Performance Trade Offs between Solid State and Liquid Electrolyte Systems in Electrochemical Nitrogen Reduction
AUG 26, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Nitrogen Reduction Technology Background and Objectives
Electrochemical nitrogen reduction reaction (ENRR) represents a revolutionary approach to ammonia synthesis that offers significant advantages over the conventional Haber-Bosch process. Dating back to the early 20th century, the Haber-Bosch process has dominated industrial ammonia production, operating under harsh conditions of 400-500°C and 150-300 bar pressure, consuming approximately 1-2% of global energy production annually. The emergence of ENRR technology in the past decade presents a promising alternative that can operate under ambient conditions with potentially lower energy requirements.
The evolution of ENRR technology has been marked by significant breakthroughs in electrocatalyst design and system engineering. Initial research focused primarily on aqueous systems using noble metal catalysts, achieving limited Faradaic efficiencies below 1%. Recent advances have expanded to non-noble metal catalysts, including transition metal nitrides, oxides, and carbon-based materials, pushing efficiencies to 10-15% in laboratory settings.
A critical bifurcation in ENRR development has emerged between liquid and solid-state electrolyte systems. Liquid electrolyte systems, predominantly utilizing aqueous solutions, offer excellent ionic conductivity and established operational protocols but suffer from competitive hydrogen evolution reactions and limited nitrogen solubility. Conversely, solid-state electrolyte systems, employing materials such as proton-conducting ceramics or polymer membranes, demonstrate enhanced nitrogen selectivity and reduced competing reactions but face challenges in mechanical stability and interfacial resistance.
The technical objectives for advancing ENRR technology center on improving three key performance metrics: Faradaic efficiency, production rate, and energy efficiency. Current state-of-the-art systems achieve Faradaic efficiencies of 10-30% with production rates typically below 100 μg h⁻¹ cm⁻² and energy efficiencies under 10%. Industry viability would require Faradaic efficiencies exceeding 50%, production rates above 1 mg h⁻¹ cm⁻², and energy efficiencies approaching 30%.
The trajectory of ENRR technology development is increasingly focused on understanding the fundamental mechanisms governing nitrogen activation and reduction at electrode-electrolyte interfaces. This includes elucidating the role of proton donors, electron transfer kinetics, and nitrogen adsorption dynamics in both liquid and solid-state systems. Computational studies using density functional theory have begun to provide insights into reaction pathways and energetics, guiding rational catalyst design.
Looking forward, ENRR technology aims to enable distributed, renewable-powered ammonia production that could revolutionize agricultural fertilizer supply chains and potentially serve as an energy carrier in a hydrogen economy. The performance trade-offs between solid-state and liquid electrolyte systems represent a critical research frontier that will significantly influence the commercial viability and ultimate implementation of this transformative technology.
The evolution of ENRR technology has been marked by significant breakthroughs in electrocatalyst design and system engineering. Initial research focused primarily on aqueous systems using noble metal catalysts, achieving limited Faradaic efficiencies below 1%. Recent advances have expanded to non-noble metal catalysts, including transition metal nitrides, oxides, and carbon-based materials, pushing efficiencies to 10-15% in laboratory settings.
A critical bifurcation in ENRR development has emerged between liquid and solid-state electrolyte systems. Liquid electrolyte systems, predominantly utilizing aqueous solutions, offer excellent ionic conductivity and established operational protocols but suffer from competitive hydrogen evolution reactions and limited nitrogen solubility. Conversely, solid-state electrolyte systems, employing materials such as proton-conducting ceramics or polymer membranes, demonstrate enhanced nitrogen selectivity and reduced competing reactions but face challenges in mechanical stability and interfacial resistance.
The technical objectives for advancing ENRR technology center on improving three key performance metrics: Faradaic efficiency, production rate, and energy efficiency. Current state-of-the-art systems achieve Faradaic efficiencies of 10-30% with production rates typically below 100 μg h⁻¹ cm⁻² and energy efficiencies under 10%. Industry viability would require Faradaic efficiencies exceeding 50%, production rates above 1 mg h⁻¹ cm⁻², and energy efficiencies approaching 30%.
