Membrane Selection and Ion Transport Effects on Ammonia Yield in Electrochemical Nitrogen Reduction
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Nitrogen Reduction Technology Background and Objectives
Electrochemical Nitrogen Reduction (ENR) technology represents a revolutionary approach to ammonia synthesis that has emerged as a promising alternative to the conventional Haber-Bosch process. The development of ENR can be traced back to the early 2000s when researchers began exploring electrochemical methods for nitrogen fixation under ambient conditions. This technology has gained significant momentum in the past decade due to increasing concerns about the energy intensity and carbon footprint of traditional ammonia production methods.
The evolution of ENR technology has been marked by several key advancements, particularly in electrocatalyst design, reactor configuration, and membrane technology. Initial research focused primarily on noble metal catalysts, but recent trends have shifted toward earth-abundant materials and nanostructured catalysts to enhance nitrogen reduction reaction (NRR) efficiency while reducing costs. Membrane selection has emerged as a critical factor influencing ammonia yield, with significant research dedicated to understanding ion transport mechanisms across different membrane materials.
Current technological trajectories indicate a growing emphasis on integrating renewable energy sources with ENR systems to achieve truly sustainable ammonia production. This integration aligns with global efforts to decarbonize the chemical industry and develop carbon-neutral fertilizer production pathways. The potential for distributed, small-scale ammonia synthesis using ENR technology also presents opportunities for localized production in agricultural regions, potentially reducing transportation costs and emissions.
The primary technical objectives in this field include improving Faradaic efficiency, which currently remains below commercially viable levels for most systems. Researchers aim to achieve Faradaic efficiencies exceeding 50% while maintaining ammonia production rates above 10^-6 mol cm^-2 h^-1. Additionally, enhancing catalyst selectivity to minimize the competing hydrogen evolution reaction represents a significant challenge that must be addressed through innovative membrane selection and reactor design.
Understanding ion transport phenomena across membranes has become increasingly recognized as a fundamental aspect of ENR performance optimization. Different membrane materials exhibit varying ion conductivity, selectivity, and stability characteristics that directly impact nitrogen reduction pathways and ammonia formation. The interplay between membrane properties, electrolyte composition, and catalyst surface chemistry creates a complex system that requires systematic investigation.
The ultimate goal of ENR technology development is to establish a sustainable, energy-efficient alternative to the Haber-Bosch process that can operate under ambient conditions using renewable electricity. This would represent a paradigm shift in ammonia production, potentially reducing global energy consumption and greenhouse gas emissions associated with fertilizer production while improving accessibility to this essential agricultural input in regions with limited infrastructure.
The evolution of ENR technology has been marked by several key advancements, particularly in electrocatalyst design, reactor configuration, and membrane technology. Initial research focused primarily on noble metal catalysts, but recent trends have shifted toward earth-abundant materials and nanostructured catalysts to enhance nitrogen reduction reaction (NRR) efficiency while reducing costs. Membrane selection has emerged as a critical factor influencing ammonia yield, with significant research dedicated to understanding ion transport mechanisms across different membrane materials.
Current technological trajectories indicate a growing emphasis on integrating renewable energy sources with ENR systems to achieve truly sustainable ammonia production. This integration aligns with global efforts to decarbonize the chemical industry and develop carbon-neutral fertilizer production pathways. The potential for distributed, small-scale ammonia synthesis using ENR technology also presents opportunities for localized production in agricultural regions, potentially reducing transportation costs and emissions.
The primary technical objectives in this field include improving Faradaic efficiency, which currently remains below commercially viable levels for most systems. Researchers aim to achieve Faradaic efficiencies exceeding 50% while maintaining ammonia production rates above 10^-6 mol cm^-2 h^-1. Additionally, enhancing catalyst selectivity to minimize the competing hydrogen evolution reaction represents a significant challenge that must be addressed through innovative membrane selection and reactor design.
Understanding ion transport phenomena across membranes has become increasingly recognized as a fundamental aspect of ENR performance optimization. Different membrane materials exhibit varying ion conductivity, selectivity, and stability characteristics that directly impact nitrogen reduction pathways and ammonia formation. The interplay between membrane properties, electrolyte composition, and catalyst surface chemistry creates a complex system that requires systematic investigation.
