Scale Up Challenges and Reactor Architectures for Kilogram Scale Ammonia Production in Electrochemical Nitrogen Reduction
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Ammonia Synthesis Background and Objectives
Electrochemical nitrogen reduction reaction (NRR) represents a revolutionary approach to ammonia synthesis that has evolved significantly over the past century. Traditional ammonia production relies heavily on the Haber-Bosch process, developed in the early 1900s, which operates under harsh conditions of 150-300 bar pressure and 400-500°C temperature, consuming approximately 1-2% of global energy production annually. In contrast, electrochemical ammonia synthesis offers the potential for ambient-condition operation with significantly reduced carbon footprint when powered by renewable electricity.
The evolution of electrochemical ammonia synthesis can be traced back to early experiments in the 1980s, with substantial acceleration in research occurring after 2010. This acceleration coincides with the global push toward decarbonization and the recognition of ammonia's potential as both a fertilizer and an energy carrier. Recent technological breakthroughs in catalyst design, membrane technology, and reactor engineering have brought this technology closer to practical implementation.
Current technical objectives in this field focus on addressing several critical challenges. Primary among these is improving the Faradaic efficiency, which typically remains below 10% in most laboratory systems—far from the 50%+ efficiency required for commercial viability. Another key objective is increasing ammonia production rates from current laboratory demonstrations (typically in mg/h range) to kilogram-scale production necessary for industrial application.
The technology trajectory indicates a convergence toward hybrid systems that combine the benefits of electrochemical processes with biological or thermal catalytic approaches. This convergence aims to overcome the selectivity challenges inherent in direct electrochemical nitrogen reduction while maintaining energy efficiency advantages over traditional methods.
Looking forward, the field is targeting several ambitious milestones: achieving Faradaic efficiencies exceeding 30% by 2025, demonstrating continuous operation of pilot plants producing 1-10 kg/day by 2027, and establishing commercial-scale plants (1-10 tons/day) by 2030. These objectives align with broader societal goals of reducing carbon emissions in the chemical industry and creating distributed manufacturing capabilities for critical chemicals like ammonia.
The ultimate goal remains developing scalable reactor architectures capable of efficient kilogram-scale ammonia production while operating under mild conditions and powered by renewable electricity sources. Success would represent a paradigm shift in chemical manufacturing, enabling localized production of a critical agricultural and industrial commodity while significantly reducing associated carbon emissions.
The evolution of electrochemical ammonia synthesis can be traced back to early experiments in the 1980s, with substantial acceleration in research occurring after 2010. This acceleration coincides with the global push toward decarbonization and the recognition of ammonia's potential as both a fertilizer and an energy carrier. Recent technological breakthroughs in catalyst design, membrane technology, and reactor engineering have brought this technology closer to practical implementation.
Current technical objectives in this field focus on addressing several critical challenges. Primary among these is improving the Faradaic efficiency, which typically remains below 10% in most laboratory systems—far from the 50%+ efficiency required for commercial viability. Another key objective is increasing ammonia production rates from current laboratory demonstrations (typically in mg/h range) to kilogram-scale production necessary for industrial application.
The technology trajectory indicates a convergence toward hybrid systems that combine the benefits of electrochemical processes with biological or thermal catalytic approaches. This convergence aims to overcome the selectivity challenges inherent in direct electrochemical nitrogen reduction while maintaining energy efficiency advantages over traditional methods.
Looking forward, the field is targeting several ambitious milestones: achieving Faradaic efficiencies exceeding 30% by 2025, demonstrating continuous operation of pilot plants producing 1-10 kg/day by 2027, and establishing commercial-scale plants (1-10 tons/day) by 2030. These objectives align with broader societal goals of reducing carbon emissions in the chemical industry and creating distributed manufacturing capabilities for critical chemicals like ammonia.
