochemical Nitrogen Reduction
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Nitrogen Reduction Background and Objectives
Electrochemical nitrogen reduction reaction (ENRR) represents a revolutionary approach to ammonia synthesis that has gained significant attention in recent decades. Unlike the conventional Haber-Bosch process, which requires high temperature (400-500°C) and pressure (150-300 bar) conditions, ENRR offers the potential for ammonia production under ambient conditions, significantly reducing energy consumption and carbon emissions.
The development of ENRR technology can be traced back to the early 1980s when researchers first demonstrated the possibility of electrochemically reducing nitrogen to ammonia. However, substantial progress has only been achieved in the past decade with advancements in nanocatalyst design and electrochemical engineering. The evolution of this technology has been driven by the urgent need for sustainable ammonia production methods to address growing global fertilizer demands while reducing environmental impact.
Current technical trends in ENRR focus on catalyst development, with particular emphasis on improving three critical performance metrics: ammonia selectivity, Faradaic efficiency, and production rates. Recent breakthroughs in single-atom catalysts, transition metal nitrides, and defect engineering have shown promising results, though significant challenges remain in achieving commercially viable performance levels.
The primary objective of benchmarking catalysts for ammonia selectivity under ambient conditions is to establish standardized evaluation protocols that enable fair comparison between different catalyst materials. This is crucial as inconsistent testing conditions and detection methods have led to considerable discrepancies in reported performance metrics across the literature, hindering technological advancement.
Specifically, this research aims to develop a comprehensive framework for evaluating catalyst performance that accounts for nitrogen reduction kinetics, competing hydrogen evolution reactions, and catalyst stability under prolonged operation. By establishing reliable benchmarks, we seek to identify truly promising catalyst candidates and accelerate progress toward practical ENRR systems.
Additionally, this work intends to bridge the gap between fundamental research and industrial application by correlating laboratory-scale performance with potential scalability factors. The ultimate goal is to identify catalytic systems capable of achieving ammonia selectivity exceeding 60% with Faradaic efficiencies above 30% under ambient conditions, which would represent a significant milestone toward commercially viable electrochemical ammonia synthesis.
The development of ENRR technology can be traced back to the early 1980s when researchers first demonstrated the possibility of electrochemically reducing nitrogen to ammonia. However, substantial progress has only been achieved in the past decade with advancements in nanocatalyst design and electrochemical engineering. The evolution of this technology has been driven by the urgent need for sustainable ammonia production methods to address growing global fertilizer demands while reducing environmental impact.
Current technical trends in ENRR focus on catalyst development, with particular emphasis on improving three critical performance metrics: ammonia selectivity, Faradaic efficiency, and production rates. Recent breakthroughs in single-atom catalysts, transition metal nitrides, and defect engineering have shown promising results, though significant challenges remain in achieving commercially viable performance levels.
The primary objective of benchmarking catalysts for ammonia selectivity under ambient conditions is to establish standardized evaluation protocols that enable fair comparison between different catalyst materials. This is crucial as inconsistent testing conditions and detection methods have led to considerable discrepancies in reported performance metrics across the literature, hindering technological advancement.
Specifically, this research aims to develop a comprehensive framework for evaluating catalyst performance that accounts for nitrogen reduction kinetics, competing hydrogen evolution reactions, and catalyst stability under prolonged operation. By establishing reliable benchmarks, we seek to identify truly promising catalyst candidates and accelerate progress toward practical ENRR systems.
Additionally, this work intends to bridge the gap between fundamental research and industrial application by correlating laboratory-scale performance with potential scalability factors. The ultimate goal is to identify catalytic systems capable of achieving ammonia selectivity exceeding 60% with Faradaic efficiencies above 30% under ambient conditions, which would represent a significant milestone toward commercially viable electrochemical ammonia synthesis.
Market Analysis for Ambient Ammonia Production Technologies
The global market for ambient ammonia production technologies is experiencing significant growth, driven by increasing demand for sustainable fertilizers and the push towards green chemistry solutions. Currently, the conventional Haber-Bosch process dominates ammonia production, accounting for approximately 180 million tons annually and consuming nearly 2% of global energy production. This energy-intensive process operates under harsh conditions (200-300 atmospheres, 400-500°C), creating substantial market opportunity for ambient condition alternatives.