The ultimate goal of ENR technology development is to establish a sustainable, energy-efficient alternative to the Haber-Bosch process that can operate under ambient conditions using renewable electricity. This would represent a paradigm shift in ammonia production, potentially reducing global energy consumption and greenhouse gas emissions associated with fertilizer production while improving accessibility to this essential agricultural input in regions with limited infrastructure.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability concerns and technological innovations. Traditional ammonia production via the Haber-Bosch process consumes approximately 2% of global energy and contributes substantially to greenhouse gas emissions. This creates a compelling market opportunity for sustainable alternatives such as electrochemical nitrogen reduction reaction (ENRR) technologies.
Current market valuation for conventional ammonia production stands at approximately $70 billion annually, with production volumes exceeding 180 million metric tons. Growth projections indicate this market could reach $95 billion by 2030, primarily driven by agricultural applications which consume over 80% of produced ammonia for fertilizers.
The sustainable ammonia segment, though currently representing less than 1% of the total market, is projected to grow at a CAGR of 35% through 2030. This acceleration is fueled by stringent environmental regulations, carbon pricing mechanisms, and corporate sustainability commitments across industrial sectors.
Key market drivers for membrane-based ENRR technologies include the agricultural sector's need for localized, small-scale ammonia production facilities that reduce transportation costs and emissions. Additionally, the hydrogen economy's development creates synergistic opportunities, as green hydrogen production infrastructure can complement electrochemical ammonia synthesis systems.
Regional market analysis reveals particularly strong growth potential in Europe, where carbon pricing mechanisms and ambitious climate targets create favorable economics for sustainable ammonia. Asia-Pacific represents the largest volume market, with China and India's agricultural sectors driving demand. North America shows increasing interest in decentralized ammonia production systems for agricultural applications.
Investment trends indicate growing venture capital interest in ENRR startups, with funding rounds increasing from $50 million in 2018 to over $300 million in 2022. Strategic partnerships between technology developers and industrial gas companies are emerging as a dominant commercialization pathway.
Market barriers include cost competitiveness against conventional ammonia (currently 2-3 times more expensive), scaling challenges for membrane technologies, and regulatory uncertainties regarding certification of green ammonia. However, declining renewable electricity costs and carbon pricing mechanisms are rapidly improving the economic equation.
Customer segments show differentiated needs: agricultural users prioritize cost and reliability, while industrial users focus on carbon intensity and supply chain resilience. The premium green ammonia market is currently driven by specialty chemical applications and demonstration projects in shipping and energy storage.
Current market valuation for conventional ammonia production stands at approximately $70 billion annually, with production volumes exceeding 180 million metric tons. Growth projections indicate this market could reach $95 billion by 2030, primarily driven by agricultural applications which consume over 80% of produced ammonia for fertilizers.
The sustainable ammonia segment, though currently representing less than 1% of the total market, is projected to grow at a CAGR of 35% through 2030. This acceleration is fueled by stringent environmental regulations, carbon pricing mechanisms, and corporate sustainability commitments across industrial sectors.
Key market drivers for membrane-based ENRR technologies include the agricultural sector's need for localized, small-scale ammonia production facilities that reduce transportation costs and emissions. Additionally, the hydrogen economy's development creates synergistic opportunities, as green hydrogen production infrastructure can complement electrochemical ammonia synthesis systems.
Regional market analysis reveals particularly strong growth potential in Europe, where carbon pricing mechanisms and ambitious climate targets create favorable economics for sustainable ammonia. Asia-Pacific represents the largest volume market, with China and India's agricultural sectors driving demand. North America shows increasing interest in decentralized ammonia production systems for agricultural applications.
Investment trends indicate growing venture capital interest in ENRR startups, with funding rounds increasing from $50 million in 2018 to over $300 million in 2022. Strategic partnerships between technology developers and industrial gas companies are emerging as a dominant commercialization pathway.
