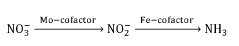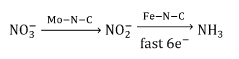Effects of Electrode Morphology on Nitrogen Adsorption and Reduction Pathways in Electrochemical Nitrogen Reduction
AUG 26, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrode Morphology in Nitrogen Reduction: Background & Objectives
Electrochemical nitrogen reduction reaction (NRR) represents a groundbreaking approach to ammonia synthesis that operates under ambient conditions, offering a sustainable alternative to the energy-intensive Haber-Bosch process. The development of this technology has gained significant momentum over the past decade, driven by increasing global demands for sustainable fertilizer production and clean energy carriers.
The morphology of electrodes plays a crucial role in determining the efficiency and selectivity of nitrogen adsorption and subsequent reduction pathways. Historical developments in this field reveal that early NRR research primarily focused on catalyst composition rather than structural design. However, recent breakthroughs have demonstrated that electrode morphology can significantly influence reaction kinetics, active site availability, and mass transport properties.
The evolution of electrode design has progressed from simple planar structures to complex three-dimensional architectures with precisely controlled porosity, surface roughness, and hierarchical features. This progression reflects growing recognition that nitrogen molecules require specific spatial configurations for optimal adsorption and activation, particularly given the strength of the N≡N triple bond (941 kJ/mol) that must be overcome.
Current technological trends indicate a shift toward nanoscale engineering of electrode surfaces, with particular emphasis on creating high-density active sites while maintaining efficient pathways for reactant diffusion and product desorption. Advanced fabrication techniques including electrodeposition, chemical vapor deposition, and template-assisted synthesis have enabled unprecedented control over electrode morphology at multiple length scales.
The primary technical objectives in this field include enhancing nitrogen adsorption capacity, optimizing the binding energy of reaction intermediates, and promoting the desired reaction pathways while suppressing competing reactions—particularly the hydrogen evolution reaction that often dominates in aqueous electrolytes. Achieving these objectives requires systematic investigation of how specific morphological features affect each step of the nitrogen reduction mechanism.
Additionally, researchers aim to establish clear structure-property relationships that can guide rational electrode design. This includes understanding how parameters such as pore size distribution, surface curvature, defect concentration, and crystallographic orientation influence the energetics of nitrogen adsorption and subsequent protonation steps.
The ultimate goal is to develop electrode architectures that can achieve ammonia production rates exceeding 10^-6 mol cm^-2 h^-1 with Faradaic efficiencies above 50% under ambient conditions—metrics that would represent significant progress toward commercial viability of electrochemical nitrogen fixation technology.
The morphology of electrodes plays a crucial role in determining the efficiency and selectivity of nitrogen adsorption and subsequent reduction pathways. Historical developments in this field reveal that early NRR research primarily focused on catalyst composition rather than structural design. However, recent breakthroughs have demonstrated that electrode morphology can significantly influence reaction kinetics, active site availability, and mass transport properties.
The evolution of electrode design has progressed from simple planar structures to complex three-dimensional architectures with precisely controlled porosity, surface roughness, and hierarchical features. This progression reflects growing recognition that nitrogen molecules require specific spatial configurations for optimal adsorption and activation, particularly given the strength of the N≡N triple bond (941 kJ/mol) that must be overcome.
Current technological trends indicate a shift toward nanoscale engineering of electrode surfaces, with particular emphasis on creating high-density active sites while maintaining efficient pathways for reactant diffusion and product desorption. Advanced fabrication techniques including electrodeposition, chemical vapor deposition, and template-assisted synthesis have enabled unprecedented control over electrode morphology at multiple length scales.
The primary technical objectives in this field include enhancing nitrogen adsorption capacity, optimizing the binding energy of reaction intermediates, and promoting the desired reaction pathways while suppressing competing reactions—particularly the hydrogen evolution reaction that often dominates in aqueous electrolytes. Achieving these objectives requires systematic investigation of how specific morphological features affect each step of the nitrogen reduction mechanism.
Additionally, researchers aim to establish clear structure-property relationships that can guide rational electrode design. This includes understanding how parameters such as pore size distribution, surface curvature, defect concentration, and crystallographic orientation influence the energetics of nitrogen adsorption and subsequent protonation steps.
