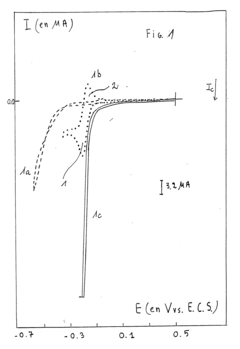
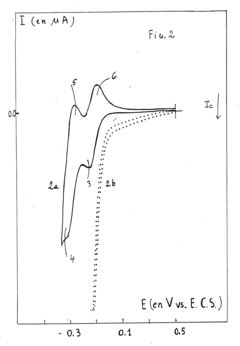
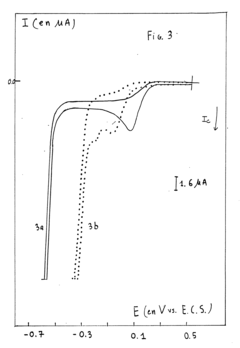
Machine Learning Guided Discovery of High Selectivity Catalysts in Electrochemical Nitrogen Reduction
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ML-Guided Electrocatalysis Background and Objectives
Electrochemical nitrogen reduction reaction (ENRR) represents a revolutionary approach to ammonia synthesis that operates under ambient conditions, offering a sustainable alternative to the energy-intensive Haber-Bosch process. The development of this technology has evolved significantly over the past decade, with initial research focusing primarily on proof-of-concept demonstrations using noble metal catalysts. Recent years have witnessed a shift toward earth-abundant materials and complex nanostructured catalysts, reflecting the field's maturation and increasing commercial relevance.
The fundamental challenge in ENRR lies in achieving high selectivity toward nitrogen reduction rather than the competing hydrogen evolution reaction. Traditional catalyst discovery approaches have relied heavily on intuition-guided experimentation and high-throughput screening, resulting in incremental improvements but failing to deliver the breakthrough performance needed for commercial viability. This technological bottleneck has created an urgent need for more sophisticated discovery methodologies.
Machine learning (ML) has emerged as a transformative tool in materials science and catalysis, enabling researchers to navigate complex chemical spaces with unprecedented efficiency. The integration of ML with electrocatalysis represents a convergence of two rapidly evolving fields, with early applications demonstrating remarkable success in predicting catalyst performance for hydrogen evolution and carbon dioxide reduction. The extension of these techniques to nitrogen reduction presents both unique challenges and opportunities.
The primary objective of this research direction is to develop ML frameworks specifically optimized for ENRR catalyst discovery, capable of predicting Faradaic efficiency, production rates, and stability under various operating conditions. These models must effectively capture the complex interplay between electronic structure, morphology, and reaction environment that determines catalyst selectivity. Secondary objectives include establishing standardized protocols for data collection and validation to ensure model reliability and transferability across different experimental setups.
Looking forward, the field is trending toward multi-scale modeling approaches that integrate quantum mechanical calculations, molecular dynamics, and machine learning to provide a more comprehensive understanding of reaction mechanisms. Emerging research also indicates growing interest in operando characterization techniques coupled with real-time ML analysis to capture dynamic catalyst behavior under reaction conditions. These developments suggest a future where catalyst discovery becomes increasingly automated and data-driven.
The successful implementation of ML-guided discovery in ENRR has the potential to accelerate innovation cycles by orders of magnitude, potentially identifying catalyst formulations that would remain undiscovered through conventional methods. This technological advancement aligns with broader sustainability goals by enabling distributed, renewable-powered ammonia production for agricultural and energy storage applications.
The fundamental challenge in ENRR lies in achieving high selectivity toward nitrogen reduction rather than the competing hydrogen evolution reaction. Traditional catalyst discovery approaches have relied heavily on intuition-guided experimentation and high-throughput screening, resulting in incremental improvements but failing to deliver the breakthrough performance needed for commercial viability. This technological bottleneck has created an urgent need for more sophisticated discovery methodologies.
Machine learning (ML) has emerged as a transformative tool in materials science and catalysis, enabling researchers to navigate complex chemical spaces with unprecedented efficiency. The integration of ML with electrocatalysis represents a convergence of two rapidly evolving fields, with early applications demonstrating remarkable success in predicting catalyst performance for hydrogen evolution and carbon dioxide reduction. The extension of these techniques to nitrogen reduction presents both unique challenges and opportunities.