Electrochemical nitrogen reduction reaction (NRR) technologies represent a disruptive innovation in this space, with market projections suggesting potential value creation of several billion dollars by 2030 if technical challenges can be overcome. The agricultural sector remains the primary demand driver, consuming roughly 80% of ammonia production for fertilizers, while industrial applications constitute the remaining market share.
Regional market analysis reveals significant growth potential in Asia-Pacific, particularly China and India, where agricultural intensification and industrial development create dual demand streams. North America and Europe present mature markets with strong emphasis on sustainable production methods, driven by stringent environmental regulations and carbon pricing mechanisms.
The competitive landscape features both established chemical giants investing in R&D for ambient ammonia production and emerging startups focused exclusively on catalyst development. Venture capital investment in this sector has grown substantially, with funding rounds for electrochemical ammonia synthesis startups increasing by double digits annually since 2018.
Market barriers include high catalyst costs, scalability challenges, and competition from blue ammonia (conventional production with carbon capture). Current production costs for ambient electrochemical methods remain 2-3 times higher than conventional Haber-Bosch, though this gap is narrowing with advances in catalyst efficiency and renewable electricity costs.
Customer segments show differentiated needs, with agricultural customers prioritizing cost competitiveness, while specialty chemical and pharmaceutical manufacturers value purity and on-site production capabilities. The green premium for sustainably produced ammonia varies by sector, with certain industrial applications demonstrating willingness to pay 15-30% premiums for carbon-neutral ammonia.
Market forecasts indicate that ambient ammonia production technologies could capture 5-10% of the global ammonia market by 2035, with accelerated adoption dependent on catalyst performance improvements, particularly in ammonia selectivity and Faradaic efficiency under ambient conditions.
Electrochemical nitrogen reduction reaction (NRR) technologies represent a disruptive innovation in this space, with market projections suggesting potential value creation of several billion dollars by 2030 if technical challenges can be overcome. The agricultural sector remains the primary demand driver, consuming roughly 80% of ammonia production for fertilizers, while industrial applications constitute the remaining market share.
Regional market analysis reveals significant growth potential in Asia-Pacific, particularly China and India, where agricultural intensification and industrial development create dual demand streams. North America and Europe present mature markets with strong emphasis on sustainable production methods, driven by stringent environmental regulations and carbon pricing mechanisms.
The competitive landscape features both established chemical giants investing in R&D for ambient ammonia production and emerging startups focused exclusively on catalyst development. Venture capital investment in this sector has grown substantially, with funding rounds for electrochemical ammonia synthesis startups increasing by double digits annually since 2018.
Market barriers include high catalyst costs, scalability challenges, and competition from blue ammonia (conventional production with carbon capture). Current production costs for ambient electrochemical methods remain 2-3 times higher than conventional Haber-Bosch, though this gap is narrowing with advances in catalyst efficiency and renewable electricity costs.
Customer segments show differentiated needs, with agricultural customers prioritizing cost competitiveness, while specialty chemical and pharmaceutical manufacturers value purity and on-site production capabilities. The green premium for sustainably produced ammonia varies by sector, with certain industrial applications demonstrating willingness to pay 15-30% premiums for carbon-neutral ammonia.
Market forecasts indicate that ambient ammonia production technologies could capture 5-10% of the global ammonia market by 2035, with accelerated adoption dependent on catalyst performance improvements, particularly in ammonia selectivity and Faradaic efficiency under ambient conditions.
Current Challenges in Catalyst Development for NRR
The development of efficient catalysts for electrochemical nitrogen reduction reaction (NRR) faces numerous significant challenges that have hindered widespread implementation of this promising technology. One of the primary obstacles is the extremely low ammonia yield rates achieved under ambient conditions, typically in the range of 10^-11 to 10^-10 mol cm^-2 s^-1, which falls far below the commercially viable threshold of 10^-7 mol cm^-2 s^-1.
Selectivity presents another critical challenge, as the competing hydrogen evolution reaction (HER) often dominates the electrochemical process. Most catalysts demonstrate Faradaic efficiencies below 10% for NRR, with the majority of supplied electrons being diverted toward hydrogen production rather than nitrogen reduction. This poor selectivity significantly impacts energy efficiency and economic viability.