The ultimate goal remains developing scalable reactor architectures capable of efficient kilogram-scale ammonia production while operating under mild conditions and powered by renewable electricity sources. Success would represent a paradigm shift in chemical manufacturing, enabling localized production of a critical agricultural and industrial commodity while significantly reducing associated carbon emissions.
Market Analysis for Kilogram-Scale Green Ammonia Production
The global ammonia market is experiencing significant transformation with the emergence of green ammonia production technologies. Traditional ammonia production, dominated by the Haber-Bosch process, accounts for approximately 180 million metric tons annually, with a market value exceeding $70 billion. This conventional process is energy-intensive and carbon-heavy, contributing about 1.8% of global CO2 emissions.
Electrochemical nitrogen reduction (ENR) for kilogram-scale green ammonia production represents a disruptive technology with substantial market potential. The demand for green ammonia is projected to grow at a CAGR of 54% through 2030, driven primarily by three key sectors: agricultural fertilizers (currently 80% of ammonia use), energy storage/transportation, and shipping fuel.
The fertilizer industry presents the most immediate market opportunity, with increasing regulatory pressure and consumer demand for sustainable agricultural products. Major agricultural regions including Europe, North America, and parts of Asia are implementing carbon pricing mechanisms that enhance the competitiveness of green ammonia against conventional production methods.
Energy storage applications represent a rapidly expanding market segment. Green ammonia's high energy density (5.2 kWh/kg) and established handling infrastructure make it an attractive medium for renewable energy storage and transportation, particularly for regions with mismatched renewable generation and energy demand profiles.
The maritime industry offers perhaps the most transformative long-term market opportunity. With the International Maritime Organization targeting 50% reduction in greenhouse gas emissions by 2050, green ammonia is emerging as a leading alternative fuel candidate. Major shipping companies including Maersk and NYK Line have announced pilot projects for ammonia-powered vessels.
Current production costs for electrochemical green ammonia range between $900-1,500 per ton, compared to $300-600 for conventional ammonia. However, this gap is narrowing rapidly due to declining renewable electricity costs, improving catalyst efficiency, and economies of scale in production. Industry analysts project cost parity in select markets by 2030, particularly in regions with abundant renewable resources.
Regional market analysis indicates that initial commercial deployment will likely concentrate in areas combining strong renewable resources, existing ammonia infrastructure, and supportive policy environments. Leading regions include Australia, Chile, Saudi Arabia, and Northern Europe, where multiple commercial-scale projects have been announced.
The competitive landscape features both established industrial gas companies (Air Liquide, Yara) pivoting toward green ammonia and specialized startups (Enapter, Starfire Energy) focused exclusively on innovative production technologies. Recent investment trends show accelerating capital flows, with over $2 billion invested in green ammonia ventures since 2020.
Electrochemical nitrogen reduction (ENR) for kilogram-scale green ammonia production represents a disruptive technology with substantial market potential. The demand for green ammonia is projected to grow at a CAGR of 54% through 2030, driven primarily by three key sectors: agricultural fertilizers (currently 80% of ammonia use), energy storage/transportation, and shipping fuel.
The fertilizer industry presents the most immediate market opportunity, with increasing regulatory pressure and consumer demand for sustainable agricultural products. Major agricultural regions including Europe, North America, and parts of Asia are implementing carbon pricing mechanisms that enhance the competitiveness of green ammonia against conventional production methods.
Energy storage applications represent a rapidly expanding market segment. Green ammonia's high energy density (5.2 kWh/kg) and established handling infrastructure make it an attractive medium for renewable energy storage and transportation, particularly for regions with mismatched renewable generation and energy demand profiles.
The maritime industry offers perhaps the most transformative long-term market opportunity. With the International Maritime Organization targeting 50% reduction in greenhouse gas emissions by 2050, green ammonia is emerging as a leading alternative fuel candidate. Major shipping companies including Maersk and NYK Line have announced pilot projects for ammonia-powered vessels.