The primary objective of this research direction is to develop ML frameworks specifically optimized for ENRR catalyst discovery, capable of predicting Faradaic efficiency, production rates, and stability under various operating conditions. These models must effectively capture the complex interplay between electronic structure, morphology, and reaction environment that determines catalyst selectivity. Secondary objectives include establishing standardized protocols for data collection and validation to ensure model reliability and transferability across different experimental setups.
Looking forward, the field is trending toward multi-scale modeling approaches that integrate quantum mechanical calculations, molecular dynamics, and machine learning to provide a more comprehensive understanding of reaction mechanisms. Emerging research also indicates growing interest in operando characterization techniques coupled with real-time ML analysis to capture dynamic catalyst behavior under reaction conditions. These developments suggest a future where catalyst discovery becomes increasingly automated and data-driven.
The successful implementation of ML-guided discovery in ENRR has the potential to accelerate innovation cycles by orders of magnitude, potentially identifying catalyst formulations that would remain undiscovered through conventional methods. This technological advancement aligns with broader sustainability goals by enabling distributed, renewable-powered ammonia production for agricultural and energy storage applications.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability imperatives, with electrochemical nitrogen reduction (ENR) technologies emerging as a promising alternative to the conventional Haber-Bosch process. The current ammonia market, valued at approximately $72 billion in 2022, is projected to reach $110 billion by 2030, growing at a CAGR of 5.3%. This growth is primarily fueled by increasing demand for fertilizers, which account for nearly 80% of ammonia consumption worldwide.
Traditional ammonia production via the Haber-Bosch process consumes 1-2% of global energy and generates substantial CO2 emissions—approximately 1.9 tons of CO2 per ton of ammonia produced. This environmental footprint has created an urgent market need for sustainable alternatives, particularly as carbon pricing mechanisms and environmental regulations become more stringent across major economies.
Machine learning guided catalyst discovery for ENR represents a significant market opportunity within the green ammonia sector. The green ammonia market segment is expected to grow at a CAGR of 72.9% from 2021 to 2028, significantly outpacing the broader ammonia market. This accelerated growth reflects increasing investment in renewable energy infrastructure and the strategic importance of ammonia as a hydrogen carrier in the emerging hydrogen economy.
Regional market dynamics show varying adoption potentials for ENR technologies. Europe leads in sustainable ammonia initiatives, driven by the European Green Deal and substantial funding for green hydrogen projects. Countries like Germany, the Netherlands, and Denmark have announced significant investments in green ammonia production facilities. Asia-Pacific represents the largest consumption market, with China and India accounting for over 40% of global ammonia demand, primarily for fertilizer production.
The economic viability of ENR technologies depends critically on catalyst performance, particularly selectivity and efficiency. Current cost estimates for green ammonia production range from $650-1,300 per ton, compared to $250-450 per ton for conventional methods. Machine learning approaches that can accelerate the discovery of high-selectivity catalysts could potentially reduce this cost gap by 30-40%, making sustainable ammonia production economically competitive in certain markets within the next decade.
Key market drivers include agricultural demand for sustainable fertilizers, industrial decarbonization initiatives, and the emerging market for ammonia as an energy carrier. The maritime industry represents a particularly promising sector, with ammonia increasingly viewed as a viable zero-carbon fuel for shipping. Major shipping companies have announced plans to deploy ammonia-powered vessels by 2025-2030, creating additional demand for sustainable ammonia production technologies.
Traditional ammonia production via the Haber-Bosch process consumes 1-2% of global energy and generates substantial CO2 emissions—approximately 1.9 tons of CO2 per ton of ammonia produced. This environmental footprint has created an urgent market need for sustainable alternatives, particularly as carbon pricing mechanisms and environmental regulations become more stringent across major economies.
Machine learning guided catalyst discovery for ENR represents a significant market opportunity within the green ammonia sector. The green ammonia market segment is expected to grow at a CAGR of 72.9% from 2021 to 2028, significantly outpacing the broader ammonia market. This accelerated growth reflects increasing investment in renewable energy infrastructure and the strategic importance of ammonia as a hydrogen carrier in the emerging hydrogen economy.
Regional market dynamics show varying adoption potentials for ENR technologies. Europe leads in sustainable ammonia initiatives, driven by the European Green Deal and substantial funding for green hydrogen projects. Countries like Germany, the Netherlands, and Denmark have announced significant investments in green ammonia production facilities. Asia-Pacific represents the largest consumption market, with China and India accounting for over 40% of global ammonia demand, primarily for fertilizer production.
