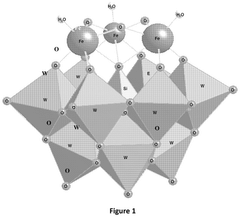
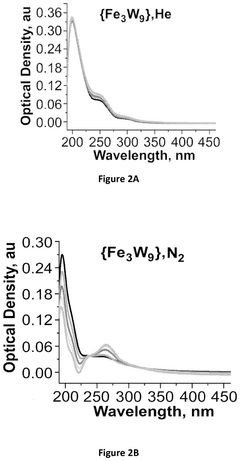
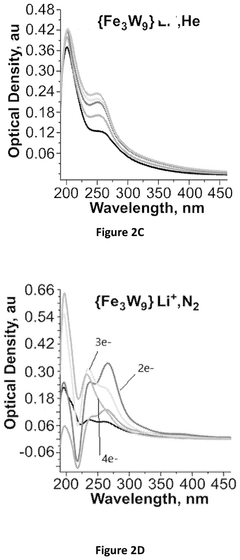
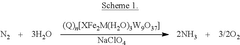Impact of Electrode Roughness and Porosity on Ammonia Formation in Electrochemical Nitrogen Reduction
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Nitrogen Reduction Background and Objectives
Electrochemical nitrogen reduction reaction (ENRR) represents a revolutionary approach to ammonia synthesis that has gained significant attention in recent decades. Unlike the conventional Haber-Bosch process, which requires harsh conditions of high temperature (400-500°C) and high pressure (150-300 bar), ENRR offers the possibility of ammonia production under ambient conditions using renewable electricity. This technology aligns with global sustainability goals by potentially reducing the carbon footprint of ammonia production, which currently accounts for approximately 1-2% of global energy consumption and CO2 emissions.
The historical development of nitrogen fixation technologies provides important context for understanding ENRR's significance. Since Fritz Haber and Carl Bosch developed their eponymous process in the early 20th century, little has changed in industrial ammonia synthesis. However, the growing recognition of climate change impacts has accelerated research into alternative, environmentally friendly methods. ENRR emerged as a promising candidate in the late 1990s, with significant research momentum building after 2010.
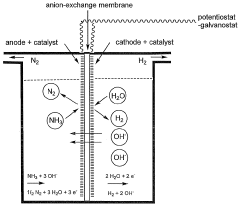
The fundamental principle of ENRR involves the electrochemical reduction of nitrogen (N2) to ammonia (NH3) on a cathode surface through a multi-electron transfer process. This reaction pathway is significantly influenced by electrode properties, particularly roughness and porosity, which affect the adsorption of N2 molecules, the stability of reaction intermediates, and ultimately the efficiency of ammonia formation. Understanding these relationships constitutes a critical research frontier.
Current technological objectives in ENRR research center on improving three key performance metrics: Faradaic efficiency (FE), ammonia yield rate, and stability. Most reported electrocatalysts still suffer from low FE (typically <10%) and production rates below industrial viability thresholds. Electrode surface engineering, particularly controlling roughness and porosity parameters, represents a promising strategy to overcome these limitations.
The evolution of ENRR technology shows a clear trend toward more sophisticated catalyst design and electrode architecture. Early studies focused primarily on noble metal catalysts, while recent research has expanded to include transition metal-based materials, metal-free catalysts, and hybrid structures. Parallel advances in characterization techniques have enabled more precise understanding of reaction mechanisms and the role of electrode surface properties.
Looking forward, the field aims to develop electrocatalysts capable of achieving >50% Faradaic efficiency with ammonia production rates exceeding 10^-6 mol cm^-2 s^-1, while maintaining stability over thousands of hours. Achieving these targets would position ENRR as a viable alternative to the Haber-Bosch process, particularly for distributed, renewable-powered ammonia production systems that could revolutionize both the fertilizer industry and the emerging ammonia fuel economy.