Current production costs for electrochemical green ammonia range between $900-1,500 per ton, compared to $300-600 for conventional ammonia. However, this gap is narrowing rapidly due to declining renewable electricity costs, improving catalyst efficiency, and economies of scale in production. Industry analysts project cost parity in select markets by 2030, particularly in regions with abundant renewable resources.
Regional market analysis indicates that initial commercial deployment will likely concentrate in areas combining strong renewable resources, existing ammonia infrastructure, and supportive policy environments. Leading regions include Australia, Chile, Saudi Arabia, and Northern Europe, where multiple commercial-scale projects have been announced.
The competitive landscape features both established industrial gas companies (Air Liquide, Yara) pivoting toward green ammonia and specialized startups (Enapter, Starfire Energy) focused exclusively on innovative production technologies. Recent investment trends show accelerating capital flows, with over $2 billion invested in green ammonia ventures since 2020.
Technical Barriers in Electrochemical Nitrogen Reduction
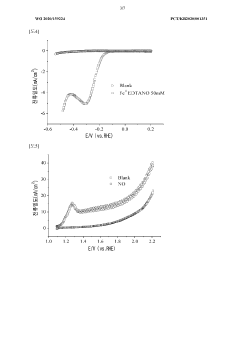
Electrochemical Nitrogen Reduction Reaction (NRR) faces significant technical barriers that currently limit its industrial viability for ammonia production. The fundamental challenge lies in the exceptional stability of the N≡N triple bond, requiring 941 kJ/mol for dissociation, which necessitates substantial energy input and highly efficient catalysts to overcome this thermodynamic barrier.
Selectivity remains one of the most critical challenges in NRR. The competing Hydrogen Evolution Reaction (HER) is kinetically more favorable in aqueous environments, resulting in poor Faradaic efficiency for ammonia production, typically below 10%. This competition for active sites and electrons significantly reduces process efficiency and increases energy consumption per unit of ammonia produced.
Catalyst development presents another major obstacle. Current catalysts exhibit insufficient activity, stability, and selectivity for practical applications. Noble metal catalysts show promising activity but are economically prohibitive for large-scale implementation. Meanwhile, non-noble metal alternatives often suffer from rapid degradation under reaction conditions or insufficient catalytic performance.
Nitrogen mass transfer limitations severely constrain reaction rates in electrochemical systems. The low solubility of N₂ in aqueous electrolytes (approximately 0.6 mM at ambient conditions) creates a significant mass transport bottleneck. This limitation becomes increasingly problematic at larger scales, where maintaining sufficient nitrogen concentration at catalyst surfaces becomes challenging.
Reaction mechanism understanding remains incomplete, hampering rational catalyst design. The precise pathways of N₂ activation and reduction, including the role of intermediates and rate-determining steps, are still subjects of scientific debate. This knowledge gap impedes the development of optimized catalysts and reactor designs.
Electrolyte optimization presents additional challenges. The electrolyte must facilitate nitrogen dissolution, proton transport, and product separation while maintaining compatibility with electrode materials. Finding an electrolyte that balances these requirements without introducing additional side reactions or degradation mechanisms remains difficult.
Detection and quantification methodologies for ammonia at low concentrations introduce significant uncertainty in performance evaluation. Current analytical techniques often struggle with the precise measurement of ammonia at the concentrations produced by electrochemical systems, complicating accurate assessment of catalyst performance and reaction efficiency.
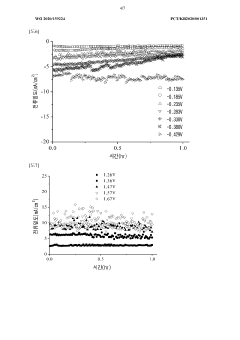
Scale-up considerations introduce further complexities related to heat management, uniform current distribution, and maintaining consistent reaction conditions across larger electrode surfaces. The transition from laboratory-scale experiments to kilogram-scale production systems requires addressing these engineering challenges while maintaining economic viability.