The economic viability of ENR technologies depends critically on catalyst performance, particularly selectivity and efficiency. Current cost estimates for green ammonia production range from $650-1,300 per ton, compared to $250-450 per ton for conventional methods. Machine learning approaches that can accelerate the discovery of high-selectivity catalysts could potentially reduce this cost gap by 30-40%, making sustainable ammonia production economically competitive in certain markets within the next decade.
Key market drivers include agricultural demand for sustainable fertilizers, industrial decarbonization initiatives, and the emerging market for ammonia as an energy carrier. The maritime industry represents a particularly promising sector, with ammonia increasingly viewed as a viable zero-carbon fuel for shipping. Major shipping companies have announced plans to deploy ammonia-powered vessels by 2025-2030, creating additional demand for sustainable ammonia production technologies.
Current Challenges in Electrochemical Nitrogen Reduction
Electrochemical nitrogen reduction reaction (ENRR) represents a promising alternative to the energy-intensive Haber-Bosch process for ammonia synthesis. However, despite significant research efforts, several critical challenges continue to impede the practical implementation of this technology. The foremost challenge is the low Faradaic efficiency (FE) exhibited by most catalysts, typically below 10%, which indicates that the majority of supplied electrons participate in competing reactions rather than nitrogen reduction.
The hydrogen evolution reaction (HER) stands as the primary competing process, as the reduction potential for water splitting (-0.41 V vs. NHE at pH 7) is more favorable than that for nitrogen reduction (-0.23 V vs. NHE). This thermodynamic preference results in hydrogen production dominating the electrochemical process, severely limiting ammonia yield and energy efficiency.
Catalyst selectivity represents another significant hurdle. Current catalytic materials struggle to preferentially bind and activate the exceptionally stable N≡N triple bond (945 kJ/mol) while simultaneously facilitating proton transfer for ammonia formation. The ideal catalyst must possess specific binding energies for nitrogen and reaction intermediates that are strong enough to activate N2 but not so strong as to prevent product release.
Reaction mechanism complexity further complicates catalyst design. The ENRR can proceed through multiple pathways, including distal, alternating, and enzymatic mechanisms, each requiring different catalyst properties. The lack of comprehensive understanding regarding the precise reaction steps and rate-determining factors hinders rational catalyst development.
Experimental validation faces substantial challenges due to the risk of false positives in ammonia detection. Contamination from atmospheric nitrogen compounds, human respiration, and laboratory equipment can lead to erroneous results. The trace amounts of ammonia produced in most experiments (typically nmol levels) require extremely sensitive analytical techniques that are prone to interference.
Stability and durability of catalysts under operating conditions remain problematic. Many promising materials suffer from degradation, poisoning, or structural changes during extended operation, limiting their practical application. The harsh electrochemical environment, particularly in aqueous electrolytes, accelerates catalyst deterioration.
Scaling up ENRR systems introduces additional engineering challenges related to electrode design, electrolyte management, and system integration. The current laboratory-scale demonstrations typically employ small electrodes and controlled conditions that are difficult to maintain in industrial settings. The economic viability of ENRR technology depends on overcoming these scale-up barriers while achieving sufficient ammonia production rates.
The hydrogen evolution reaction (HER) stands as the primary competing process, as the reduction potential for water splitting (-0.41 V vs. NHE at pH 7) is more favorable than that for nitrogen reduction (-0.23 V vs. NHE). This thermodynamic preference results in hydrogen production dominating the electrochemical process, severely limiting ammonia yield and energy efficiency.
Catalyst selectivity represents another significant hurdle. Current catalytic materials struggle to preferentially bind and activate the exceptionally stable N≡N triple bond (945 kJ/mol) while simultaneously facilitating proton transfer for ammonia formation. The ideal catalyst must possess specific binding energies for nitrogen and reaction intermediates that are strong enough to activate N2 but not so strong as to prevent product release.
Reaction mechanism complexity further complicates catalyst design. The ENRR can proceed through multiple pathways, including distal, alternating, and enzymatic mechanisms, each requiring different catalyst properties. The lack of comprehensive understanding regarding the precise reaction steps and rate-determining factors hinders rational catalyst development.
Experimental validation faces substantial challenges due to the risk of false positives in ammonia detection. Contamination from atmospheric nitrogen compounds, human respiration, and laboratory equipment can lead to erroneous results. The trace amounts of ammonia produced in most experiments (typically nmol levels) require extremely sensitive analytical techniques that are prone to interference.