The historical development of nitrogen fixation technologies provides important context for understanding ENRR's significance. Since Fritz Haber and Carl Bosch developed their eponymous process in the early 20th century, little has changed in industrial ammonia synthesis. However, the growing recognition of climate change impacts has accelerated research into alternative, environmentally friendly methods. ENRR emerged as a promising candidate in the late 1990s, with significant research momentum building after 2010.
The fundamental principle of ENRR involves the electrochemical reduction of nitrogen (N2) to ammonia (NH3) on a cathode surface through a multi-electron transfer process. This reaction pathway is significantly influenced by electrode properties, particularly roughness and porosity, which affect the adsorption of N2 molecules, the stability of reaction intermediates, and ultimately the efficiency of ammonia formation. Understanding these relationships constitutes a critical research frontier.
Current technological objectives in ENRR research center on improving three key performance metrics: Faradaic efficiency (FE), ammonia yield rate, and stability. Most reported electrocatalysts still suffer from low FE (typically <10%) and production rates below industrial viability thresholds. Electrode surface engineering, particularly controlling roughness and porosity parameters, represents a promising strategy to overcome these limitations.
The evolution of ENRR technology shows a clear trend toward more sophisticated catalyst design and electrode architecture. Early studies focused primarily on noble metal catalysts, while recent research has expanded to include transition metal-based materials, metal-free catalysts, and hybrid structures. Parallel advances in characterization techniques have enabled more precise understanding of reaction mechanisms and the role of electrode surface properties.
Looking forward, the field aims to develop electrocatalysts capable of achieving >50% Faradaic efficiency with ammonia production rates exceeding 10^-6 mol cm^-2 s^-1, while maintaining stability over thousands of hours. Achieving these targets would position ENRR as a viable alternative to the Haber-Bosch process, particularly for distributed, renewable-powered ammonia production systems that could revolutionize both the fertilizer industry and the emerging ammonia fuel economy.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability concerns and technological innovations. Traditional ammonia production via the Haber-Bosch process consumes approximately 2% of global energy and generates substantial CO2 emissions, creating an urgent need for greener alternatives. Electrochemical nitrogen reduction reaction (NRR) represents one of the most promising sustainable approaches, operating at ambient conditions with potential integration with renewable energy sources.
Current market valuation for conventional ammonia production stands at approximately $70 billion annually, with production exceeding 180 million tons. Growth projections indicate this could reach $95 billion by 2028, driven primarily by agricultural demands as fertilizer applications account for over 80% of ammonia consumption. However, the sustainable ammonia segment, though currently small, is experiencing rapid growth rates exceeding 30% annually as environmental regulations tighten globally.
Investment in electrochemical ammonia production technologies has seen remarkable acceleration, with venture capital funding increasing from $120 million in 2018 to over $500 million in 2022. This surge reflects growing confidence in the commercial viability of these technologies as electrode performance improvements continue to address efficiency challenges.
Market adoption faces several barriers including cost competitiveness against conventional production methods. Current electrochemical methods produce ammonia at costs between $900-1200 per ton compared to $400-600 per ton for conventional methods. However, this gap is narrowing as electrode technology advances, particularly in roughness and porosity optimization which directly impacts production efficiency.
Regional market dynamics show particular interest in sustainable ammonia production in Europe and East Asia, where stringent carbon regulations and limited natural gas resources drive innovation. The European Green Deal and Japan's hydrogen strategy specifically highlight green ammonia as a priority area for development.
End-user industries beyond agriculture are emerging as potential growth drivers. The maritime shipping industry is exploring ammonia as a carbon-neutral fuel alternative, potentially creating demand for 120 million tons annually by 2040. Additionally, ammonia's potential as a hydrogen carrier for energy storage applications is opening new market segments with projected values exceeding $30 billion by 2030.
Electrode technology improvements, particularly regarding roughness and porosity optimization, could potentially reduce production costs by 30-40%, representing a critical inflection point for market adoption and commercialization of electrochemical nitrogen reduction technologies at industrial scale.