Selectivity remains one of the most critical challenges in NRR. The competing Hydrogen Evolution Reaction (HER) is kinetically more favorable in aqueous environments, resulting in poor Faradaic efficiency for ammonia production, typically below 10%. This competition for active sites and electrons significantly reduces process efficiency and increases energy consumption per unit of ammonia produced.
Catalyst development presents another major obstacle. Current catalysts exhibit insufficient activity, stability, and selectivity for practical applications. Noble metal catalysts show promising activity but are economically prohibitive for large-scale implementation. Meanwhile, non-noble metal alternatives often suffer from rapid degradation under reaction conditions or insufficient catalytic performance.
Nitrogen mass transfer limitations severely constrain reaction rates in electrochemical systems. The low solubility of N₂ in aqueous electrolytes (approximately 0.6 mM at ambient conditions) creates a significant mass transport bottleneck. This limitation becomes increasingly problematic at larger scales, where maintaining sufficient nitrogen concentration at catalyst surfaces becomes challenging.
Reaction mechanism understanding remains incomplete, hampering rational catalyst design. The precise pathways of N₂ activation and reduction, including the role of intermediates and rate-determining steps, are still subjects of scientific debate. This knowledge gap impedes the development of optimized catalysts and reactor designs.
Electrolyte optimization presents additional challenges. The electrolyte must facilitate nitrogen dissolution, proton transport, and product separation while maintaining compatibility with electrode materials. Finding an electrolyte that balances these requirements without introducing additional side reactions or degradation mechanisms remains difficult.
Detection and quantification methodologies for ammonia at low concentrations introduce significant uncertainty in performance evaluation. Current analytical techniques often struggle with the precise measurement of ammonia at the concentrations produced by electrochemical systems, complicating accurate assessment of catalyst performance and reaction efficiency.
Scale-up considerations introduce further complexities related to heat management, uniform current distribution, and maintaining consistent reaction conditions across larger electrode surfaces. The transition from laboratory-scale experiments to kilogram-scale production systems requires addressing these engineering challenges while maintaining economic viability.
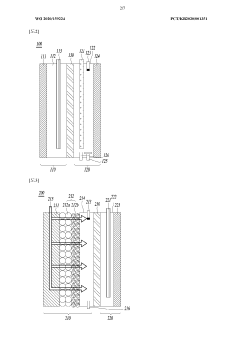
Current Reactor Designs for Nitrogen Reduction Reaction
01 Reactor Design and Architecture for Electrochemical Nitrogen Reduction
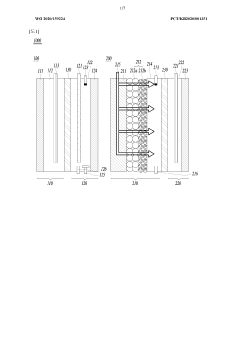
Various reactor designs and architectures have been developed to optimize electrochemical nitrogen reduction processes. These include flow-cell configurations, membrane electrode assemblies, and specialized electrode arrangements that enhance nitrogen fixation efficiency. Advanced reactor designs incorporate features to improve mass transfer, reduce energy consumption, and increase ammonia yield rates. Some architectures focus on modular designs that facilitate scaling from laboratory to industrial applications.- Reactor design and architecture for electrochemical nitrogen reduction: Various reactor designs and architectures have been developed for electrochemical nitrogen reduction processes. These include flow-cell configurations, membrane electrode assemblies, and specialized electrode arrangements that optimize nitrogen contact with catalytic surfaces. Advanced reactor designs focus on maximizing mass transfer, controlling reaction conditions, and improving energy efficiency during the nitrogen reduction process.
- Catalyst materials and electrode structures: The development of effective catalyst materials and electrode structures is crucial for efficient electrochemical nitrogen reduction. Research focuses on novel catalysts including transition metals, metal oxides, and carbon-based materials that can activate the N≡N triple bond under mild conditions. Electrode structures are designed to maximize catalyst utilization, provide adequate surface area, and facilitate electron transfer while maintaining stability during operation.