The trajectory of ENRR technology development is increasingly focused on understanding the fundamental mechanisms governing nitrogen activation and reduction at electrode-electrolyte interfaces. This includes elucidating the role of proton donors, electron transfer kinetics, and nitrogen adsorption dynamics in both liquid and solid-state systems. Computational studies using density functional theory have begun to provide insights into reaction pathways and energetics, guiding rational catalyst design.
Looking forward, ENRR technology aims to enable distributed, renewable-powered ammonia production that could revolutionize agricultural fertilizer supply chains and potentially serve as an energy carrier in a hydrogen economy. The performance trade-offs between solid-state and liquid electrolyte systems represent a critical research frontier that will significantly influence the commercial viability and ultimate implementation of this transformative technology.
Market Analysis for Nitrogen Fixation Technologies
The global nitrogen fixation market is experiencing significant growth, driven by increasing demand for fertilizers in agriculture and various industrial applications. Currently valued at approximately 32 billion USD, this market is projected to expand at a compound annual growth rate of 3.5% through 2030, reaching an estimated 45 billion USD. The traditional Haber-Bosch process dominates industrial nitrogen fixation, accounting for over 90% of fixed nitrogen production worldwide, despite its high energy consumption and carbon footprint.
Electrochemical nitrogen reduction (ENR) technologies represent an emerging segment within this market, with particular interest in both solid-state and liquid electrolyte systems. The market potential for these technologies is substantial, especially as environmental regulations tighten and industries seek more sustainable production methods. Current market penetration remains limited, with ENR technologies primarily in research and development phases rather than commercial deployment.
Regional analysis reveals Asia-Pacific as the fastest-growing market for nitrogen fixation technologies, driven by China and India's expanding agricultural sectors. North America and Europe lead in research and development of alternative nitrogen fixation methods, including electrochemical approaches, supported by substantial government funding and corporate investment in green technologies.
Market segmentation shows distinct applications across agricultural fertilizers (68%), industrial chemicals (22%), and specialty applications including pharmaceuticals and electronics (10%). The agricultural sector remains the primary driver of market growth, though industrial applications are expanding at a faster rate due to diversification of nitrogen compound usage in manufacturing processes.
Consumer trends indicate increasing preference for sustainably produced fertilizers and chemicals, creating market pull for alternatives to Haber-Bosch. This shift is particularly evident in developed economies where environmental considerations increasingly influence purchasing decisions. Premium pricing for "green ammonia" and other sustainably fixed nitrogen products is becoming viable in certain market segments.
Competitive analysis reveals traditional chemical companies investing in electrochemical nitrogen reduction research, while startups focused specifically on solid-state or liquid electrolyte systems are attracting significant venture capital. Strategic partnerships between technology developers and end-users are emerging as a key market entry strategy, allowing for application-specific optimization of ENR technologies.
Market barriers include high capital costs for new technologies, technical challenges in achieving commercially viable conversion rates, and entrenched infrastructure supporting conventional processes. However, regulatory incentives for carbon reduction and potential cost advantages through distributed production models are creating favorable conditions for market entry of novel nitrogen fixation technologies.
Electrochemical nitrogen reduction (ENR) technologies represent an emerging segment within this market, with particular interest in both solid-state and liquid electrolyte systems. The market potential for these technologies is substantial, especially as environmental regulations tighten and industries seek more sustainable production methods. Current market penetration remains limited, with ENR technologies primarily in research and development phases rather than commercial deployment.
Regional analysis reveals Asia-Pacific as the fastest-growing market for nitrogen fixation technologies, driven by China and India's expanding agricultural sectors. North America and Europe lead in research and development of alternative nitrogen fixation methods, including electrochemical approaches, supported by substantial government funding and corporate investment in green technologies.
Market segmentation shows distinct applications across agricultural fertilizers (68%), industrial chemicals (22%), and specialty applications including pharmaceuticals and electronics (10%). The agricultural sector remains the primary driver of market growth, though industrial applications are expanding at a faster rate due to diversification of nitrogen compound usage in manufacturing processes.