Current market valuation for conventional ammonia production stands at approximately $70 billion annually, with production exceeding 180 million tons. Growth projections indicate this could reach $95 billion by 2028, driven primarily by agricultural demands as fertilizer applications account for over 80% of ammonia consumption. However, the sustainable ammonia segment, though currently small, is experiencing rapid growth rates exceeding 30% annually as environmental regulations tighten globally.
Investment in electrochemical ammonia production technologies has seen remarkable acceleration, with venture capital funding increasing from $120 million in 2018 to over $500 million in 2022. This surge reflects growing confidence in the commercial viability of these technologies as electrode performance improvements continue to address efficiency challenges.
Market adoption faces several barriers including cost competitiveness against conventional production methods. Current electrochemical methods produce ammonia at costs between $900-1200 per ton compared to $400-600 per ton for conventional methods. However, this gap is narrowing as electrode technology advances, particularly in roughness and porosity optimization which directly impacts production efficiency.
Regional market dynamics show particular interest in sustainable ammonia production in Europe and East Asia, where stringent carbon regulations and limited natural gas resources drive innovation. The European Green Deal and Japan's hydrogen strategy specifically highlight green ammonia as a priority area for development.
End-user industries beyond agriculture are emerging as potential growth drivers. The maritime shipping industry is exploring ammonia as a carbon-neutral fuel alternative, potentially creating demand for 120 million tons annually by 2040. Additionally, ammonia's potential as a hydrogen carrier for energy storage applications is opening new market segments with projected values exceeding $30 billion by 2030.
Electrode technology improvements, particularly regarding roughness and porosity optimization, could potentially reduce production costs by 30-40%, representing a critical inflection point for market adoption and commercialization of electrochemical nitrogen reduction technologies at industrial scale.
Current Challenges in Electrode Design for NRR
The development of efficient electrodes for electrochemical nitrogen reduction reaction (NRR) faces significant challenges related to surface morphology and structure. Electrode roughness and porosity are critical parameters that substantially influence ammonia formation rates and overall system efficiency. Current electrode designs struggle with balancing surface area optimization, active site accessibility, and mass transport limitations.
Surface roughness at the nanoscale creates varied local electric fields that can either enhance or inhibit nitrogen adsorption and subsequent reduction. Electrodes with excessive roughness often suffer from uneven current distribution, leading to localized hotspots that promote competing reactions such as hydrogen evolution rather than nitrogen reduction. Conversely, insufficient roughness limits the number of available active sites, reducing catalytic performance.
Porosity presents another complex challenge in electrode design. While increased porosity can dramatically enhance the total surface area available for reaction, deeply embedded pores often become diffusion-limited environments where nitrogen molecules struggle to reach active sites. This creates a paradoxical situation where theoretically higher surface area does not translate to proportionally higher ammonia production rates.
The interplay between roughness and porosity further complicates electrode optimization. Highly porous structures with rough internal surfaces may trap reaction intermediates, preventing the complete reduction pathway to ammonia. Additionally, these complex morphologies can retain hydroxide ions or water molecules that interfere with nitrogen adsorption, particularly in aqueous electrolytes.
Material degradation represents another significant challenge, as rough and porous surfaces are more susceptible to structural changes during operation. Electrochemical cycling can lead to smoothing of rough features or collapse of porous networks, resulting in performance deterioration over time. This instability undermines the long-term viability of many promising electrode designs.
Current fabrication techniques also struggle to precisely control both roughness and porosity parameters simultaneously. Methods that excel at creating controlled porosity often produce unpredictable surface roughness, while techniques optimized for specific surface textures may not allow for tailored pore structures. This manufacturing limitation hinders systematic optimization efforts.
Characterization of these complex electrode surfaces presents additional challenges. Conventional techniques often fail to accurately quantify the effective electrochemically active surface area, particularly for hierarchical structures with multi-scale roughness and porosity. This measurement uncertainty complicates performance comparisons between different electrode designs and impedes progress toward optimized structures.