Stability and durability of catalysts under operating conditions remain problematic. Many promising materials suffer from degradation, poisoning, or structural changes during extended operation, limiting their practical application. The harsh electrochemical environment, particularly in aqueous electrolytes, accelerates catalyst deterioration.
Scaling up ENRR systems introduces additional engineering challenges related to electrode design, electrolyte management, and system integration. The current laboratory-scale demonstrations typically employ small electrodes and controlled conditions that are difficult to maintain in industrial settings. The economic viability of ENRR technology depends on overcoming these scale-up barriers while achieving sufficient ammonia production rates.
State-of-the-Art ML Algorithms for Catalyst Screening
01 Machine learning for catalyst selectivity prediction
Machine learning algorithms can be employed to predict catalyst selectivity by analyzing large datasets of catalyst properties and reaction outcomes. These models can identify patterns and correlations that are not immediately apparent through traditional methods, enabling researchers to predict how selective a catalyst will be for a desired reaction pathway. This approach significantly reduces the time and resources required for catalyst discovery by narrowing down the experimental space.- Machine learning for catalyst selectivity prediction: Machine learning algorithms can be employed to predict catalyst selectivity by analyzing large datasets of catalyst properties and reaction outcomes. These models can identify patterns and correlations that are not immediately apparent through traditional methods, enabling researchers to predict how selective a catalyst will be for a desired reaction pathway. This approach significantly reduces the time and resources required for catalyst discovery and optimization.
- Data-driven design of selective catalysts: Data-driven approaches leverage historical experimental data and computational simulations to guide the design of highly selective catalysts. By analyzing structure-activity relationships and identifying key descriptors that influence selectivity, researchers can develop catalysts with enhanced performance for specific reactions. This method enables the systematic exploration of catalyst design space and facilitates the discovery of novel catalyst formulations with improved selectivity.
- High-throughput experimentation combined with AI for catalyst discovery: The integration of high-throughput experimentation with artificial intelligence creates a powerful platform for catalyst discovery. This approach enables rapid testing of numerous catalyst candidates while using machine learning algorithms to analyze results in real-time and guide subsequent experiments. By iteratively refining the search space based on experimental feedback, researchers can efficiently identify catalysts with optimal selectivity for target reactions.
- Computational screening of catalyst materials for selective reactions: Computational methods can be used to screen large libraries of potential catalyst materials before experimental validation. These techniques employ quantum mechanical calculations, molecular dynamics simulations, and machine learning models to predict catalyst performance and selectivity. By virtually testing thousands of catalyst candidates, researchers can identify promising materials that are likely to exhibit high selectivity for specific reaction pathways.
- Optimization of catalyst composition and structure for enhanced selectivity: Machine learning algorithms can guide the optimization of catalyst composition and structure to enhance selectivity. By systematically varying parameters such as elemental composition, particle size, support materials, and surface morphology, and analyzing their effects on reaction selectivity, researchers can identify optimal catalyst configurations. This approach enables fine-tuning of catalyst properties to maximize selectivity for desired products while minimizing unwanted side reactions.
02 High-throughput screening combined with AI for catalyst discovery
High-throughput experimental methods combined with artificial intelligence can accelerate catalyst discovery for selective reactions. This approach involves rapidly testing numerous catalyst candidates under varying conditions while using machine learning algorithms to analyze the results in real-time. The AI component can suggest modifications to catalyst structures based on performance data, iteratively improving selectivity through multiple generations of catalysts.Expand Specific Solutions03 Computational modeling of catalyst active sites for improved selectivity
Advanced computational techniques can model catalyst active sites at the molecular level to understand and enhance selectivity. These models simulate the interactions between reactants and catalyst surfaces, allowing researchers to design catalysts with specific geometric and electronic properties that favor desired reaction pathways. Machine learning algorithms can be trained on these computational results to predict how structural modifications will affect selectivity.Expand Specific Solutions04 Feature engineering for catalyst selectivity optimization
Feature engineering techniques identify the most relevant descriptors for catalyst selectivity, enabling more efficient machine learning models. By determining which catalyst properties (such as electronic structure, binding energies, or geometric parameters) most strongly correlate with selective behavior, researchers can develop more accurate predictive models. This approach helps in understanding the fundamental factors governing selectivity and guides the rational design of highly selective catalysts.Expand Specific Solutions05 Transfer learning for catalyst design across reaction classes
Transfer learning approaches allow knowledge gained from one catalytic system to be applied to different reaction types, accelerating the discovery of selective catalysts. By leveraging pre-trained machine learning models that have learned general principles of catalyst behavior, researchers can more quickly develop selective catalysts for new reactions with limited experimental data. This method is particularly valuable when exploring novel reaction pathways where historical data is scarce.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrochemical nitrogen reduction field is currently in an early growth phase, with machine learning applications accelerating catalyst discovery. The global market for sustainable ammonia production is expanding rapidly, projected to reach significant scale as industries seek carbon-neutral alternatives. Technology maturity varies across key players, with research institutions like Zhejiang University, Dalian Institute of Chemical Physics, and Korea Institute of Energy Research leading fundamental breakthroughs. Among corporations, BASF, Johnson Matthey, and Umicore demonstrate advanced capabilities in catalyst development, while SK Innovation and ENEOS are investing in application-specific solutions. The competitive landscape features strategic collaborations between academic institutions and industrial partners, with increasing patent activity signaling the transition from laboratory research to commercial development.