Consumer trends indicate increasing preference for sustainably produced fertilizers and chemicals, creating market pull for alternatives to Haber-Bosch. This shift is particularly evident in developed economies where environmental considerations increasingly influence purchasing decisions. Premium pricing for "green ammonia" and other sustainably fixed nitrogen products is becoming viable in certain market segments.
Competitive analysis reveals traditional chemical companies investing in electrochemical nitrogen reduction research, while startups focused specifically on solid-state or liquid electrolyte systems are attracting significant venture capital. Strategic partnerships between technology developers and end-users are emerging as a key market entry strategy, allowing for application-specific optimization of ENR technologies.
Market barriers include high capital costs for new technologies, technical challenges in achieving commercially viable conversion rates, and entrenched infrastructure supporting conventional processes. However, regulatory incentives for carbon reduction and potential cost advantages through distributed production models are creating favorable conditions for market entry of novel nitrogen fixation technologies.
Current Challenges in Solid-State vs Liquid Electrolyte Systems
The electrochemical nitrogen reduction reaction (NRR) represents a promising approach for sustainable ammonia production, but faces significant challenges in both solid-state and liquid electrolyte systems. In liquid electrolyte systems, the primary challenge remains the competitive hydrogen evolution reaction (HER), which dominates due to the aqueous environment and significantly reduces Faradaic efficiency for nitrogen reduction. This competition is particularly problematic as the standard reduction potential for nitrogen reduction (-0.23 V vs. SHE) is close to that of hydrogen evolution (0 V vs. SHE), making selective catalysis extremely difficult.
Liquid electrolyte systems also suffer from limited nitrogen solubility (approximately 20 mg/L at room temperature and atmospheric pressure), creating mass transport limitations that restrict reaction rates. The three-phase interface requirement—where gaseous N₂, liquid electrolyte, and solid catalyst must meet—creates additional complexity in reactor design and operation. Furthermore, the stability of catalysts in liquid environments often deteriorates due to leaching, poisoning, and structural degradation during extended operation.
Solid-state electrolyte systems have emerged as potential alternatives but face their own set of challenges. The primary limitation is their significantly lower ionic conductivity compared to liquid electrolytes, typically 1-3 orders of magnitude lower at room temperature. This conductivity gap necessitates operation at elevated temperatures (often >400°C) to achieve practical reaction rates, introducing thermal management complexities and energy inefficiencies.
Interface resistance between the solid electrolyte and electrodes represents another major hurdle, as imperfect contact creates high resistance pathways that limit current density and overall system performance. Mechanical stability issues are also prevalent, with solid electrolytes prone to cracking during thermal cycling or extended operation due to mechanical stress and volume changes during ion transport.
Manufacturing scalability presents additional challenges for solid-state systems. Current fabrication techniques for high-quality solid electrolytes with minimal defects and high conductivity remain costly and difficult to scale, limiting commercial viability. The integration of solid electrolytes into practical devices with appropriate sealing and connections adds further complexity.
Both systems face catalyst selectivity challenges, though in different contexts. In liquid systems, selectivity must overcome the HER competition, while solid-state systems must address competing reactions like NOx formation at elevated temperatures. Additionally, both approaches struggle with long-term stability under operating conditions, though the degradation mechanisms differ significantly between the two electrolyte environments.
Recent research has begun exploring hybrid approaches that combine aspects of both systems, such as polymer electrolytes with controlled water content or supported ionic liquid membranes, which may offer pathways to overcome the limitations inherent to either pure solid-state or liquid electrolyte configurations.
Liquid electrolyte systems also suffer from limited nitrogen solubility (approximately 20 mg/L at room temperature and atmospheric pressure), creating mass transport limitations that restrict reaction rates. The three-phase interface requirement—where gaseous N₂, liquid electrolyte, and solid catalyst must meet—creates additional complexity in reactor design and operation. Furthermore, the stability of catalysts in liquid environments often deteriorates due to leaching, poisoning, and structural degradation during extended operation.