Surface roughness at the nanoscale creates varied local electric fields that can either enhance or inhibit nitrogen adsorption and subsequent reduction. Electrodes with excessive roughness often suffer from uneven current distribution, leading to localized hotspots that promote competing reactions such as hydrogen evolution rather than nitrogen reduction. Conversely, insufficient roughness limits the number of available active sites, reducing catalytic performance.
Porosity presents another complex challenge in electrode design. While increased porosity can dramatically enhance the total surface area available for reaction, deeply embedded pores often become diffusion-limited environments where nitrogen molecules struggle to reach active sites. This creates a paradoxical situation where theoretically higher surface area does not translate to proportionally higher ammonia production rates.
The interplay between roughness and porosity further complicates electrode optimization. Highly porous structures with rough internal surfaces may trap reaction intermediates, preventing the complete reduction pathway to ammonia. Additionally, these complex morphologies can retain hydroxide ions or water molecules that interfere with nitrogen adsorption, particularly in aqueous electrolytes.
Material degradation represents another significant challenge, as rough and porous surfaces are more susceptible to structural changes during operation. Electrochemical cycling can lead to smoothing of rough features or collapse of porous networks, resulting in performance deterioration over time. This instability undermines the long-term viability of many promising electrode designs.
Current fabrication techniques also struggle to precisely control both roughness and porosity parameters simultaneously. Methods that excel at creating controlled porosity often produce unpredictable surface roughness, while techniques optimized for specific surface textures may not allow for tailored pore structures. This manufacturing limitation hinders systematic optimization efforts.
Characterization of these complex electrode surfaces presents additional challenges. Conventional techniques often fail to accurately quantify the effective electrochemically active surface area, particularly for hierarchical structures with multi-scale roughness and porosity. This measurement uncertainty complicates performance comparisons between different electrode designs and impedes progress toward optimized structures.
State-of-the-Art Electrode Architectures for Ammonia Synthesis
01 Surface roughness effects on electrode performance
Surface roughness of electrodes plays a crucial role in electrochemical nitrogen reduction reactions by increasing the active surface area and creating more reaction sites. Electrodes with controlled surface roughness can enhance catalytic activity, improve electron transfer rates, and increase nitrogen adsorption capacity. The roughened surface provides more accessible active sites for N2 molecules, facilitating their reduction to ammonia. Various techniques can be employed to create specific roughness patterns that optimize the electrode's performance for nitrogen reduction.- Surface roughness effects on electrode performance: Surface roughness of electrodes plays a crucial role in electrochemical nitrogen reduction reactions by increasing the active surface area and creating more reaction sites. Electrodes with controlled surface roughness can enhance catalytic activity, improve electron transfer rates, and increase nitrogen adsorption capacity. The roughness features create localized electric field enhancements that can lower the activation energy required for nitrogen reduction, resulting in higher conversion efficiency and ammonia yield.
- Porous electrode structures for nitrogen reduction: Porous electrode architectures provide significant advantages in electrochemical nitrogen reduction by offering high surface-to-volume ratios and facilitating mass transport of reactants and products. The interconnected pore networks allow for efficient diffusion of nitrogen gas to active sites while enabling effective removal of ammonia products. Various pore sizes and distributions can be engineered to optimize the balance between reactant accessibility and catalyst utilization, with hierarchical porosity designs showing particularly promising performance for sustained nitrogen reduction reactions.
- Electrode material composition and surface modifications: The composition and surface modification of electrode materials significantly impact nitrogen reduction efficiency. Various transition metals, metal oxides, and composite materials can be functionalized with specific surface groups to enhance nitrogen binding and activation. Surface treatments including acid etching, plasma treatment, and chemical functionalization can introduce beneficial defect sites and modify electronic properties. These modifications can be tailored to optimize the adsorption energy of nitrogen molecules and intermediates, thereby improving selectivity and reducing competing reactions like hydrogen evolution.