Market barriers include cost competitiveness against conventional ammonia (currently 2-3 times more expensive), scaling challenges for membrane technologies, and regulatory uncertainties regarding certification of green ammonia. However, declining renewable electricity costs and carbon pricing mechanisms are rapidly improving the economic equation.
Customer segments show differentiated needs: agricultural users prioritize cost and reliability, while industrial users focus on carbon intensity and supply chain resilience. The premium green ammonia market is currently driven by specialty chemical applications and demonstration projects in shipping and energy storage.
Current Membrane Technology Challenges in eNRR
The electrochemical nitrogen reduction reaction (eNRR) represents a promising approach for sustainable ammonia production, yet membrane technology remains a critical bottleneck limiting practical implementation. Current membrane systems face significant challenges in balancing ion selectivity, conductivity, and stability under eNRR operating conditions. Conventional ion exchange membranes, while effective in other electrochemical systems, often exhibit insufficient nitrogen permeability or undesired proton transfer that favors the competing hydrogen evolution reaction (HER).
Cation exchange membranes (CEMs) typically used in eNRR systems suffer from proton crossover issues, which significantly reduce Faradaic efficiency for ammonia production. When protons migrate too readily across the membrane, they become available for hydrogen evolution rather than nitrogen reduction, resulting in ammonia yields below 10% Faradaic efficiency in many systems. Conversely, anion exchange membranes (AEMs) face degradation challenges in the highly alkaline environments often employed to suppress HER.
Membrane fouling presents another substantial challenge, particularly in continuous operation scenarios. Intermediate reaction species and contaminants from feedstock gases can accumulate on membrane surfaces, progressively blocking ion transport channels and reducing system performance over time. Current membrane materials lack sufficient resistance to these fouling mechanisms, necessitating frequent replacement or regeneration procedures that impact economic viability.
The mechanical and chemical stability of membranes under eNRR conditions represents a significant hurdle. The combination of applied electrical potential, reactive nitrogen species, and often extreme pH conditions creates a highly demanding environment for membrane materials. Most commercially available membranes experience accelerated degradation under these conditions, with performance deterioration observed after only tens or hundreds of hours of operation.
Ion transport selectivity remains perhaps the most fundamental challenge. Ideal membranes for eNRR should selectively transport desired ions while blocking competing species, but achieving this selective permeability has proven exceptionally difficult. Current membranes exhibit poor discrimination between nitrogen-containing species and competing ions, limiting reaction specificity and yield.
Water management across the membrane interface presents additional complications. Excessive water transport can dilute reactants and products, while insufficient hydration reduces ionic conductivity. Existing membranes struggle to maintain optimal water balance across varying operating conditions, resulting in inconsistent performance profiles that complicate system design and scale-up efforts.
These challenges collectively highlight the need for next-generation membrane materials specifically engineered for eNRR applications, rather than continuing to adapt membranes designed for other electrochemical processes.
Cation exchange membranes (CEMs) typically used in eNRR systems suffer from proton crossover issues, which significantly reduce Faradaic efficiency for ammonia production. When protons migrate too readily across the membrane, they become available for hydrogen evolution rather than nitrogen reduction, resulting in ammonia yields below 10% Faradaic efficiency in many systems. Conversely, anion exchange membranes (AEMs) face degradation challenges in the highly alkaline environments often employed to suppress HER.
Membrane fouling presents another substantial challenge, particularly in continuous operation scenarios. Intermediate reaction species and contaminants from feedstock gases can accumulate on membrane surfaces, progressively blocking ion transport channels and reducing system performance over time. Current membrane materials lack sufficient resistance to these fouling mechanisms, necessitating frequent replacement or regeneration procedures that impact economic viability.
The mechanical and chemical stability of membranes under eNRR conditions represents a significant hurdle. The combination of applied electrical potential, reactive nitrogen species, and often extreme pH conditions creates a highly demanding environment for membrane materials. Most commercially available membranes experience accelerated degradation under these conditions, with performance deterioration observed after only tens or hundreds of hours of operation.