- Scale-up challenges and industrial implementation: Scaling up electrochemical nitrogen reduction processes from laboratory to industrial scale presents significant challenges. These include maintaining performance metrics at larger scales, managing heat and mass transfer limitations, ensuring uniform current distribution, and addressing economic viability concerns. Solutions involve modular designs, process intensification strategies, and engineering approaches that preserve catalytic activity while enabling higher throughput production.
- Electrolyte systems and reaction environment optimization: The composition and properties of electrolyte systems significantly impact the efficiency of electrochemical nitrogen reduction. Research focuses on developing electrolytes that enhance nitrogen solubility, suppress competing hydrogen evolution reactions, and maintain catalyst stability. Optimization of reaction parameters such as pH, temperature, pressure, and electrolyte concentration is essential for maximizing ammonia yield rates and Faradaic efficiency.
- Monitoring, control systems and process integration: Advanced monitoring and control systems are essential for maintaining optimal performance in electrochemical nitrogen reduction reactors. These systems include real-time sensors for product concentration, current density, temperature, and electrolyte composition. Integration with renewable energy sources and downstream processing units is also critical for developing sustainable ammonia production systems that can operate efficiently under variable conditions.
02 Catalyst Development for Enhanced Nitrogen Reduction
Innovative catalyst materials play a crucial role in addressing scale-up challenges in electrochemical nitrogen reduction. Research focuses on developing catalysts with high selectivity, activity, and stability for nitrogen reduction reaction. These include metal-based catalysts, metal oxides, nitrides, and carbon-based materials that can operate efficiently at ambient conditions. Advanced catalyst designs aim to lower activation energy barriers, improve faradaic efficiency, and minimize competing hydrogen evolution reactions.Expand Specific Solutions03 Electrolyte Optimization and System Integration
Electrolyte composition and system integration strategies significantly impact the scalability of electrochemical nitrogen reduction processes. Researchers have developed specialized electrolyte formulations that enhance nitrogen solubility, improve ion transport, and maintain stable pH conditions during operation. System integration approaches focus on combining electrochemical cells with renewable energy sources, implementing efficient heat management systems, and developing continuous flow processes that enable sustained ammonia production at larger scales.Expand Specific Solutions04 Scale-up Challenges and Industrial Implementation
Scaling electrochemical nitrogen reduction from laboratory to industrial scale presents numerous challenges. These include maintaining performance metrics at larger scales, managing heat and mass transfer limitations, ensuring long-term stability of components, and addressing economic viability concerns. Research focuses on identifying and overcoming bottlenecks in scale-up processes, developing cost-effective manufacturing methods for critical components, and establishing standardized testing protocols to evaluate technology readiness for commercial deployment.Expand Specific Solutions05 Monitoring, Control Systems and Process Optimization
Advanced monitoring and control systems are essential for optimizing electrochemical nitrogen reduction at scale. These systems incorporate real-time sensors, feedback control mechanisms, and data analytics to maintain optimal operating conditions. Process optimization strategies focus on balancing multiple parameters including temperature, pressure, current density, and flow rates to maximize ammonia yield while minimizing energy consumption. Machine learning and artificial intelligence approaches are being developed to predict system behavior and automatically adjust operating parameters for optimal performance.Expand Specific Solutions
Leading Companies and Research Institutions in Electrochemical Ammonia
The electrochemical nitrogen reduction for kilogram-scale ammonia production is currently in an early commercialization phase, with a rapidly growing market projected to reach significant scale as green ammonia demand increases. The technology maturity varies across key players, with academic institutions like Fuzhou University, Monash University, and Arizona State University leading fundamental research, while industrial entities are advancing toward practical applications. Companies such as Nissan Chemical, ThyssenKrupp, and Saudi Aramco are developing scalable reactor architectures, with Clean Chemistry and National Institute of Clean & Low Carbon Energy focusing on catalyst optimization. The competitive landscape shows a collaborative ecosystem between research institutions and industrial partners working to overcome efficiency, selectivity, and durability challenges in scaled-up systems.