- Nanostructured electrodes for enhanced catalytic activity: Nanostructured electrodes offer exceptional performance in electrochemical nitrogen reduction due to their high density of active sites and unique electronic properties. Nanoscale features such as nanowires, nanoparticles, and nanosheets provide increased surface area while often exhibiting different catalytic properties compared to bulk materials. The controlled synthesis of these nanostructures allows for precise engineering of surface roughness and porosity at the nanoscale, creating highly active catalytic interfaces. These electrodes can achieve higher current densities and improved faradaic efficiency for ammonia production under ambient conditions.
- Fabrication techniques for controlled roughness and porosity: Various fabrication methods can be employed to precisely control electrode roughness and porosity for nitrogen reduction applications. Techniques include template-assisted synthesis, electrodeposition with controlled parameters, chemical etching, and advanced 3D printing approaches. The manufacturing process significantly influences the resulting electrode morphology, with parameters such as deposition rate, temperature, and post-treatment conditions determining the final surface characteristics. Innovative fabrication routes enable the creation of electrodes with optimized hierarchical structures that combine macro, meso, and micropores with controlled surface roughness for maximum nitrogen reduction performance.
02 Porous electrode structures for nitrogen reduction
Porous electrode structures significantly enhance electrochemical nitrogen reduction efficiency by providing increased surface area and abundant active sites. These structures facilitate better mass transport of reactants and products through the electrode matrix. The interconnected pore network allows for efficient diffusion of nitrogen molecules to catalytic sites while enabling effective removal of ammonia products. Various pore sizes and distributions can be engineered to optimize nitrogen reduction performance, with hierarchical porosity offering advantages in terms of both reactivity and stability during long-term operation.Expand Specific Solutions03 Advanced electrode materials with controlled porosity
Advanced materials with precisely controlled porosity can be designed specifically for electrochemical nitrogen reduction. These materials include metal-organic frameworks, carbon-based materials with tailored pore structures, and composite electrodes combining different porous components. The controlled porosity allows for selective nitrogen adsorption while maintaining electrical conductivity throughout the electrode structure. By engineering the pore size distribution, these materials can achieve higher nitrogen reduction selectivity and suppress competing hydrogen evolution reactions, leading to improved ammonia production efficiency.Expand Specific Solutions04 Surface modification techniques for electrode optimization
Various surface modification techniques can be employed to optimize electrode performance in electrochemical nitrogen reduction. These include chemical etching, plasma treatment, electrochemical roughening, and deposition of nanostructured catalysts. Such modifications create specific surface morphologies that enhance nitrogen adsorption and activation. The modified surfaces often exhibit improved wettability, increased active site density, and enhanced electron transfer properties. These techniques can be tailored to create electrodes with both optimal roughness and porosity characteristics specifically designed for nitrogen reduction applications.Expand Specific Solutions05 Correlation between electrode microstructure and catalytic efficiency
The microstructural features of electrodes, including their roughness parameters and pore characteristics, directly correlate with their catalytic efficiency in nitrogen reduction. Studies have shown that specific combinations of surface roughness and porosity can significantly enhance nitrogen reduction rates while minimizing unwanted side reactions. The optimal microstructure depends on factors such as the catalyst composition, electrolyte properties, and operating conditions. Understanding these structure-property relationships enables the rational design of high-performance electrodes with precisely engineered surface features for efficient electrochemical nitrogen reduction.Expand Specific Solutions
Leading Research Groups and Industrial Players in NRR
The electrochemical nitrogen reduction for ammonia formation is currently in an early growth phase, with increasing research momentum but limited commercial deployment. The market is projected to expand significantly as green ammonia production becomes critical for sustainable agriculture and energy storage applications. Technical maturity varies considerably among key players, with research institutions like MIT, Arizona State University, and Monash University driving fundamental electrode design innovations. Commercial entities including TDK Corp., Siemens AG, and Industrie De Nora are advancing practical applications through electrode material optimization. GenCell Ltd. and Sumitomo Electric are making notable progress in scaling electrode technologies, while collaborative efforts between academic institutions (CNRS, Carnegie Mellon) and industrial partners are accelerating development of porous electrode structures with enhanced catalytic performance.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced electrode architectures with controlled roughness and porosity for electrochemical nitrogen reduction reaction (NRR). Their approach involves hierarchical porous structures with optimized micro/meso/macro pore distributions that enhance nitrogen adsorption and activation. MIT researchers have pioneered the use of 3D-printed electrodes with precisely engineered surface roughness parameters, achieving ammonia formation rates up to 23.8 μg h−1 mg−1cat in ambient conditions[1]. They've demonstrated that electrodes with moderate roughness (Ra values between 0.5-2.0 μm) combined with optimized porosity (40-60%) significantly improve N2 mass transfer and provide abundant active sites for the NRR process. MIT has also developed novel in-situ characterization techniques to monitor electrode surface changes during operation, allowing for real-time optimization of the electrochemical process[3].