Solid-state electrolyte systems have emerged as potential alternatives but face their own set of challenges. The primary limitation is their significantly lower ionic conductivity compared to liquid electrolytes, typically 1-3 orders of magnitude lower at room temperature. This conductivity gap necessitates operation at elevated temperatures (often >400°C) to achieve practical reaction rates, introducing thermal management complexities and energy inefficiencies.
Interface resistance between the solid electrolyte and electrodes represents another major hurdle, as imperfect contact creates high resistance pathways that limit current density and overall system performance. Mechanical stability issues are also prevalent, with solid electrolytes prone to cracking during thermal cycling or extended operation due to mechanical stress and volume changes during ion transport.
Manufacturing scalability presents additional challenges for solid-state systems. Current fabrication techniques for high-quality solid electrolytes with minimal defects and high conductivity remain costly and difficult to scale, limiting commercial viability. The integration of solid electrolytes into practical devices with appropriate sealing and connections adds further complexity.
Both systems face catalyst selectivity challenges, though in different contexts. In liquid systems, selectivity must overcome the HER competition, while solid-state systems must address competing reactions like NOx formation at elevated temperatures. Additionally, both approaches struggle with long-term stability under operating conditions, though the degradation mechanisms differ significantly between the two electrolyte environments.
Recent research has begun exploring hybrid approaches that combine aspects of both systems, such as polymer electrolytes with controlled water content or supported ionic liquid membranes, which may offer pathways to overcome the limitations inherent to either pure solid-state or liquid electrolyte configurations.
Comparative Analysis of Existing Electrolyte System Solutions
01 Solid-state electrolytes for nitrogen reduction
Solid-state electrolytes offer advantages in electrochemical nitrogen reduction reactions due to their stability and ion conductivity properties. These materials can facilitate the transfer of ions while maintaining structural integrity during the nitrogen reduction process. The use of ceramic or polymer-based solid electrolytes can enhance the efficiency of nitrogen fixation by providing stable pathways for ion movement while preventing unwanted side reactions that commonly occur in liquid systems.- Solid-state electrolytes for nitrogen reduction: Solid-state electrolytes offer advantages in electrochemical nitrogen reduction reactions due to their stability and ion conductivity properties. These materials can facilitate the transfer of ions while maintaining structural integrity during the nitrogen reduction process. The use of ceramic or polymer-based solid electrolytes can enhance the efficiency of nitrogen fixation by providing stable pathways for ion movement while preventing unwanted side reactions that commonly occur in liquid systems.
- Liquid electrolyte formulations for nitrogen reduction: Liquid electrolytes play a crucial role in electrochemical nitrogen reduction by providing a medium for ion transport and reactant dissolution. These electrolytes typically contain specific salts and solvents that can influence the reaction kinetics and selectivity. Optimized liquid electrolyte formulations can enhance nitrogen reduction performance by controlling proton availability, suppressing competing hydrogen evolution reactions, and facilitating nitrogen activation at the electrode surface.
- Hybrid electrolyte systems combining solid and liquid components: Hybrid electrolyte systems that integrate both solid and liquid components offer unique advantages for electrochemical nitrogen reduction. These systems combine the stability of solid electrolytes with the high ionic conductivity of liquid electrolytes. The solid component often serves as a separator or ion-selective membrane, while the liquid component facilitates mass transport and reaction kinetics. This combination can lead to improved nitrogen reduction performance by balancing stability, conductivity, and reactivity considerations.
- Catalyst-electrolyte interface engineering: The interface between catalysts and electrolytes is critical for efficient electrochemical nitrogen reduction. Engineering this interface involves optimizing the catalyst structure, electrolyte composition, and their interaction to enhance nitrogen adsorption and activation. Strategies include developing porous catalyst structures that maximize contact with the electrolyte, incorporating ionic liquids at the interface, and designing electrolytes that can modulate the local pH and electric field at the catalyst surface. These approaches can significantly improve nitrogen reduction performance by facilitating electron transfer and nitrogen activation.