Catalyst stability under operating conditions remains problematic, with many materials showing performance degradation after only hours of operation. Metal-based catalysts often suffer from leaching, poisoning, or structural changes during extended electrochemical cycling, while carbon-based materials may undergo oxidation or restructuring that alters their active sites.
Mechanistic understanding of the NRR pathway on different catalyst surfaces is still incomplete, hampering rational catalyst design. The precise role of surface defects, coordination environments, and electronic structures in activating the exceptionally stable N≡N triple bond remains unclear, making systematic improvement difficult.
Accurate detection and quantification of produced ammonia presents a methodological challenge that complicates catalyst benchmarking. Current colorimetric methods like indophenol blue and Nessler's reagent are susceptible to interference, while more advanced techniques such as nuclear magnetic resonance spectroscopy and ion chromatography require specialized equipment not universally available.
Scalability issues persist as many high-performing catalysts rely on precious metals or complex nanostructures that are difficult to produce at industrial scale. The translation of laboratory results to practical applications is further complicated by the gap between idealized testing conditions and real-world operating environments.
Standardization of testing protocols represents another significant hurdle, as variations in experimental setups, electrolyte compositions, and control experiments make direct comparison between different catalysts nearly impossible. The lack of universally accepted benchmarking procedures has led to inconsistent and sometimes contradictory reports in the literature.
Environmental considerations also pose challenges, as some catalyst materials contain toxic elements or require environmentally harmful synthesis procedures, potentially offsetting the sustainability benefits of ambient ammonia production.
Selectivity presents another critical challenge, as the competing hydrogen evolution reaction (HER) often dominates the electrochemical process. Most catalysts demonstrate Faradaic efficiencies below 10% for NRR, with the majority of supplied electrons being diverted toward hydrogen production rather than nitrogen reduction. This poor selectivity significantly impacts energy efficiency and economic viability.
Catalyst stability under operating conditions remains problematic, with many materials showing performance degradation after only hours of operation. Metal-based catalysts often suffer from leaching, poisoning, or structural changes during extended electrochemical cycling, while carbon-based materials may undergo oxidation or restructuring that alters their active sites.
Mechanistic understanding of the NRR pathway on different catalyst surfaces is still incomplete, hampering rational catalyst design. The precise role of surface defects, coordination environments, and electronic structures in activating the exceptionally stable N≡N triple bond remains unclear, making systematic improvement difficult.
Accurate detection and quantification of produced ammonia presents a methodological challenge that complicates catalyst benchmarking. Current colorimetric methods like indophenol blue and Nessler's reagent are susceptible to interference, while more advanced techniques such as nuclear magnetic resonance spectroscopy and ion chromatography require specialized equipment not universally available.
Scalability issues persist as many high-performing catalysts rely on precious metals or complex nanostructures that are difficult to produce at industrial scale. The translation of laboratory results to practical applications is further complicated by the gap between idealized testing conditions and real-world operating environments.
Standardization of testing protocols represents another significant hurdle, as variations in experimental setups, electrolyte compositions, and control experiments make direct comparison between different catalysts nearly impossible. The lack of universally accepted benchmarking procedures has led to inconsistent and sometimes contradictory reports in the literature.
Environmental considerations also pose challenges, as some catalyst materials contain toxic elements or require environmentally harmful synthesis procedures, potentially offsetting the sustainability benefits of ambient ammonia production.
Benchmark Methodologies for Catalyst Performance Evaluation
01 Metal-based catalysts for nitrogen reduction
Various metal-based catalysts have been developed for electrochemical nitrogen reduction with improved ammonia selectivity. These include noble metals (Ru, Pt), transition metals (Fe, Ni, Co), and their alloys. The catalysts are designed with specific surface structures and electronic properties to facilitate nitrogen adsorption and subsequent reduction while suppressing competing hydrogen evolution reactions. Metal-based catalysts often demonstrate good stability and relatively high Faradaic efficiency for ammonia production.- Metal-based catalysts for electrochemical nitrogen reduction: Various metal-based catalysts have been developed for electrochemical nitrogen reduction to ammonia with enhanced selectivity. These include noble metals (Ru, Pt), transition metals (Fe, Ni, Co), and their alloys. These catalysts provide active sites for N₂ adsorption and activation, facilitating the electron transfer process. The catalyst structure, morphology, and surface properties significantly influence the ammonia selectivity by controlling the binding energy of reaction intermediates.