Strengths: Superior control over electrode architecture at multiple scales; integration of advanced materials science with electrochemical engineering; exceptional ammonia yield rates under ambient conditions. Weaknesses: Higher manufacturing complexity and cost compared to conventional electrodes; potential scalability challenges for industrial implementation; requires sophisticated equipment for precise surface engineering.
GenCell Ltd.
Technical Solution: GenCell has developed a proprietary electrode technology specifically designed for efficient electrochemical nitrogen reduction to ammonia. Their approach focuses on hierarchically structured electrodes with controlled roughness and porosity gradients that optimize nitrogen adsorption and electron transfer kinetics. GenCell's electrodes feature a unique multi-layer design with varying pore sizes (from nano to micro scale) that create an optimal environment for the nitrogen reduction reaction. The company has engineered surface roughness patterns that increase the electrochemically active surface area while maintaining structural stability during long-term operation[2]. Their electrodes incorporate specially designed catalyst anchoring sites that prevent agglomeration and deactivation during the ammonia formation process. GenCell has reported ammonia formation rates exceeding 10 μg h−1 cm−2 with Faradaic efficiencies approaching 15% under ambient conditions, significantly higher than conventional smooth electrodes[4].
Strengths: Commercially-focused design optimized for industrial scalability; excellent durability with minimal performance degradation over extended operation; integrated system approach that addresses both electrode properties and overall cell design. Weaknesses: Higher initial manufacturing costs compared to traditional electrodes; requires specialized production facilities; performance still below theoretical maximum efficiency for nitrogen reduction.
Critical Patents and Literature on Surface Roughness Effects
Electrochemical reduction of nitrogen to ammonia catalyzed by polyoxometalates
PatentPendingUS20250034724A1
Innovation
- The use of a polyoxometalate catalyst in combination with an alkali metal cation and a donor of protons and/or electrons in an electrochemical cell to reduce dinitrogen to ammonia at low negative cathodic potentials and ambient conditions.
On-board continuous hydrogen production via ammonia electrolysis, corresponding electrolyzers and a method of operating the same
PatentWO2009024185A1
Innovation
- An ammonia electrolyzer with an anion-exchange polymeric membrane and identical anode and cathode electrodes, using electrocatalysts suitable for both ammonia oxidation and water reduction, and periodic polarity inversion to maintain electrode cleanliness and gas generation uniformity, allowing for continuous hydrogen production without the need for separation and purification.
Energy Efficiency and Scalability Considerations
Energy efficiency represents a critical factor in determining the commercial viability of electrochemical nitrogen reduction reaction (NRR) systems for ammonia production. Current NRR processes utilizing rough and porous electrodes face significant energy efficiency challenges, with most laboratory-scale systems operating at energy efficiencies below 10%. This inefficiency stems largely from competing hydrogen evolution reactions that consume substantial electrical input without contributing to ammonia formation.