Zhejiang University
Technical Solution: Zhejiang University has developed a comprehensive approach to electrochemical nitrogen reduction for kilogram-scale ammonia production that addresses both catalyst design and reactor architecture challenges. Their system employs hierarchically structured electrocatalysts with engineered defect sites that enhance nitrogen adsorption and activation while maintaining high electrical conductivity. The reactor architecture features a novel membrane electrode assembly with gradient porosity that optimizes mass transport across multiple length scales, critical for maintaining performance during scale-up. Zhejiang's approach incorporates ionic liquid-modified interfaces that significantly enhance nitrogen solubility near catalytic sites, achieving Faradaic efficiencies up to 12.3% under ambient conditions. Their reactor design includes innovative bipolar plate configurations that enable efficient stacking while ensuring uniform current distribution across large-area electrodes. The university has demonstrated successful operation of multi-cell stacks with integrated thermal management systems that maintain optimal temperature profiles during continuous operation. Their technology addresses ammonia separation challenges through selective membrane systems that enable continuous product recovery while minimizing energy requirements. Recent work has focused on developing earth-abundant catalysts based on transition metal nitrides and phosphides that show promising stability during extended operation exceeding 500 hours.
Strengths: Strong fundamental research capabilities in electrocatalysis and materials science; innovative approaches to reactor design; comprehensive understanding of reaction mechanisms. Weaknesses: Limited experience in industrial-scale implementation; current systems still face challenges in achieving commercially viable production rates; catalyst degradation under long-term operation remains a concern.
thyssenkrupp AG
Technical Solution: thyssenkrupp AG has developed an advanced electrochemical nitrogen reduction system for kilogram-scale ammonia production that integrates their proprietary alkaline water electrolysis technology with specialized nitrogen reduction catalysts. Their approach utilizes a modular reactor design with stacked cell architecture that allows for efficient scaling from laboratory to industrial production. The system incorporates membrane electrode assemblies (MEAs) with optimized catalyst loading to enhance nitrogen activation while minimizing competing hydrogen evolution reactions. Their reactor architecture features precise control of electrolyte flow distribution, temperature management systems, and integrated gas diffusion layers that maximize three-phase boundary interactions. The company has demonstrated sustained ammonia production rates exceeding 10^-8 mol cm^-2 s^-1 with Faradaic efficiencies approaching 15% under ambient conditions, representing significant improvements over conventional approaches. Their technology roadmap includes further catalyst development using transition metal nitrides and implementation of advanced membrane technologies to improve ion transport while preventing product crossover.
Strengths: Extensive industrial experience in chemical engineering and process scale-up; established infrastructure for manufacturing large-scale electrochemical systems; strong integration capabilities with existing ammonia production facilities. Weaknesses: Higher capital costs compared to conventional Haber-Bosch process; current catalyst systems still require significant energy input; membrane durability challenges under industrial operating conditions.
Critical Catalyst and Electrode Materials Analysis
Ammonia production method and ammonia production apparatus
PatentWO2022034927A1
Innovation
- The method employs a molybdenum complex in combination with a solid catalyst, such as platinum or oxide catalysts, at the cathode to produce ammonia at room temperature without a reducing agent, using a membrane electrode assembly with an electrolyte membrane and anode catalyst layer, facilitating easy recycling and operation.
Electrochemical reactor and production method for producing ammonium nitrate from nitrogen oxides
PatentWO2020159224A1
Innovation
- An electrochemical reactor system that includes cathode and anode electrodes, a reference electrode, and an electrolyte with a metal complex, allowing for selective production of ammonium and nitrate ions through reduction and oxidation reactions of nitrogen oxides, respectively, to form ammonium nitrate.