The ultimate goal is to develop electrode architectures that can achieve ammonia production rates exceeding 10^-6 mol cm^-2 h^-1 with Faradaic efficiencies above 50% under ambient conditions—metrics that would represent significant progress toward commercial viability of electrochemical nitrogen fixation technology.
Market Analysis for Electrochemical Nitrogen Fixation Technologies
The global market for electrochemical nitrogen fixation technologies is experiencing significant growth, driven by increasing demand for sustainable fertilizer production methods. Traditional nitrogen fixation via the Haber-Bosch process consumes approximately 1-2% of global energy production and generates substantial greenhouse gas emissions. This creates a compelling market opportunity for electrochemical nitrogen reduction reaction (NRR) technologies that can operate at ambient conditions with renewable electricity.
Current market estimates value the industrial nitrogen fixation sector at approximately $150 billion annually, with fertilizer production representing the largest segment. The electrochemical nitrogen fixation market, while still nascent, is projected to grow at a compound annual growth rate of 25-30% over the next decade as the technology matures and production costs decrease.
Regional analysis indicates that Asia-Pacific, particularly China and India, represents the largest potential market due to their agricultural intensity and growing focus on sustainable farming practices. North America and Europe follow closely, driven by stringent environmental regulations and investment in green technologies. These regions are also home to most early-stage companies and research institutions developing NRR technologies.
Market segmentation reveals diverse application potential beyond traditional fertilizers. Emerging applications include on-site ammonia production for remote agricultural operations, integration with renewable energy systems for energy storage, and specialized chemical manufacturing. The pharmaceutical and specialty chemicals sectors represent premium market segments where high-purity, small-scale nitrogen fixation commands significant price premiums.
Investor interest in the sector has grown substantially, with venture capital funding for electrochemical nitrogen fixation startups exceeding $300 million in the past three years. Strategic investments from established agricultural technology companies and chemical manufacturers signal growing confidence in the commercial viability of these technologies.
Key market drivers include increasing environmental regulations on conventional fertilizer production, volatility in natural gas prices affecting traditional ammonia synthesis costs, and growing consumer demand for sustainably produced agricultural products. The carbon credit market also presents a significant economic incentive, as electrochemical nitrogen fixation could potentially generate carbon offset credits when powered by renewable energy.
Market barriers include scaling challenges, electrode durability issues in real-world applications, and competition from incremental improvements to the Haber-Bosch process. The market also faces adoption hurdles related to integration with existing agricultural supply chains and infrastructure limitations for renewable electricity in many agricultural regions.
Current market estimates value the industrial nitrogen fixation sector at approximately $150 billion annually, with fertilizer production representing the largest segment. The electrochemical nitrogen fixation market, while still nascent, is projected to grow at a compound annual growth rate of 25-30% over the next decade as the technology matures and production costs decrease.
Regional analysis indicates that Asia-Pacific, particularly China and India, represents the largest potential market due to their agricultural intensity and growing focus on sustainable farming practices. North America and Europe follow closely, driven by stringent environmental regulations and investment in green technologies. These regions are also home to most early-stage companies and research institutions developing NRR technologies.
Market segmentation reveals diverse application potential beyond traditional fertilizers. Emerging applications include on-site ammonia production for remote agricultural operations, integration with renewable energy systems for energy storage, and specialized chemical manufacturing. The pharmaceutical and specialty chemicals sectors represent premium market segments where high-purity, small-scale nitrogen fixation commands significant price premiums.
Investor interest in the sector has grown substantially, with venture capital funding for electrochemical nitrogen fixation startups exceeding $300 million in the past three years. Strategic investments from established agricultural technology companies and chemical manufacturers signal growing confidence in the commercial viability of these technologies.
Key market drivers include increasing environmental regulations on conventional fertilizer production, volatility in natural gas prices affecting traditional ammonia synthesis costs, and growing consumer demand for sustainably produced agricultural products. The carbon credit market also presents a significant economic incentive, as electrochemical nitrogen fixation could potentially generate carbon offset credits when powered by renewable energy.
Market barriers include scaling challenges, electrode durability issues in real-world applications, and competition from incremental improvements to the Haber-Bosch process. The market also faces adoption hurdles related to integration with existing agricultural supply chains and infrastructure limitations for renewable electricity in many agricultural regions.