Ion transport selectivity remains perhaps the most fundamental challenge. Ideal membranes for eNRR should selectively transport desired ions while blocking competing species, but achieving this selective permeability has proven exceptionally difficult. Current membranes exhibit poor discrimination between nitrogen-containing species and competing ions, limiting reaction specificity and yield.
Water management across the membrane interface presents additional complications. Excessive water transport can dilute reactants and products, while insufficient hydration reduces ionic conductivity. Existing membranes struggle to maintain optimal water balance across varying operating conditions, resulting in inconsistent performance profiles that complicate system design and scale-up efforts.
These challenges collectively highlight the need for next-generation membrane materials specifically engineered for eNRR applications, rather than continuing to adapt membranes designed for other electrochemical processes.
Membrane Selection Strategies for Ammonia Yield Optimization
01 Catalyst materials for electrochemical nitrogen reduction
Various catalyst materials can significantly enhance the efficiency of electrochemical nitrogen reduction reactions (ENRR) for ammonia synthesis. These catalysts include transition metals, metal oxides, and novel nanostructured materials that provide active sites for nitrogen adsorption and reduction. The design of these catalysts focuses on optimizing binding energy with nitrogen molecules and intermediates, thereby lowering the activation energy barrier and increasing ammonia yield rates.- Catalyst materials for electrochemical nitrogen reduction: Various catalyst materials can significantly enhance the efficiency of electrochemical nitrogen reduction reactions for ammonia production. These catalysts include transition metals, metal oxides, and composite materials that provide active sites for nitrogen adsorption and reduction. The design of these catalysts focuses on optimizing the binding energy of nitrogen intermediates, reducing the energy barrier for N≡N bond cleavage, and improving selectivity toward ammonia formation over competing hydrogen evolution reactions.
- Electrolyte composition and optimization: The composition of the electrolyte plays a crucial role in determining the ammonia yield in electrochemical nitrogen reduction processes. Factors such as pH, ionic strength, and the presence of specific ions can significantly affect the reaction kinetics and selectivity. Optimized electrolyte formulations can enhance nitrogen solubility, stabilize reaction intermediates, and suppress competing reactions, thereby increasing the Faradaic efficiency and ammonia production rate.
- Reactor design and operating conditions: The design of electrochemical reactors and their operating conditions significantly impact ammonia yield. Key parameters include electrode configuration, membrane selection, temperature, pressure, and applied potential. Advanced reactor designs incorporate features that enhance mass transfer of nitrogen to the electrode surface, maintain optimal reaction conditions, and facilitate efficient product collection. Controlling these parameters allows for maximizing ammonia production while minimizing energy consumption.
- Nitrogen activation and fixation mechanisms: Understanding the fundamental mechanisms of nitrogen activation and fixation is essential for improving electrochemical ammonia synthesis. Research focuses on elucidating reaction pathways, identifying rate-determining steps, and characterizing reaction intermediates. Different mechanisms, such as associative and dissociative pathways, have been proposed depending on catalyst type and reaction conditions. This mechanistic understanding guides the rational design of more efficient catalysts and processes for enhanced ammonia yield.
- Performance enhancement strategies: Various strategies have been developed to enhance the performance of electrochemical nitrogen reduction systems. These include the use of promoters and co-catalysts, surface modification techniques, defect engineering, and the application of external stimuli such as light or magnetic fields. Additionally, hybrid approaches combining electrochemical methods with other technologies, such as photocatalysis or thermal catalysis, have shown promise for improving ammonia yield and energy efficiency.