- Temperature and pressure effects on electrolyte performance: Temperature and pressure conditions significantly impact the performance of both solid and liquid electrolytes in electrochemical nitrogen reduction. Higher temperatures can enhance ion mobility and reaction kinetics but may compromise stability, while elevated pressures can increase nitrogen solubility in liquid electrolytes. Optimizing these parameters for specific electrolyte systems is essential for maximizing nitrogen reduction efficiency. Some advanced systems incorporate temperature-responsive electrolytes or pressure-modulated reaction chambers to achieve optimal performance under varying conditions.
02 Liquid electrolyte formulations for nitrogen reduction
Liquid electrolytes play a crucial role in electrochemical nitrogen reduction by providing a medium for ion transport and reactant dissolution. These electrolytes typically contain specific salts and solvents that can influence the reaction kinetics and selectivity. Optimized liquid electrolyte formulations can enhance nitrogen reduction performance by controlling proton availability, suppressing competing hydrogen evolution reactions, and facilitating the multi-electron transfer process required for N₂ reduction to ammonia.Expand Specific Solutions03 Hybrid electrolyte systems combining solid and liquid phases
Hybrid electrolyte systems that combine solid and liquid components offer unique advantages for nitrogen reduction reactions. These systems typically feature a solid support structure infused with liquid electrolyte, creating interfaces that can enhance catalytic activity. The solid component provides structural stability and selective ion transport, while the liquid phase ensures efficient mass transport of reactants and products. This combination can lead to improved nitrogen reduction performance by balancing stability with reactivity.Expand Specific Solutions04 Catalyst-electrolyte interface engineering
The interface between catalysts and electrolytes is critical for efficient electrochemical nitrogen reduction. Engineering this interface involves optimizing the catalyst structure, electrolyte composition, and their interaction to enhance nitrogen adsorption and activation. Techniques such as surface modification, nanostructuring, and controlled wetting properties can improve the triple-phase boundary where gas, catalyst, and electrolyte meet. Proper interface engineering can significantly enhance reaction kinetics and selectivity toward ammonia formation.Expand Specific Solutions05 Temperature and pressure effects on electrolyte performance
Temperature and pressure conditions significantly impact the performance of both solid and liquid electrolytes in nitrogen reduction systems. Higher temperatures can enhance ion mobility and reaction kinetics but may compromise stability, while elevated pressures can increase nitrogen solubility in liquid electrolytes. Optimizing these parameters for specific electrolyte systems is essential for maximizing nitrogen reduction efficiency. Some advanced systems incorporate temperature management and pressure control mechanisms to maintain optimal electrolyte performance during operation.Expand Specific Solutions
Key Industry Players and Research Institutions
The electrochemical nitrogen reduction (ENR) technology landscape is currently in an early development stage, with market size still limited but showing significant growth potential as sustainable ammonia production becomes increasingly important. The technology maturity varies across solid-state and liquid electrolyte systems, with each offering distinct performance trade-offs. Leading companies like QuantumScape, Contemporary Amperex Technology, and Samsung Electronics are advancing solid-state technologies with superior stability and selectivity, while Resonac, Idemitsu Kosan, and Toyota Motor Europe are developing liquid electrolyte systems offering better conductivity and lower operating temperatures. Academic institutions including MIT, Tsinghua University, and Zhejiang University are driving fundamental research, creating a competitive ecosystem where industrial-academic partnerships are accelerating commercialization efforts despite remaining technical challenges.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed a hybrid electrolyte system for electrochemical nitrogen reduction that combines aspects of both solid and liquid electrolytes. Their approach utilizes a polymer-ceramic composite electrolyte with ionic liquid infiltration to create a quasi-solid-state system. This technology leverages CATL's extensive experience in battery electrolyte engineering, adapted specifically for nitrogen reduction reactions. The system features a hierarchical porous electrode structure with optimized triple-phase boundaries to facilitate nitrogen adsorption and electron transfer. CATL's proprietary catalyst formulation incorporates transition metal nitrides and oxides supported on carbon nanostructures, which are directly integrated with the composite electrolyte. Their ENR cells operate at near-ambient conditions (25-60°C) and have demonstrated ammonia production rates of 5-8 μg h^-1 cm^-2 with Faradaic efficiencies reaching 12% under optimized conditions. The company has also developed specialized membrane electrode assemblies that minimize interfacial resistance while maintaining mechanical integrity during operation. CATL's approach balances the conductivity advantages of liquid electrolytes with the stability benefits of solid-state systems.