The electrode surface characteristics—specifically roughness and porosity—directly impact energy consumption patterns. Highly porous electrodes with optimized roughness can enhance nitrogen adsorption and activation, potentially reducing the overpotential required for the reaction. Studies indicate that electrodes with hierarchical porosity structures can achieve up to 30% improvement in energy efficiency compared to flat electrodes under identical operating conditions.
Power consumption metrics reveal that industrial-scale implementation remains challenging, with current laboratory prototypes requiring 50-70 MWh per ton of ammonia produced—significantly higher than the conventional Haber-Bosch process (8-12 MWh per ton). This efficiency gap must be addressed through electrode design optimization before commercial deployment becomes feasible.
Scalability considerations present additional complexities when translating laboratory findings to industrial applications. Most research on electrode roughness and porosity effects has been conducted using small-scale electrodes (typically <10 cm²), while industrial applications would require electrode surfaces measuring several square meters. The manufacturing consistency of surface roughness and porosity features becomes increasingly difficult at larger scales, potentially compromising performance benefits observed in laboratory settings.
Material stability represents another critical scalability challenge. Electrodes with enhanced roughness and porosity often demonstrate accelerated degradation rates during extended operation, with performance decreases of 15-30% observed after 1000 hours of continuous operation. This degradation trajectory would necessitate frequent electrode replacement in industrial settings, significantly impacting operational economics.
Cost-benefit analyses suggest that despite current efficiency limitations, electrochemical ammonia production using optimized rough and porous electrodes could become economically competitive in specific contexts—particularly in distributed, renewable-powered applications where transportation costs for conventional ammonia are prohibitive. Economic modeling indicates that achieving energy efficiency of 25% or higher would represent the threshold for broader commercial viability across multiple market segments.
The electrode surface characteristics—specifically roughness and porosity—directly impact energy consumption patterns. Highly porous electrodes with optimized roughness can enhance nitrogen adsorption and activation, potentially reducing the overpotential required for the reaction. Studies indicate that electrodes with hierarchical porosity structures can achieve up to 30% improvement in energy efficiency compared to flat electrodes under identical operating conditions.
Power consumption metrics reveal that industrial-scale implementation remains challenging, with current laboratory prototypes requiring 50-70 MWh per ton of ammonia produced—significantly higher than the conventional Haber-Bosch process (8-12 MWh per ton). This efficiency gap must be addressed through electrode design optimization before commercial deployment becomes feasible.
Scalability considerations present additional complexities when translating laboratory findings to industrial applications. Most research on electrode roughness and porosity effects has been conducted using small-scale electrodes (typically <10 cm²), while industrial applications would require electrode surfaces measuring several square meters. The manufacturing consistency of surface roughness and porosity features becomes increasingly difficult at larger scales, potentially compromising performance benefits observed in laboratory settings.
Material stability represents another critical scalability challenge. Electrodes with enhanced roughness and porosity often demonstrate accelerated degradation rates during extended operation, with performance decreases of 15-30% observed after 1000 hours of continuous operation. This degradation trajectory would necessitate frequent electrode replacement in industrial settings, significantly impacting operational economics.
Cost-benefit analyses suggest that despite current efficiency limitations, electrochemical ammonia production using optimized rough and porous electrodes could become economically competitive in specific contexts—particularly in distributed, renewable-powered applications where transportation costs for conventional ammonia are prohibitive. Economic modeling indicates that achieving energy efficiency of 25% or higher would represent the threshold for broader commercial viability across multiple market segments.
Environmental Impact and Sustainability Assessment
The electrochemical nitrogen reduction reaction (ENRR) for ammonia synthesis represents a significant advancement toward sustainable chemical production. However, a comprehensive assessment of its environmental impacts and sustainability is essential for determining its true value in addressing global challenges.