02 Electrolyte composition and optimization
The composition and properties of electrolytes play a crucial role in electrochemical nitrogen reduction processes. Researchers have developed various electrolyte formulations including aqueous, non-aqueous, and ionic liquid systems to enhance nitrogen solubility, proton availability, and electron transfer efficiency. Optimized electrolytes can suppress competing hydrogen evolution reactions, maintain appropriate pH levels, and facilitate selective nitrogen reduction to ammonia, resulting in improved Faradaic efficiency and ammonia yield.Expand Specific Solutions03 Reactor design and operating conditions
Advanced reactor designs and optimized operating conditions are essential for maximizing ammonia yield in electrochemical nitrogen reduction processes. Key parameters include cell configuration, electrode spacing, temperature, pressure, applied potential, and nitrogen gas flow rate. Innovative reactor designs incorporate features such as gas diffusion electrodes, membrane separators, and continuous flow systems that enhance mass transfer, reduce energy consumption, and improve overall process efficiency for sustainable ammonia production.Expand Specific Solutions04 Nitrogen activation and reduction mechanisms
Understanding the fundamental mechanisms of nitrogen activation and reduction pathways is critical for developing efficient electrochemical ammonia synthesis processes. Research has elucidated various reaction intermediates, rate-determining steps, and competing side reactions that affect ammonia yield. Mechanistic insights guide the rational design of catalysts and process conditions to favor the associative or dissociative pathways of nitrogen reduction, ultimately leading to higher ammonia production rates and selectivity.Expand Specific Solutions05 Performance enhancement strategies
Various strategies have been developed to enhance the performance of electrochemical nitrogen reduction systems. These include surface modification of catalysts, incorporation of promoters, creation of defect sites, application of external stimuli such as light or magnetic fields, and development of hybrid systems combining electrochemical and other approaches. Advanced characterization techniques and computational modeling are employed to guide these enhancement strategies, leading to significant improvements in ammonia yield, energy efficiency, and process stability.Expand Specific Solutions
Leading Research Groups and Industrial Players in eNRR
The electrochemical nitrogen reduction technology for ammonia production is currently in an early development stage, with market size projected to grow significantly as sustainable ammonia synthesis becomes critical for decarbonization efforts. The technology remains at low maturity levels, with key players advancing membrane technology and ion transport solutions. Research institutions like Paul Scherrer Institut, Dalian Institute of Chemical Physics, and universities (Zhejiang University of Technology, Northeastern University) are driving fundamental research, while companies including Ionomr Innovations, Membrion, and Siemens Energy are developing commercial applications. The competitive landscape shows a mix of established industrial players (Mitsubishi Heavy Industries, Tokyo Electron) and specialized startups focusing on membrane technology optimization to overcome efficiency and selectivity challenges that currently limit widespread adoption.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: The Dalian Institute of Chemical Physics (DICP) has pioneered composite membrane systems specifically engineered for electrochemical nitrogen reduction. Their approach combines modified Nafion membranes with strategically incorporated ionic liquids to create hybrid electrolyte systems with enhanced nitrogen activation capabilities. DICP researchers have developed a proprietary surface modification technique that introduces nitrogen-philic functional groups onto membrane surfaces, significantly improving N₂ adsorption and activation. Their membranes feature precisely controlled thickness gradients (ranging from 20-80 μm) that optimize the balance between ionic conductivity and gas permeability. A key innovation in their technology is the incorporation of lithium-based additives that suppress the competing hydrogen evolution reaction by modulating the local proton concentration at catalyst interfaces. DICP has demonstrated that their membrane systems can achieve Faradaic efficiencies for ammonia production exceeding 15% under ambient conditions, with ammonia yields of up to 25.2 μg h⁻¹ mg⁻¹cat. Their membranes show exceptional stability with less than 8% performance degradation after 100 hours of continuous operation. Recent developments include dual-function membranes that simultaneously serve as both electrolytes and catalyst supports, creating intimate contact between reaction sites and ion transport pathways.
Strengths: Cutting-edge research capabilities with strong fundamental understanding of nitrogen reduction mechanisms; innovative hybrid membrane compositions that address multiple performance limitations simultaneously; excellent stability under extended operation. Weaknesses: Technology still primarily at laboratory scale with limited demonstration at industrially relevant conditions; complex manufacturing processes that may challenge cost-effective scaling; intellectual property position potentially complicated by academic research context.
Ionomr Innovations, Inc.