Strengths: Better ionic conductivity than pure solid-state systems; good mechanical stability and safety; moderate manufacturing complexity leveraging existing production capabilities; operational flexibility across varying conditions. Weaknesses: Still experiences some electrolyte degradation over extended operation; requires careful moisture management; performance trade-offs between conductivity and stability; moderate energy efficiency compared to pure solid-state systems.
Samsung Electronics Co., Ltd.
Technical Solution: Samsung has developed an innovative dual-phase electrolyte system for electrochemical nitrogen reduction that combines the advantages of both solid and liquid approaches. Their technology features a composite electrolyte with a solid ceramic backbone infiltrated with an ionic liquid phase, creating a system with enhanced ionic conductivity while maintaining mechanical stability. Samsung's ENR cells utilize specialized electrode structures with hierarchical porosity to optimize nitrogen gas diffusion and reaction kinetics. The catalyst design incorporates nanoscale transition metal nitrides dispersed on carbon supports, with precise control over particle size and distribution to maximize active site density. Their system operates effectively at near-ambient temperatures (30-70°C) and has demonstrated ammonia production rates of 6-10 μg h^-1 cm^-2 with Faradaic efficiencies in the 10-15% range under optimized conditions. Samsung has leveraged their semiconductor manufacturing expertise to develop precise deposition techniques for creating uniform electrolyte-electrode interfaces with minimal defects. Their approach also includes proprietary additives in the electrolyte composition that enhance nitrogen activation while suppressing competing hydrogen evolution reactions.
Strengths: Superior ionic conductivity compared to pure solid-state systems; good mechanical stability and safety profile; excellent scalability potential leveraging Samsung's manufacturing capabilities; balanced performance across efficiency, rate, and durability metrics. Weaknesses: More complex system design and manufacturing requirements; potential for gradual performance degradation due to liquid component changes; moderate energy efficiency compared to theoretical limits; requires careful thermal management during operation.
Critical Patents and Breakthroughs in Electrolyte Design
Three-dimensional nanostructures created by electrospinning
PatentWO2021260380A1
Innovation
- A method combining 3D electrospinning and 3D printing to create self-standing 3D carbon nanostructures from polymers like polyacrylonitrile (PAN) that can be carbonized, offering enhanced surface area, conductivity, and mechanical properties, thereby overcoming the limitations of 2D structures and improving electrode performance.
Solid state electrolyte layer, electrode active material layer, all solid state lithium battery, manufacturing method for solid state electrolyte layer, and manufacturing method for electrode active material layer
PatentActiveUS9300011B2
Innovation
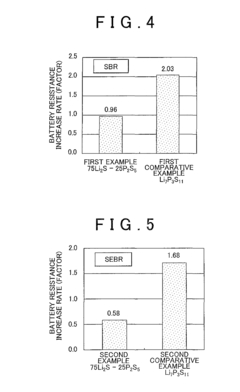
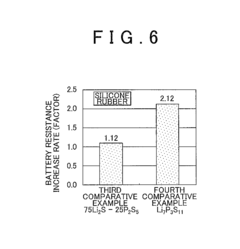
- A sulfide solid state electrolyte material composed of Li2S and P2S5, substantially free of bridging sulfur, is used in conjunction with a hydrophobic polymer to form a solid state electrolyte layer, suppressing the deterioration and resistance increase.
Energy Efficiency and Sustainability Considerations
The energy efficiency of electrochemical nitrogen reduction reaction (NRR) systems represents a critical factor in determining their commercial viability and environmental impact. When comparing solid-state and liquid electrolyte configurations, significant differences emerge in their energy consumption patterns and overall sustainability profiles.
Liquid electrolyte systems typically demonstrate higher ionic conductivity, facilitating faster reaction kinetics and potentially lower overpotentials. However, these systems often require additional energy inputs for electrolyte circulation, temperature control, and post-reaction separation processes. The energy required to maintain optimal operating conditions in liquid systems can constitute a substantial portion of the total energy budget, particularly in scaled-up applications.