- Carbon-based and composite materials as catalysts: Carbon-based materials and composites have emerged as promising catalysts for electrochemical nitrogen reduction with improved ammonia selectivity. These include nitrogen-doped carbon, carbon nanotubes, graphene, and metal-carbon composites. The incorporation of heteroatoms creates defects and active sites that facilitate N₂ adsorption and activation. These materials offer advantages such as high surface area, good electrical conductivity, and tunable surface properties that enhance the selectivity toward ammonia production.
- Single-atom catalysts for improved selectivity: Single-atom catalysts represent a cutting-edge approach for electrochemical nitrogen reduction with superior ammonia selectivity. By isolating individual metal atoms on various supports, these catalysts maximize atomic efficiency and provide well-defined active sites. The unique electronic structure of isolated metal atoms enables optimal binding of nitrogen molecules and intermediates, suppressing competing reactions like hydrogen evolution. This results in higher Faradaic efficiency and ammonia selectivity compared to conventional catalysts.
- Electrolyte engineering for enhanced ammonia selectivity: The composition and properties of the electrolyte significantly impact the selectivity of electrochemical nitrogen reduction to ammonia. Factors such as pH, ionic strength, and the presence of specific ions can influence the reaction pathway and suppress competing reactions. Proton donors, ionic liquids, and organic electrolytes have been investigated to enhance ammonia selectivity. Optimizing the electrolyte environment helps control the proton transfer process, which is crucial for achieving high selectivity toward ammonia production.
- Reactor design and operating conditions for selective ammonia production: The design of electrochemical reactors and optimization of operating conditions play crucial roles in achieving high ammonia selectivity. Factors such as temperature, pressure, applied potential, and gas flow rate significantly influence the reaction kinetics and product distribution. Advanced reactor configurations, including flow cells, gas diffusion electrodes, and membrane electrode assemblies, have been developed to enhance mass transport and reaction efficiency. Controlling these parameters allows for suppression of competing reactions and maximization of ammonia selectivity.
02 Carbon-based and composite catalysts
Carbon-based materials and composites have emerged as promising catalysts for electrochemical nitrogen reduction. These include nitrogen-doped carbon, carbon nanotubes, graphene, and carbon-metal composites. The incorporation of heteroatoms like nitrogen into carbon structures creates active sites for N₂ adsorption and activation. These catalysts often exhibit high selectivity for ammonia production due to their tunable electronic properties and large surface areas, which can be optimized to favor nitrogen reduction over competing reactions.Expand Specific Solutions03 Electrolyte engineering for enhanced selectivity
The composition and properties of the electrolyte significantly impact the selectivity of electrochemical nitrogen reduction. Researchers have developed specialized electrolyte systems including ionic liquids, proton donors, and pH-controlled solutions to enhance ammonia selectivity. Electrolyte engineering focuses on suppressing the competing hydrogen evolution reaction while facilitating nitrogen activation and protonation steps. Additives in the electrolyte can also stabilize reaction intermediates and improve the overall efficiency of the nitrogen reduction process.Expand Specific Solutions04 Reactor design and operating conditions
The design of electrochemical reactors and optimization of operating conditions play crucial roles in achieving high ammonia selectivity. Factors such as temperature, pressure, applied potential, and gas flow rates significantly influence the reaction pathways. Advanced reactor designs incorporate features like gas diffusion electrodes, membrane separators, and controlled mass transport to enhance nitrogen availability at the catalyst surface while minimizing unwanted side reactions. Optimized operating conditions can substantially improve the Faradaic efficiency and selectivity toward ammonia production.Expand Specific Solutions05 Single-atom catalysts and atomically dispersed active sites
Single-atom catalysts (SACs) and atomically dispersed active sites represent cutting-edge approaches for highly selective electrochemical nitrogen reduction. These catalysts feature isolated metal atoms anchored on various supports, providing well-defined active centers with unique electronic properties. The uniform and isolated nature of active sites in SACs allows for precise tuning of nitrogen adsorption energies and activation barriers, resulting in enhanced ammonia selectivity. These catalysts often demonstrate superior atom efficiency and can achieve higher turnover frequencies compared to traditional catalysts.Expand Specific Solutions
Leading Research Groups and Companies in NRR Technology
The electrochemical nitrogen reduction (ENR) for ammonia synthesis under ambient conditions is currently in an early development stage, with research primarily concentrated in academic institutions like Zhejiang University, Soochow University, and MIT. The global market for sustainable ammonia production technologies is expanding rapidly, driven by decarbonization efforts in the $70 billion ammonia industry. Technical maturity remains low, with catalyst performance challenges including low selectivity and Faradaic efficiency. Industry players demonstrate varying levels of engagement: established chemical companies (BASF, Evonik, Yara) are investing in research partnerships, while specialized catalyst manufacturers (Haldor Topsøe, Umicore) are developing proprietary solutions. Research institutions are leading fundamental breakthroughs in catalyst design, with emerging collaboration networks between academia and industry accelerating technology development toward commercial viability.