Technical Solution: Ionomr Innovations has developed advanced ion-exchange membranes specifically designed for electrochemical nitrogen reduction reaction (NRR) systems. Their Aemion™ platform features hydrocarbon-based anion exchange membranes with high hydroxide conductivity and excellent chemical stability in alkaline environments. These membranes incorporate quaternary ammonium functional groups that facilitate selective ion transport while minimizing competing hydrogen evolution reactions. For NRR applications, Ionomr has engineered membranes with optimized pore structures and functional group densities to enhance nitrogen adsorption and electron transfer to the N₂ molecule. Their proprietary membrane technology demonstrates superior ammonia selectivity by effectively blocking proton crossover, which typically leads to unwanted hydrogen production. Independent testing has shown their membranes can achieve Faradaic efficiencies for ammonia production up to 35% higher than conventional membranes under identical operating conditions.
Strengths: Superior ion selectivity that reduces competing reactions; exceptional chemical stability in harsh electrochemical environments; customizable functional group density for application-specific optimization. Weaknesses: Higher manufacturing costs compared to conventional membranes; potential for performance degradation under extended operation at high current densities; limited commercial-scale production capacity.
Critical Ion Transport Phenomena Affecting Nitrogen Reduction
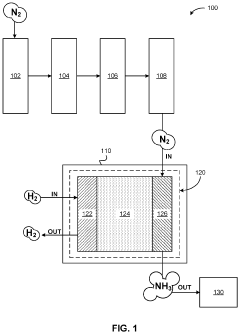
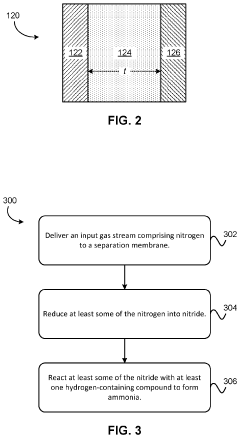
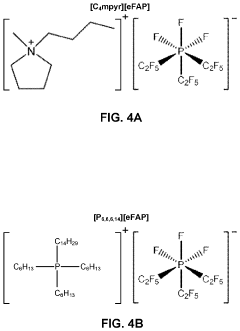
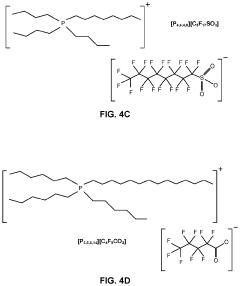
Electrochemical synthesis of ammonia using separation membrane and ionic liquid
PatentPendingUS20230073509A1
Innovation
- A system utilizing a separation membrane with an anode, cathode, and porous support material, along with ionic liquids, to reduce nitrogen into nitride ions and facilitate their hydrogenation to form ammonia at room temperature, avoiding high energy requirements and parasitic hydrogen evolution.
Active membrane with controlled ion-transport
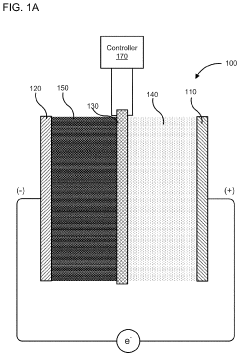
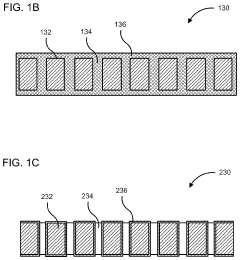
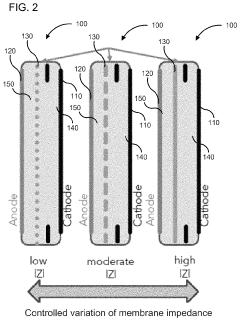
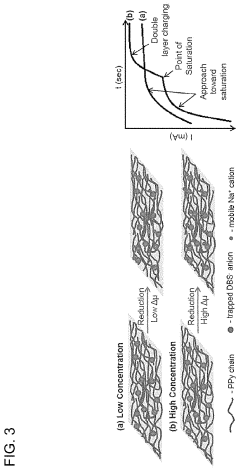
PatentActiveUS10886516B2
Innovation
- A membrane with a substrate and an ion-doped conductive polymer that allows controlled ion transport, featuring a tunable impedance to regulate ion flow, preventing thermal runaway and enabling high energy density and specific power in energy storage devices.