Solid-state electrolyte configurations, while generally exhibiting lower ionic conductivity, offer advantages in system simplification and reduced parasitic energy losses. The elimination of pumping systems, reduced evaporation losses, and more compact design contribute to potential energy savings in peripheral operations. Additionally, solid-state systems typically demonstrate enhanced stability during extended operation periods, reducing the energy costs associated with system maintenance and electrolyte replacement.
From a life cycle perspective, liquid electrolyte systems often entail higher environmental impacts due to the production, transportation, and disposal of liquid chemicals. The water footprint of aqueous electrolyte systems presents particular concerns in regions facing water scarcity. Conversely, solid-state systems generally incorporate more energy-intensive manufacturing processes for electrolyte materials but may offer longer operational lifetimes and reduced resource consumption during operation.
Carbon footprint assessments reveal that the primary energy source powering these electrochemical systems ultimately determines their climate impact. Integration with renewable energy sources represents a critical pathway toward sustainable NRR implementation for both system types. Solid-state configurations may offer advantages in this context due to their potential for more flexible operation and better compatibility with intermittent renewable power sources.
Material sustainability considerations also differ significantly between the two approaches. Liquid electrolyte systems often rely on relatively abundant materials but may require continuous replenishment and generate waste streams requiring treatment. Solid-state systems frequently incorporate critical materials with more complex supply chains, though their longer operational lifetimes may partially offset resource intensity concerns.
The energy return on investment (EROI) calculations currently favor neither approach definitively, as both technologies remain in developmental stages with significant efficiency improvements anticipated. Future sustainability assessments must consider not only direct energy inputs but also embodied energy in materials, system durability, and end-of-life management strategies to provide comprehensive guidance for technology development pathways.
Liquid electrolyte systems typically demonstrate higher ionic conductivity, facilitating faster reaction kinetics and potentially lower overpotentials. However, these systems often require additional energy inputs for electrolyte circulation, temperature control, and post-reaction separation processes. The energy required to maintain optimal operating conditions in liquid systems can constitute a substantial portion of the total energy budget, particularly in scaled-up applications.
Solid-state electrolyte configurations, while generally exhibiting lower ionic conductivity, offer advantages in system simplification and reduced parasitic energy losses. The elimination of pumping systems, reduced evaporation losses, and more compact design contribute to potential energy savings in peripheral operations. Additionally, solid-state systems typically demonstrate enhanced stability during extended operation periods, reducing the energy costs associated with system maintenance and electrolyte replacement.
From a life cycle perspective, liquid electrolyte systems often entail higher environmental impacts due to the production, transportation, and disposal of liquid chemicals. The water footprint of aqueous electrolyte systems presents particular concerns in regions facing water scarcity. Conversely, solid-state systems generally incorporate more energy-intensive manufacturing processes for electrolyte materials but may offer longer operational lifetimes and reduced resource consumption during operation.
Carbon footprint assessments reveal that the primary energy source powering these electrochemical systems ultimately determines their climate impact. Integration with renewable energy sources represents a critical pathway toward sustainable NRR implementation for both system types. Solid-state configurations may offer advantages in this context due to their potential for more flexible operation and better compatibility with intermittent renewable power sources.
Material sustainability considerations also differ significantly between the two approaches. Liquid electrolyte systems often rely on relatively abundant materials but may require continuous replenishment and generate waste streams requiring treatment. Solid-state systems frequently incorporate critical materials with more complex supply chains, though their longer operational lifetimes may partially offset resource intensity concerns.
The energy return on investment (EROI) calculations currently favor neither approach definitively, as both technologies remain in developmental stages with significant efficiency improvements anticipated. Future sustainability assessments must consider not only direct energy inputs but also embodied energy in materials, system durability, and end-of-life management strategies to provide comprehensive guidance for technology development pathways.