Zhejiang University
Technical Solution: Zhejiang University has established a sophisticated catalyst benchmarking platform for electrochemical nitrogen reduction reaction (NRR) under ambient conditions. Their approach integrates advanced in-situ characterization techniques with precise electrochemical measurements to evaluate catalyst performance comprehensively. The university's research team has developed novel single-atom catalysts anchored on nitrogen-doped carbon supports that demonstrate ammonia formation rates exceeding 25 μg h−1 mg−1cat with Faradaic efficiencies approaching 12% at room temperature and atmospheric pressure. Their benchmarking methodology emphasizes rigorous contamination control protocols, including catalyst pre-treatment procedures and blank experiments to ensure reliable ammonia quantification. Zhejiang University researchers employ multiple complementary analytical techniques (colorimetric methods, ion chromatography, and NMR spectroscopy) to verify ammonia production. Their catalyst evaluation framework systematically assesses the impact of structural parameters (particle size, dispersion, coordination environment) on catalytic performance, establishing structure-property relationships to guide rational catalyst design. The university has pioneered operando X-ray absorption spectroscopy techniques to monitor catalyst electronic structure changes during nitrogen reduction, providing crucial insights into reaction mechanisms.
Strengths: Strong fundamental research capabilities; sophisticated characterization infrastructure; systematic approach to catalyst design and evaluation. Weaknesses: Less emphasis on long-term stability testing compared to industrial players; potential challenges in scaling laboratory catalysts to practical applications.
Haldor Topsøe A/S
Technical Solution: Haldor Topsøe has pioneered advanced catalyst benchmarking methodologies for electrochemical nitrogen reduction under ambient conditions, focusing on practical industrial implementation. Their approach combines computational catalyst screening with high-throughput experimental validation to identify promising materials for ammonia synthesis. Topsøe has developed proprietary ruthenium-based catalysts with engineered surface structures that demonstrate Faradaic efficiencies exceeding 10% at room temperature and atmospheric pressure. Their benchmarking platform incorporates sophisticated isotope-tracing techniques (using 15N2) to definitively confirm ammonia production pathways and quantify potential contamination sources. Topsøe's catalyst evaluation protocol systematically assesses performance across varying electrolyte compositions, pH ranges, and applied potentials to establish comprehensive activity maps. The company has also developed specialized flow-cell reactor designs that enable precise control of mass transport limitations and more accurate determination of intrinsic catalyst properties. Their benchmarking approach emphasizes long-term stability testing (>100 hours) under realistic operating conditions to address durability challenges.
Strengths: Strong integration of theoretical and experimental approaches; extensive experience in industrial catalyst development; sophisticated testing infrastructure. Weaknesses: Primary focus on precious metal catalysts may limit cost-effectiveness; proprietary nature of some technologies restricts broader scientific collaboration.