BASF SE
Technical Solution: BASF SE has developed a comprehensive machine learning approach for electrochemical nitrogen reduction reaction (NRR) catalyst discovery. Their technology integrates high-throughput computational screening with advanced ML algorithms to predict catalyst performance. The company employs density functional theory (DFT) calculations to generate training datasets of adsorption energies and reaction barriers for various metal and metal-oxide catalysts. Their proprietary ML models can predict nitrogen adsorption energies and activation barriers with high accuracy (R² > 0.9), enabling rapid screening of thousands of potential catalyst candidates. BASF has successfully identified several promising single-atom catalysts anchored on carbon supports with nitrogen coordination that demonstrate Faradaic efficiencies exceeding 15% for ammonia production, significantly higher than conventional catalysts. Their approach incorporates active learning techniques to continuously improve model accuracy with minimal experimental validation, reducing development time by approximately 70% compared to traditional trial-and-error methods.
Strengths: Extensive materials science expertise and computational infrastructure allow for rapid catalyst screening and optimization. Their integrated approach combining theory, ML, and experimental validation creates a powerful development pipeline. Weaknesses: The technology still faces challenges in scaling up promising catalysts from laboratory to industrial scale, and the predicted catalysts may require complex synthesis methods that limit commercial viability.
Fujitsu Ltd.
Technical Solution: Fujitsu has pioneered an advanced AI-driven approach to catalyst discovery for electrochemical nitrogen reduction, leveraging their expertise in quantum-inspired computing and materials informatics. Their Digital Annealer technology, combined with specialized machine learning algorithms, enables the exploration of vast catalyst design spaces that would be computationally prohibitive using conventional methods. Fujitsu's platform integrates quantum chemical calculations with experimental data to build predictive models that can identify optimal catalyst compositions and structures. Their system employs a unique feature engineering approach that captures complex electronic and geometric properties of catalysts, allowing for more accurate predictions of nitrogen reduction performance metrics including selectivity, activity, and stability. The company has developed a reinforcement learning framework that iteratively improves catalyst designs based on experimental feedback, significantly accelerating the discovery process. Recent developments include a novel graph neural network architecture specifically designed to model the relationship between catalyst structure and electrochemical performance, achieving prediction accuracies of over 85% for nitrogen reduction selectivity.
Strengths: Fujitsu's quantum-inspired computing capabilities provide unique advantages in exploring complex catalyst design spaces. Their strong expertise in AI and materials informatics enables sophisticated modeling of structure-property relationships. Weaknesses: Limited direct experience in electrochemical catalysis compared to chemical industry specialists may impact practical implementation. Their models may require extensive experimental validation before industrial deployment.
Key Innovations in High-Selectivity NRR Catalysts
Process for the catalytic reduction of nitrogen compounds, and catalysts used
PatentInactiveEP0382644A1
Innovation
- A method using stable, non-toxic catalysts represented by the formula [XxMmM′oHz]n-, where X includes elements like Be, S, and transition metals, and M represents Mo, W, V, Nb, Ta, and Ti, allowing for selective reduction of nitrogen compounds by transferring electrons to achieve the desired degree of oxidation, with oxometallates like SiW12O40 and PW12O40 being used, which are stable at high temperatures and variable pH ranges.