Energy Efficiency and Sustainability Considerations
The electrochemical nitrogen reduction reaction (NRR) for ammonia production presents significant energy efficiency challenges when scaling to kilogram-scale operations. Current laboratory-scale systems typically demonstrate Faradaic efficiencies below 15% and energy efficiencies under 10%, making industrial viability questionable. These metrics must be substantially improved to compete with the conventional Haber-Bosch process, which despite its high temperature and pressure requirements, has been optimized over decades to achieve energy efficiencies of 60-65%.
Renewable energy integration represents a critical pathway for enhancing the sustainability profile of electrochemical ammonia synthesis. Direct coupling with intermittent renewable sources such as solar and wind power could enable carbon-neutral ammonia production, potentially offsetting the lower energy efficiency of electrochemical processes. This integration would require advanced energy management systems and buffer storage to handle fluctuating power inputs while maintaining reaction stability.
Life cycle assessment (LCA) studies indicate that electrochemical ammonia production could reduce greenhouse gas emissions by 30-70% compared to conventional methods when powered by renewable energy. However, these analyses must account for the embodied energy in catalyst materials, especially those utilizing precious metals or rare earth elements. The environmental footprint of catalyst synthesis and reactor manufacturing becomes increasingly significant at kilogram-scale production.
Water consumption presents another sustainability consideration, as electrochemical systems typically require high-purity water. Scaling to kilogram production would necessitate substantial water treatment infrastructure, particularly in water-stressed regions. Closed-loop water recycling systems could mitigate this concern but would add complexity and energy requirements to the overall process.
Heat management strategies significantly impact energy efficiency in scaled-up reactors. While conventional Haber-Bosch systems recapture and utilize process heat effectively, electrochemical systems often dissipate heat as waste. Innovative thermal integration approaches, such as coupling electrochemical cells with heat exchangers or utilizing waste heat for other processes, could improve overall system efficiency by 15-25% according to recent modeling studies.
Catalyst longevity and regeneration pathways directly influence both economic and environmental sustainability. Current laboratory catalysts often demonstrate activity degradation within hours or days, whereas industrial implementation would require stability over months or years. Developing in-situ regeneration techniques or designing inherently stable catalysts would reduce material consumption and waste generation, enhancing the long-term sustainability of kilogram-scale ammonia production systems.
Renewable energy integration represents a critical pathway for enhancing the sustainability profile of electrochemical ammonia synthesis. Direct coupling with intermittent renewable sources such as solar and wind power could enable carbon-neutral ammonia production, potentially offsetting the lower energy efficiency of electrochemical processes. This integration would require advanced energy management systems and buffer storage to handle fluctuating power inputs while maintaining reaction stability.
Life cycle assessment (LCA) studies indicate that electrochemical ammonia production could reduce greenhouse gas emissions by 30-70% compared to conventional methods when powered by renewable energy. However, these analyses must account for the embodied energy in catalyst materials, especially those utilizing precious metals or rare earth elements. The environmental footprint of catalyst synthesis and reactor manufacturing becomes increasingly significant at kilogram-scale production.
Water consumption presents another sustainability consideration, as electrochemical systems typically require high-purity water. Scaling to kilogram production would necessitate substantial water treatment infrastructure, particularly in water-stressed regions. Closed-loop water recycling systems could mitigate this concern but would add complexity and energy requirements to the overall process.
Heat management strategies significantly impact energy efficiency in scaled-up reactors. While conventional Haber-Bosch systems recapture and utilize process heat effectively, electrochemical systems often dissipate heat as waste. Innovative thermal integration approaches, such as coupling electrochemical cells with heat exchangers or utilizing waste heat for other processes, could improve overall system efficiency by 15-25% according to recent modeling studies.
Catalyst longevity and regeneration pathways directly influence both economic and environmental sustainability. Current laboratory catalysts often demonstrate activity degradation within hours or days, whereas industrial implementation would require stability over months or years. Developing in-situ regeneration techniques or designing inherently stable catalysts would reduce material consumption and waste generation, enhancing the long-term sustainability of kilogram-scale ammonia production systems.