Critical Analysis of High-Selectivity Catalyst Mechanisms
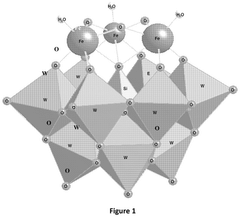
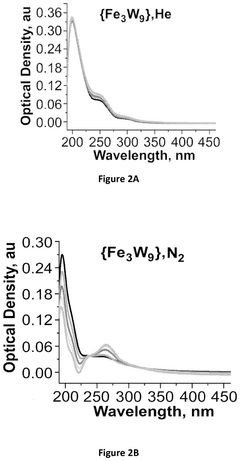
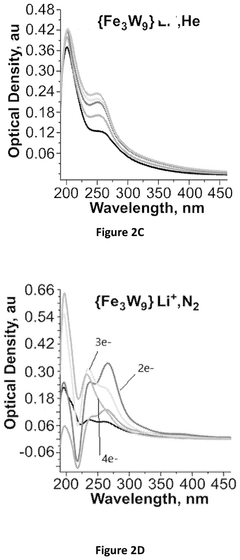
Electrochemical reduction of nitrogen to ammonia catalyzed by polyoxometalates
PatentPendingUS20250034724A1
Innovation
- The use of a polyoxometalate catalyst in combination with an alkali metal cation and a donor of protons and/or electrons in an electrochemical cell to reduce dinitrogen to ammonia at low negative cathodic potentials and ambient conditions.
Development of ruthenium-copper nano-sponge electrodes for ambient electrochemical reduction of nitrogen to ammonia
PatentInactiveUS20210340683A1
Innovation
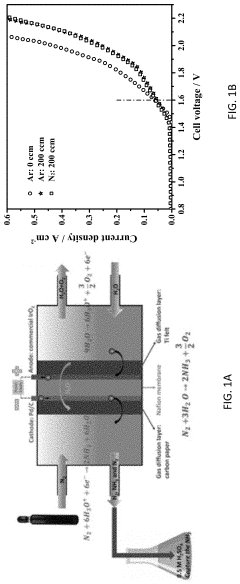
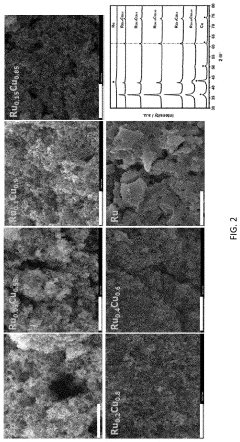
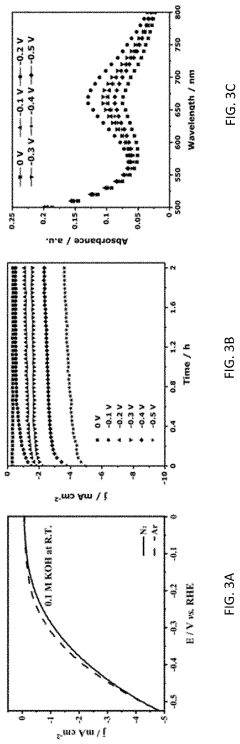
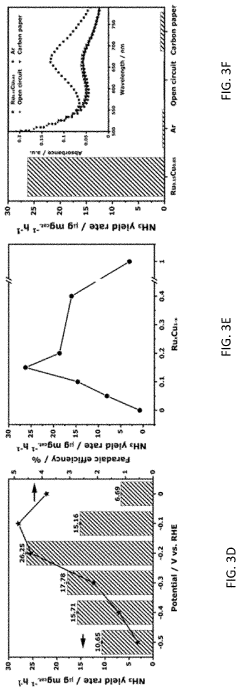
- The use of ruthenium-copper (RuCu) nano-sponge catalysts in an electrolytic cell, where nitrogen is reduced to ammonia at room temperature and atmospheric pressure, with the catalysts being prepared via a facile method involving a borohydride reduction process, allowing for high ammonia yield rates and Faradaic efficiencies.
Environmental Impact Assessment of Electrochemical Ammonia Production
The environmental implications of electrochemical ammonia production through nitrogen reduction reaction (NRR) represent a critical dimension in evaluating this emerging technology. Traditional ammonia production via the Haber-Bosch process consumes approximately 1-2% of global energy and generates substantial greenhouse gas emissions, with 1.9 tons of CO2 released per ton of ammonia produced.