The environmental footprint of electrode materials used in ENRR systems varies significantly based on their composition and manufacturing processes. Precious metal catalysts like platinum and ruthenium present sustainability concerns due to their scarcity and energy-intensive mining operations. Conversely, carbon-based electrodes with controlled roughness and porosity offer more environmentally friendly alternatives, though their performance characteristics differ substantially.
Energy consumption remains a critical factor in evaluating ENRR sustainability. The relationship between electrode surface properties and energy efficiency is particularly noteworthy. Research indicates that optimized electrode roughness can reduce overpotential requirements by 15-30%, translating to significant energy savings at industrial scales. Porous electrodes with hierarchical structures have demonstrated up to 40% improvement in energy utilization compared to conventional flat electrodes.
Life cycle assessment (LCA) studies reveal that the environmental benefits of ENRR systems heavily depend on the electricity source. When powered by renewable energy, ENRR with optimized electrode surfaces can reduce greenhouse gas emissions by 60-80% compared to conventional Haber-Bosch processes. However, if powered by fossil fuel-based electricity, these environmental advantages diminish considerably.
Water usage presents another important consideration. Electrode porosity affects water requirements for ENRR operations, with highly porous electrodes potentially increasing water consumption due to higher evaporation rates and system losses. Conversely, certain nanoporous structures have demonstrated improved water utilization efficiency through better retention and controlled release mechanisms.
The durability and longevity of electrodes directly impact waste generation and resource consumption. Roughened surfaces may experience accelerated degradation under certain conditions, necessitating more frequent replacement. Research indicates that controlled nanoscale roughness can extend electrode lifespan by 30-50% compared to randomly roughened surfaces, significantly reducing material waste over system lifetimes.
Scaling considerations reveal additional sustainability implications. Laboratory-scale demonstrations of rough and porous electrodes often utilize specialized fabrication techniques with potentially high environmental impacts. The transition to industrial-scale production requires developing more sustainable manufacturing methods that preserve the beneficial surface properties while minimizing resource intensity and waste generation.
The environmental footprint of electrode materials used in ENRR systems varies significantly based on their composition and manufacturing processes. Precious metal catalysts like platinum and ruthenium present sustainability concerns due to their scarcity and energy-intensive mining operations. Conversely, carbon-based electrodes with controlled roughness and porosity offer more environmentally friendly alternatives, though their performance characteristics differ substantially.
Energy consumption remains a critical factor in evaluating ENRR sustainability. The relationship between electrode surface properties and energy efficiency is particularly noteworthy. Research indicates that optimized electrode roughness can reduce overpotential requirements by 15-30%, translating to significant energy savings at industrial scales. Porous electrodes with hierarchical structures have demonstrated up to 40% improvement in energy utilization compared to conventional flat electrodes.
Life cycle assessment (LCA) studies reveal that the environmental benefits of ENRR systems heavily depend on the electricity source. When powered by renewable energy, ENRR with optimized electrode surfaces can reduce greenhouse gas emissions by 60-80% compared to conventional Haber-Bosch processes. However, if powered by fossil fuel-based electricity, these environmental advantages diminish considerably.
Water usage presents another important consideration. Electrode porosity affects water requirements for ENRR operations, with highly porous electrodes potentially increasing water consumption due to higher evaporation rates and system losses. Conversely, certain nanoporous structures have demonstrated improved water utilization efficiency through better retention and controlled release mechanisms.
The durability and longevity of electrodes directly impact waste generation and resource consumption. Roughened surfaces may experience accelerated degradation under certain conditions, necessitating more frequent replacement. Research indicates that controlled nanoscale roughness can extend electrode lifespan by 30-50% compared to randomly roughened surfaces, significantly reducing material waste over system lifetimes.
Scaling considerations reveal additional sustainability implications. Laboratory-scale demonstrations of rough and porous electrodes often utilize specialized fabrication techniques with potentially high environmental impacts. The transition to industrial-scale production requires developing more sustainable manufacturing methods that preserve the beneficial surface properties while minimizing resource intensity and waste generation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!