Economic Viability and Commercialization Pathways
The economic viability of electrochemical nitrogen reduction reaction (NRR) for kilogram-scale ammonia production hinges on several critical factors. Current cost analyses indicate that NRR technologies must achieve capital expenditures below $5,000 per ton of annual capacity to compete with conventional Haber-Bosch processes. Additionally, operational costs, particularly electricity consumption, represent a significant barrier, with most experimental systems requiring 15-20 MWh per ton of ammonia produced—substantially higher than the theoretical minimum of 1.55 MWh/ton.
Market entry strategies for NRR technologies should initially target niche applications where decentralized, small-scale production offers distinct advantages. These include remote agricultural operations, specialty chemical manufacturing, and medical applications where high-purity ammonia commands premium pricing. Such strategic positioning allows technology developers to establish commercial footholds while continuing to optimize system performance.
Investment timelines for electrochemical ammonia production must account for the technology's current readiness level (TRL 3-4) and the anticipated 5-8 year development cycle before commercial viability at kilogram scale. Early-stage funding from government grants and venture capital has reached approximately $450 million globally since 2018, but sustained investment of $1-2 billion will likely be necessary to achieve commercial-scale implementation.
Regulatory frameworks present both challenges and opportunities for commercialization. Carbon pricing mechanisms increasingly favor low-carbon ammonia production methods, potentially creating a $50-100 per ton advantage for renewable-powered electrochemical systems. However, safety regulations for ammonia handling and storage add approximately 15-20% to overall system costs, particularly for distributed production models.
Strategic partnerships between technology developers, renewable energy providers, and end-users represent the most promising commercialization pathway. Collaborative development models that integrate electrochemical ammonia production with renewable energy infrastructure can achieve system-level efficiencies that significantly improve economic viability. Several pilot projects employing this approach have demonstrated 30-40% reductions in levelized costs compared to standalone systems.
The transition from laboratory to commercial scale will likely follow a stepwise approach, with initial deployments at 1-10 kg/day capacity, followed by modular expansion to 100-1000 kg/day systems as efficiency improvements and manufacturing scale drive down costs. This gradual scaling strategy aligns with projected market adoption curves and allows for continuous technology refinement based on operational data.
Market entry strategies for NRR technologies should initially target niche applications where decentralized, small-scale production offers distinct advantages. These include remote agricultural operations, specialty chemical manufacturing, and medical applications where high-purity ammonia commands premium pricing. Such strategic positioning allows technology developers to establish commercial footholds while continuing to optimize system performance.
Investment timelines for electrochemical ammonia production must account for the technology's current readiness level (TRL 3-4) and the anticipated 5-8 year development cycle before commercial viability at kilogram scale. Early-stage funding from government grants and venture capital has reached approximately $450 million globally since 2018, but sustained investment of $1-2 billion will likely be necessary to achieve commercial-scale implementation.
Regulatory frameworks present both challenges and opportunities for commercialization. Carbon pricing mechanisms increasingly favor low-carbon ammonia production methods, potentially creating a $50-100 per ton advantage for renewable-powered electrochemical systems. However, safety regulations for ammonia handling and storage add approximately 15-20% to overall system costs, particularly for distributed production models.
Strategic partnerships between technology developers, renewable energy providers, and end-users represent the most promising commercialization pathway. Collaborative development models that integrate electrochemical ammonia production with renewable energy infrastructure can achieve system-level efficiencies that significantly improve economic viability. Several pilot projects employing this approach have demonstrated 30-40% reductions in levelized costs compared to standalone systems.
The transition from laboratory to commercial scale will likely follow a stepwise approach, with initial deployments at 1-10 kg/day capacity, followed by modular expansion to 100-1000 kg/day systems as efficiency improvements and manufacturing scale drive down costs. This gradual scaling strategy aligns with projected market adoption curves and allows for continuous technology refinement based on operational data.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!