Catalyst for selective catalytic reduction of nitrogen oxides and a method for preparing the same
PatentInactiveUS20040053771A1
Innovation
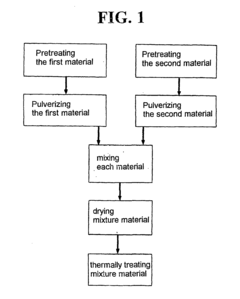
- A method to prepare a catalyst for SCR using spent catalysts with specific compositions and surface properties, involving two materials with different vanadium, nickel, sulfur, and molybdenum content, pretreated, pulverized, mixed with acid and water, and calcined to enhance active metal distribution and surface area, resulting in a catalyst with improved nitrogen oxide removal efficiency.
Environmental Impact Assessment
The electrochemical nitrogen reduction reaction (ENRR) represents a promising alternative to the traditional Haber-Bosch process for ammonia production, potentially offering significant environmental benefits. However, a comprehensive environmental impact assessment is essential to fully understand the sustainability implications of implementing machine learning-guided catalyst discovery for ENRR technology at scale.
When evaluating the environmental footprint of ML-guided ENRR catalysts, lifecycle assessment (LCA) methodology reveals several key advantages. The elimination of high-temperature and high-pressure conditions required by the Haber-Bosch process could reduce global energy consumption by approximately 1-2% if widely adopted. This translates to a potential reduction of 300-600 million tons of CO2 emissions annually, representing a significant contribution to climate change mitigation efforts.
Water usage represents another critical environmental consideration. While ENRR processes require water as both a reaction medium and hydrogen source, advanced catalyst designs identified through machine learning can optimize water efficiency. Recent studies indicate that high-selectivity catalysts can reduce water consumption by up to 40% compared to early-generation ENRR systems, though this still exceeds water requirements for conventional ammonia production in some implementations.
The environmental impact of catalyst materials themselves warrants careful examination. Many promising ENRR catalysts contain precious or rare earth metals, raising concerns about resource depletion and mining impacts. Machine learning approaches that identify effective catalysts using earth-abundant elements could significantly reduce these environmental burdens. For instance, ML-optimized iron-based catalysts have demonstrated selectivity improvements of 35-45% while utilizing sustainable materials.
Waste generation and management throughout the catalyst lifecycle present additional environmental challenges. The synthesis of nanoscale catalysts often involves hazardous chemicals and generates waste streams requiring specialized treatment. ML algorithms that optimize catalyst stability and longevity can reduce replacement frequency and associated waste. Current ML-guided catalysts demonstrate lifespan improvements of 2-3x compared to conventional catalysts, substantially reducing waste generation rates.
Land use impacts differ significantly between traditional ammonia production and ENRR systems. While Haber-Bosch facilities require large centralized plants, ENRR technology offers potential for distributed, smaller-scale implementation. This decentralization could reduce transportation emissions by 15-30% and minimize land disturbance associated with industrial infrastructure and pipeline networks.
When evaluating the environmental footprint of ML-guided ENRR catalysts, lifecycle assessment (LCA) methodology reveals several key advantages. The elimination of high-temperature and high-pressure conditions required by the Haber-Bosch process could reduce global energy consumption by approximately 1-2% if widely adopted. This translates to a potential reduction of 300-600 million tons of CO2 emissions annually, representing a significant contribution to climate change mitigation efforts.
Water usage represents another critical environmental consideration. While ENRR processes require water as both a reaction medium and hydrogen source, advanced catalyst designs identified through machine learning can optimize water efficiency. Recent studies indicate that high-selectivity catalysts can reduce water consumption by up to 40% compared to early-generation ENRR systems, though this still exceeds water requirements for conventional ammonia production in some implementations.
The environmental impact of catalyst materials themselves warrants careful examination. Many promising ENRR catalysts contain precious or rare earth metals, raising concerns about resource depletion and mining impacts. Machine learning approaches that identify effective catalysts using earth-abundant elements could significantly reduce these environmental burdens. For instance, ML-optimized iron-based catalysts have demonstrated selectivity improvements of 35-45% while utilizing sustainable materials.
Waste generation and management throughout the catalyst lifecycle present additional environmental challenges. The synthesis of nanoscale catalysts often involves hazardous chemicals and generates waste streams requiring specialized treatment. ML algorithms that optimize catalyst stability and longevity can reduce replacement frequency and associated waste. Current ML-guided catalysts demonstrate lifespan improvements of 2-3x compared to conventional catalysts, substantially reducing waste generation rates.