Electrochemical nitrogen reduction under ambient conditions offers significant environmental advantages. When powered by renewable electricity sources such as solar, wind, or hydroelectric power, this process can achieve near-zero carbon emissions. This represents a potential reduction of over 450 million tons of CO2 annually if implemented at global scale, equivalent to removing approximately 100 million passenger vehicles from roads.
Water consumption presents another important environmental consideration. While electrochemical processes require water as both solvent and proton source, they consume substantially less water than conventional production methods. Preliminary studies indicate a potential 30-40% reduction in water usage compared to Haber-Bosch facilities, particularly significant in water-stressed regions where agricultural demand for fertilizers remains high.
Land use impacts also differ significantly between conventional and electrochemical approaches. Distributed electrochemical ammonia production could reduce transportation requirements and associated emissions by enabling localized production closer to agricultural application sites. This decentralized model potentially eliminates 15-20% of current transportation-related environmental impacts in the ammonia supply chain.
Catalyst materials present both opportunities and challenges from an environmental perspective. While precious metal catalysts demonstrate superior selectivity, their environmental footprint includes resource-intensive mining operations. Recent benchmarking studies of earth-abundant catalysts such as iron-based compounds and nitrogen-doped carbon materials show promising selectivity (>10% Faradaic efficiency) while significantly reducing environmental impacts associated with catalyst production and end-of-life management.
Waste stream management represents another environmental consideration. Electrochemical systems produce fewer byproducts than conventional processes, but electrolyte degradation and catalyst deactivation require careful management. Recent catalyst benchmarking studies indicate that optimized iron phthalocyanine catalysts maintain selectivity for over 100 hours under ambient conditions, reducing waste generation from frequent catalyst replacement.
Energy efficiency remains a critical environmental factor. Current benchmarked catalysts operating at ambient conditions typically require 20-30 MWh per ton of ammonia, compared to approximately 10 MWh for optimized Haber-Bosch processes. However, this gap continues to narrow as catalyst selectivity improves, with recent iron-nitrogen-carbon catalysts demonstrating 35% Faradaic efficiency under ambient conditions.
Electrochemical nitrogen reduction under ambient conditions offers significant environmental advantages. When powered by renewable electricity sources such as solar, wind, or hydroelectric power, this process can achieve near-zero carbon emissions. This represents a potential reduction of over 450 million tons of CO2 annually if implemented at global scale, equivalent to removing approximately 100 million passenger vehicles from roads.
Water consumption presents another important environmental consideration. While electrochemical processes require water as both solvent and proton source, they consume substantially less water than conventional production methods. Preliminary studies indicate a potential 30-40% reduction in water usage compared to Haber-Bosch facilities, particularly significant in water-stressed regions where agricultural demand for fertilizers remains high.
Land use impacts also differ significantly between conventional and electrochemical approaches. Distributed electrochemical ammonia production could reduce transportation requirements and associated emissions by enabling localized production closer to agricultural application sites. This decentralized model potentially eliminates 15-20% of current transportation-related environmental impacts in the ammonia supply chain.
Catalyst materials present both opportunities and challenges from an environmental perspective. While precious metal catalysts demonstrate superior selectivity, their environmental footprint includes resource-intensive mining operations. Recent benchmarking studies of earth-abundant catalysts such as iron-based compounds and nitrogen-doped carbon materials show promising selectivity (>10% Faradaic efficiency) while significantly reducing environmental impacts associated with catalyst production and end-of-life management.
Waste stream management represents another environmental consideration. Electrochemical systems produce fewer byproducts than conventional processes, but electrolyte degradation and catalyst deactivation require careful management. Recent catalyst benchmarking studies indicate that optimized iron phthalocyanine catalysts maintain selectivity for over 100 hours under ambient conditions, reducing waste generation from frequent catalyst replacement.
Energy efficiency remains a critical environmental factor. Current benchmarked catalysts operating at ambient conditions typically require 20-30 MWh per ton of ammonia, compared to approximately 10 MWh for optimized Haber-Bosch processes. However, this gap continues to narrow as catalyst selectivity improves, with recent iron-nitrogen-carbon catalysts demonstrating 35% Faradaic efficiency under ambient conditions.