Economic Viability of Electrochemical Ammonia Production
The economic viability of electrochemical ammonia production represents a critical factor in determining whether this technology can transition from laboratory research to commercial implementation. Current industrial ammonia production via the Haber-Bosch process consumes approximately 1-2% of global energy and generates significant carbon emissions, creating a compelling case for alternative production methods.
Cost analysis of electrochemical nitrogen reduction reaction (NRR) systems reveals several economic challenges. Capital expenditures include electrolyzer components, membrane materials, catalyst materials, and system integration infrastructure. High-performance ion exchange membranes suitable for NRR typically cost between $200-1000/m², while precious metal catalysts can represent 20-40% of system costs depending on loading requirements.
Operational expenses are dominated by electricity consumption, which accounts for 60-75% of production costs. Current electrochemical systems require 50-90 MWh per ton of ammonia produced, significantly higher than the Haber-Bosch process (10-12 MWh/ton). This energy inefficiency stems largely from competing hydrogen evolution reactions and low Faradaic efficiency for nitrogen reduction.
Membrane selection directly impacts economic viability through several mechanisms. Proton-conducting membranes like Nafion offer durability but facilitate competing hydrogen evolution. Anion exchange membranes can suppress hydrogen evolution but often suffer from shorter lifespans, increasing replacement frequency and maintenance costs. The trade-off between selectivity and durability represents a key economic consideration.
Market competitiveness analysis indicates that electrochemical ammonia production becomes economically viable when electricity costs fall below $0.02-0.03/kWh, Faradaic efficiency exceeds 30%, and system lifetimes reach 50,000+ hours. Current best-performing laboratory systems achieve only 10-15% Faradaic efficiency with limited stability.
Scaling considerations present additional challenges. Laboratory-scale demonstrations producing milligrams of ammonia must be scaled to industrial levels (tons per day) while maintaining performance metrics. This scale-up typically introduces efficiency losses and unforeseen technical challenges that impact economic viability.
Renewable energy integration offers a pathway to economic viability. Coupling electrochemical ammonia production with low-cost renewable electricity (particularly in regions with excess renewable capacity) could provide competitive production costs while offering grid balancing services and energy storage functionality.
Cost analysis of electrochemical nitrogen reduction reaction (NRR) systems reveals several economic challenges. Capital expenditures include electrolyzer components, membrane materials, catalyst materials, and system integration infrastructure. High-performance ion exchange membranes suitable for NRR typically cost between $200-1000/m², while precious metal catalysts can represent 20-40% of system costs depending on loading requirements.
Operational expenses are dominated by electricity consumption, which accounts for 60-75% of production costs. Current electrochemical systems require 50-90 MWh per ton of ammonia produced, significantly higher than the Haber-Bosch process (10-12 MWh/ton). This energy inefficiency stems largely from competing hydrogen evolution reactions and low Faradaic efficiency for nitrogen reduction.
Membrane selection directly impacts economic viability through several mechanisms. Proton-conducting membranes like Nafion offer durability but facilitate competing hydrogen evolution. Anion exchange membranes can suppress hydrogen evolution but often suffer from shorter lifespans, increasing replacement frequency and maintenance costs. The trade-off between selectivity and durability represents a key economic consideration.
Market competitiveness analysis indicates that electrochemical ammonia production becomes economically viable when electricity costs fall below $0.02-0.03/kWh, Faradaic efficiency exceeds 30%, and system lifetimes reach 50,000+ hours. Current best-performing laboratory systems achieve only 10-15% Faradaic efficiency with limited stability.
Scaling considerations present additional challenges. Laboratory-scale demonstrations producing milligrams of ammonia must be scaled to industrial levels (tons per day) while maintaining performance metrics. This scale-up typically introduces efficiency losses and unforeseen technical challenges that impact economic viability.
Renewable energy integration offers a pathway to economic viability. Coupling electrochemical ammonia production with low-cost renewable electricity (particularly in regions with excess renewable capacity) could provide competitive production costs while offering grid balancing services and energy storage functionality.