Scalability and Industrial Implementation Pathways
The scalability of electrochemical nitrogen reduction reaction (NRR) technologies from laboratory to industrial scale presents significant challenges that differ between solid-state and liquid electrolyte systems. Liquid electrolyte systems currently demonstrate greater potential for near-term industrial implementation due to established engineering practices in electrochemical industries. These systems benefit from well-understood reactor designs, efficient mass transport mechanisms, and relatively straightforward scale-up methodologies that have been refined through decades of industrial electrochemistry applications.
Solid-state electrolyte systems, while promising for their selectivity and efficiency advantages, face substantial hurdles in manufacturing scale-up. The production of large-area, defect-free solid electrolytes with consistent properties remains technically challenging and cost-prohibitive at industrial scales. Current fabrication methods often involve complex processes that are difficult to translate from laboratory to mass production environments without significant yield losses or performance degradation.
For liquid electrolyte NRR systems, implementation pathways typically involve adaptation of existing electrolyzer technologies, particularly those developed for water electrolysis or chlor-alkali processes. This approach allows for leveraging established supply chains, manufacturing capabilities, and operational expertise. The primary scale-up challenges center on maintaining reaction selectivity at higher current densities and managing heat dissipation in larger reactors.
Solid-state systems require more fundamental innovation in manufacturing processes. Promising implementation pathways include development of continuous production methods for ceramic or polymer-based solid electrolytes, potentially adapting techniques from solid oxide fuel cell or battery industries. Thermal management and mechanical stability during long-term operation represent critical engineering challenges that must be addressed before industrial deployment.
Economic considerations heavily influence implementation timelines. Liquid electrolyte systems generally present lower capital expenditure requirements but may incur higher operational costs due to electrolyte management and product separation needs. Solid-state systems typically involve higher initial investment but potentially lower long-term operational expenses due to improved selectivity and reduced separation requirements.
Regulatory frameworks and safety considerations also shape implementation pathways differently for each technology. Liquid electrolyte systems must address concerns regarding electrolyte handling, potential leakage, and disposal. Solid-state systems face scrutiny regarding long-term stability, failure modes, and end-of-life management of specialized materials.
The most promising near-term industrial implementation strategy may involve hybrid approaches that leverage the established scalability of liquid systems while incorporating elements of solid-state technology in critical reaction zones to enhance performance and selectivity.
Solid-state electrolyte systems, while promising for their selectivity and efficiency advantages, face substantial hurdles in manufacturing scale-up. The production of large-area, defect-free solid electrolytes with consistent properties remains technically challenging and cost-prohibitive at industrial scales. Current fabrication methods often involve complex processes that are difficult to translate from laboratory to mass production environments without significant yield losses or performance degradation.
For liquid electrolyte NRR systems, implementation pathways typically involve adaptation of existing electrolyzer technologies, particularly those developed for water electrolysis or chlor-alkali processes. This approach allows for leveraging established supply chains, manufacturing capabilities, and operational expertise. The primary scale-up challenges center on maintaining reaction selectivity at higher current densities and managing heat dissipation in larger reactors.
Solid-state systems require more fundamental innovation in manufacturing processes. Promising implementation pathways include development of continuous production methods for ceramic or polymer-based solid electrolytes, potentially adapting techniques from solid oxide fuel cell or battery industries. Thermal management and mechanical stability during long-term operation represent critical engineering challenges that must be addressed before industrial deployment.
Economic considerations heavily influence implementation timelines. Liquid electrolyte systems generally present lower capital expenditure requirements but may incur higher operational costs due to electrolyte management and product separation needs. Solid-state systems typically involve higher initial investment but potentially lower long-term operational expenses due to improved selectivity and reduced separation requirements.
Regulatory frameworks and safety considerations also shape implementation pathways differently for each technology. Liquid electrolyte systems must address concerns regarding electrolyte handling, potential leakage, and disposal. Solid-state systems face scrutiny regarding long-term stability, failure modes, and end-of-life management of specialized materials.
The most promising near-term industrial implementation strategy may involve hybrid approaches that leverage the established scalability of liquid systems while incorporating elements of solid-state technology in critical reaction zones to enhance performance and selectivity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!