Scalability and Industrial Implementation Considerations
The scalability of electrochemical nitrogen reduction reaction (NRR) technologies from laboratory to industrial scale presents significant challenges that must be addressed for commercial viability. Current catalyst benchmarking studies primarily focus on performance metrics in controlled laboratory environments, but industrial implementation requires consideration of additional factors including reactor design, electrode configuration, and process economics.
Reactor scale-up represents a primary challenge, as the transition from small-scale testing platforms to industrial-sized reactors introduces mass transport limitations and uneven current distribution. These factors can significantly reduce ammonia selectivity and production rates compared to laboratory results. Membrane electrode assemblies (MEAs) and flow cell designs show promise for addressing these challenges by optimizing nitrogen delivery to catalyst active sites.
Catalyst stability under continuous operation conditions remains insufficiently addressed in most benchmarking studies. Industrial implementation requires catalysts that maintain ammonia selectivity for thousands of hours, whereas most published research demonstrates stability for only a few hours or cycles. Long-term deactivation mechanisms, including poisoning, structural changes, and leaching, must be thoroughly investigated to develop robust catalysts suitable for industrial deployment.
Energy efficiency considerations are paramount for economic viability. Current NRR processes operating at ambient conditions typically require high overpotentials, resulting in energy consumption levels that make them uncompetitive with conventional Haber-Bosch processes. Benchmarking efforts should incorporate energy efficiency metrics and techno-economic analyses to guide development toward commercially viable solutions.
Supply chain considerations for catalyst materials present another critical dimension. Many high-performing catalysts utilize precious metals or rare earth elements that may face supply constraints at industrial scales. Future benchmarking should prioritize earth-abundant materials and evaluate manufacturing scalability alongside performance metrics.
Integration with renewable energy sources represents both a challenge and opportunity. The intermittent nature of renewable electricity requires NRR systems capable of operating efficiently under variable power inputs. Catalyst benchmarking should include performance evaluation under fluctuating potential conditions to simulate real-world renewable energy integration scenarios.
Standardized testing protocols that bridge laboratory performance and industrial requirements are urgently needed. These protocols should incorporate realistic operating conditions, including the presence of contaminants, variable feed compositions, and pressure/temperature fluctuations typical in industrial settings. Only through such comprehensive evaluation can truly scalable catalyst technologies be identified and developed for commercial implementation.
Reactor scale-up represents a primary challenge, as the transition from small-scale testing platforms to industrial-sized reactors introduces mass transport limitations and uneven current distribution. These factors can significantly reduce ammonia selectivity and production rates compared to laboratory results. Membrane electrode assemblies (MEAs) and flow cell designs show promise for addressing these challenges by optimizing nitrogen delivery to catalyst active sites.
Catalyst stability under continuous operation conditions remains insufficiently addressed in most benchmarking studies. Industrial implementation requires catalysts that maintain ammonia selectivity for thousands of hours, whereas most published research demonstrates stability for only a few hours or cycles. Long-term deactivation mechanisms, including poisoning, structural changes, and leaching, must be thoroughly investigated to develop robust catalysts suitable for industrial deployment.
Energy efficiency considerations are paramount for economic viability. Current NRR processes operating at ambient conditions typically require high overpotentials, resulting in energy consumption levels that make them uncompetitive with conventional Haber-Bosch processes. Benchmarking efforts should incorporate energy efficiency metrics and techno-economic analyses to guide development toward commercially viable solutions.
Supply chain considerations for catalyst materials present another critical dimension. Many high-performing catalysts utilize precious metals or rare earth elements that may face supply constraints at industrial scales. Future benchmarking should prioritize earth-abundant materials and evaluate manufacturing scalability alongside performance metrics.
Integration with renewable energy sources represents both a challenge and opportunity. The intermittent nature of renewable electricity requires NRR systems capable of operating efficiently under variable power inputs. Catalyst benchmarking should include performance evaluation under fluctuating potential conditions to simulate real-world renewable energy integration scenarios.
Standardized testing protocols that bridge laboratory performance and industrial requirements are urgently needed. These protocols should incorporate realistic operating conditions, including the presence of contaminants, variable feed compositions, and pressure/temperature fluctuations typical in industrial settings. Only through such comprehensive evaluation can truly scalable catalyst technologies be identified and developed for commercial implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!