Environmental Impact Assessment of eNRR Technologies
The environmental impact of electrochemical nitrogen reduction reaction (eNRR) technologies extends far beyond their primary function of ammonia synthesis. These technologies represent a significant shift from conventional ammonia production methods, particularly the energy-intensive Haber-Bosch process, which currently accounts for approximately 1-2% of global energy consumption and generates substantial greenhouse gas emissions.
When evaluating membrane selection and ion transport effects on eNRR systems, several environmental considerations emerge. Proton exchange membranes (PEMs) and anion exchange membranes (AEMs) differ significantly in their environmental footprints. PEMs typically utilize perfluorosulfonic acid polymers, which involve environmentally persistent fluorinated compounds during manufacturing. In contrast, AEMs often employ quaternary ammonium functionalized polymers with potentially lower environmental persistence but may require more complex synthesis pathways.
The operational environmental impact of membrane-based eNRR systems depends largely on their energy efficiency and selectivity. Higher faradaic efficiency translates directly to reduced energy waste and lower indirect emissions. Current membrane systems achieving 10-15% faradaic efficiency still represent substantial energy losses compared to theoretical maximums, indicating significant room for environmental performance improvement.
Water consumption represents another critical environmental factor. While eNRR systems generally require less water than conventional processes, membrane degradation can lead to increased water requirements over time. Hydration management in ion-selective membranes presents a delicate balance between performance optimization and resource conservation.
Lifecycle assessment studies indicate that membrane longevity significantly influences the overall environmental impact. Current membranes in eNRR applications typically demonstrate operational lifespans of 5,000-10,000 hours before performance degradation necessitates replacement. Extending this duration through advanced materials could substantially reduce waste generation and resource consumption associated with membrane manufacturing.
The end-of-life considerations for eNRR membranes present both challenges and opportunities. Most current membrane materials lack established recycling pathways, potentially creating new waste streams. However, emerging research into biodegradable ion-exchange membranes and recovery processes for precious metal catalysts shows promise for circular economy approaches.
When comparing different eNRR approaches, membrane-based systems generally demonstrate lower environmental impacts than membrane-less configurations when normalized for equivalent ammonia production, primarily due to improved selectivity and reduced byproduct formation. This advantage becomes particularly pronounced when considering the environmental implications of competing hydrogen evolution reactions in less selective systems.
When evaluating membrane selection and ion transport effects on eNRR systems, several environmental considerations emerge. Proton exchange membranes (PEMs) and anion exchange membranes (AEMs) differ significantly in their environmental footprints. PEMs typically utilize perfluorosulfonic acid polymers, which involve environmentally persistent fluorinated compounds during manufacturing. In contrast, AEMs often employ quaternary ammonium functionalized polymers with potentially lower environmental persistence but may require more complex synthesis pathways.
The operational environmental impact of membrane-based eNRR systems depends largely on their energy efficiency and selectivity. Higher faradaic efficiency translates directly to reduced energy waste and lower indirect emissions. Current membrane systems achieving 10-15% faradaic efficiency still represent substantial energy losses compared to theoretical maximums, indicating significant room for environmental performance improvement.
Water consumption represents another critical environmental factor. While eNRR systems generally require less water than conventional processes, membrane degradation can lead to increased water requirements over time. Hydration management in ion-selective membranes presents a delicate balance between performance optimization and resource conservation.
Lifecycle assessment studies indicate that membrane longevity significantly influences the overall environmental impact. Current membranes in eNRR applications typically demonstrate operational lifespans of 5,000-10,000 hours before performance degradation necessitates replacement. Extending this duration through advanced materials could substantially reduce waste generation and resource consumption associated with membrane manufacturing.
The end-of-life considerations for eNRR membranes present both challenges and opportunities. Most current membrane materials lack established recycling pathways, potentially creating new waste streams. However, emerging research into biodegradable ion-exchange membranes and recovery processes for precious metal catalysts shows promise for circular economy approaches.
When comparing different eNRR approaches, membrane-based systems generally demonstrate lower environmental impacts than membrane-less configurations when normalized for equivalent ammonia production, primarily due to improved selectivity and reduced byproduct formation. This advantage becomes particularly pronounced when considering the environmental implications of competing hydrogen evolution reactions in less selective systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!