Land use impacts differ significantly between traditional ammonia production and ENRR systems. While Haber-Bosch facilities require large centralized plants, ENRR technology offers potential for distributed, smaller-scale implementation. This decentralization could reduce transportation emissions by 15-30% and minimize land disturbance associated with industrial infrastructure and pipeline networks.
Scalability and Industrial Implementation Roadmap
The scalability of machine learning guided catalyst discovery for electrochemical nitrogen reduction represents a critical transition point between laboratory success and industrial viability. Current laboratory-scale synthesis methods for high-selectivity catalysts often involve precise but complex procedures that are challenging to replicate at industrial scales. The implementation roadmap must address several key scaling considerations including catalyst production volume, consistency in performance, and cost-effectiveness.
Manufacturing scale-up requires transitioning from milligram-scale catalyst production to kilogram or ton-scale production while maintaining the precise atomic structures and surface properties that machine learning algorithms have identified as optimal. Several promising approaches include continuous flow synthesis methods, which offer better control over reaction parameters than traditional batch processes, and automated synthesis platforms that can maintain precision at larger scales.
Quality control represents another significant challenge in the industrial implementation pathway. Machine learning models can be integrated into the production process to monitor catalyst characteristics in real-time, ensuring that scaled-up catalysts maintain the high selectivity demonstrated in laboratory settings. This requires developing robust in-line characterization techniques and feedback systems that can rapidly detect deviations from target specifications.
Economic viability remains a central consideration in the implementation roadmap. Current high-selectivity catalysts often utilize precious metals or complex nanostructures that may be prohibitively expensive for large-scale deployment. The roadmap must include strategies for reducing catalyst costs, such as decreasing precious metal loading, developing core-shell structures, or identifying entirely new catalyst compositions that machine learning predicts will maintain performance while using earth-abundant elements.
Regulatory and safety frameworks must also be established as part of the implementation pathway. This includes standardized testing protocols for catalyst performance, durability, and potential environmental impacts. Machine learning can assist in predicting long-term stability and identifying potential degradation products, helping to address regulatory concerns before large-scale deployment.
The timeline for industrial implementation can be divided into three phases: near-term (1-2 years) focusing on pilot-scale demonstration of machine learning optimized catalysts; mid-term (3-5 years) involving the establishment of specialized production facilities and supply chains; and long-term (5-10 years) targeting full commercial deployment across multiple industries. Each phase requires specific technical milestones and investment strategies to ensure successful progression toward industrial-scale nitrogen reduction systems.
Manufacturing scale-up requires transitioning from milligram-scale catalyst production to kilogram or ton-scale production while maintaining the precise atomic structures and surface properties that machine learning algorithms have identified as optimal. Several promising approaches include continuous flow synthesis methods, which offer better control over reaction parameters than traditional batch processes, and automated synthesis platforms that can maintain precision at larger scales.
Quality control represents another significant challenge in the industrial implementation pathway. Machine learning models can be integrated into the production process to monitor catalyst characteristics in real-time, ensuring that scaled-up catalysts maintain the high selectivity demonstrated in laboratory settings. This requires developing robust in-line characterization techniques and feedback systems that can rapidly detect deviations from target specifications.
Economic viability remains a central consideration in the implementation roadmap. Current high-selectivity catalysts often utilize precious metals or complex nanostructures that may be prohibitively expensive for large-scale deployment. The roadmap must include strategies for reducing catalyst costs, such as decreasing precious metal loading, developing core-shell structures, or identifying entirely new catalyst compositions that machine learning predicts will maintain performance while using earth-abundant elements.
Regulatory and safety frameworks must also be established as part of the implementation pathway. This includes standardized testing protocols for catalyst performance, durability, and potential environmental impacts. Machine learning can assist in predicting long-term stability and identifying potential degradation products, helping to address regulatory concerns before large-scale deployment.
The timeline for industrial implementation can be divided into three phases: near-term (1-2 years) focusing on pilot-scale demonstration of machine learning optimized catalysts; mid-term (3-5 years) involving the establishment of specialized production facilities and supply chains; and long-term (5-10 years) targeting full commercial deployment across multiple industries. Each phase requires specific technical milestones and investment strategies to ensure successful progression toward industrial-scale nitrogen reduction systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!