Current Challenges in Electrode Design for Nitrogen Reduction
Despite significant advancements in electrochemical nitrogen reduction reaction (NRR) research, electrode design remains a critical bottleneck in achieving commercially viable nitrogen fixation systems. Current electrodes face multiple challenges that limit their practical application and efficiency in converting atmospheric nitrogen to ammonia under ambient conditions.
The primary challenge lies in the competing hydrogen evolution reaction (HER), which occurs at similar potential ranges as NRR. Most electrode materials demonstrate preferential catalytic activity toward HER rather than NRR, resulting in low Faradaic efficiency for ammonia production. This competition fundamentally limits the selectivity of the process and wastes significant energy.
Nitrogen adsorption presents another major hurdle, as the N≡N triple bond requires substantial energy input for activation. Most electrode surfaces exhibit weak nitrogen adsorption capabilities, with the molecule often binding in unfavorable orientations that hinder effective electron transfer. The relationship between surface morphology and adsorption strength/orientation remains poorly understood and difficult to control.
Reaction pathway identification and control represent significant scientific challenges. Different electrode morphologies can promote various reaction intermediates (*NH, NH2, etc.), leading to diverse reaction pathways with varying energy barriers. Current analytical techniques struggle to identify these intermediates in real-time, making mechanistic understanding and rational design difficult.
Stability issues plague many promising electrode materials, with performance degradation occurring through multiple mechanisms including surface poisoning, restructuring, and dissolution. Particularly in aqueous electrolytes, maintaining structural integrity over extended operation periods remains problematic.
Mass transport limitations significantly impact reaction kinetics, especially regarding nitrogen availability at the electrode surface. Current electrode designs often fail to optimize the three-phase boundary where gaseous nitrogen, liquid electrolyte, and solid catalyst interact. Pore structure, wettability, and gas diffusion pathways require careful engineering that current fabrication methods struggle to achieve consistently.
Scalability concerns further complicate electrode development, as many high-performing laboratory-scale electrodes utilize expensive noble metals or complex nanostructures that present significant manufacturing challenges. The trade-off between performance, cost, and scalability remains unresolved for most promising electrode materials.
Addressing these interconnected challenges requires interdisciplinary approaches combining advanced materials science, electrochemistry, and engineering to develop next-generation electrode architectures specifically optimized for nitrogen reduction pathways.
The primary challenge lies in the competing hydrogen evolution reaction (HER), which occurs at similar potential ranges as NRR. Most electrode materials demonstrate preferential catalytic activity toward HER rather than NRR, resulting in low Faradaic efficiency for ammonia production. This competition fundamentally limits the selectivity of the process and wastes significant energy.
Nitrogen adsorption presents another major hurdle, as the N≡N triple bond requires substantial energy input for activation. Most electrode surfaces exhibit weak nitrogen adsorption capabilities, with the molecule often binding in unfavorable orientations that hinder effective electron transfer. The relationship between surface morphology and adsorption strength/orientation remains poorly understood and difficult to control.
Reaction pathway identification and control represent significant scientific challenges. Different electrode morphologies can promote various reaction intermediates (*NH, NH2, etc.), leading to diverse reaction pathways with varying energy barriers. Current analytical techniques struggle to identify these intermediates in real-time, making mechanistic understanding and rational design difficult.
Stability issues plague many promising electrode materials, with performance degradation occurring through multiple mechanisms including surface poisoning, restructuring, and dissolution. Particularly in aqueous electrolytes, maintaining structural integrity over extended operation periods remains problematic.
Mass transport limitations significantly impact reaction kinetics, especially regarding nitrogen availability at the electrode surface. Current electrode designs often fail to optimize the three-phase boundary where gaseous nitrogen, liquid electrolyte, and solid catalyst interact. Pore structure, wettability, and gas diffusion pathways require careful engineering that current fabrication methods struggle to achieve consistently.
Scalability concerns further complicate electrode development, as many high-performing laboratory-scale electrodes utilize expensive noble metals or complex nanostructures that present significant manufacturing challenges. The trade-off between performance, cost, and scalability remains unresolved for most promising electrode materials.
Addressing these interconnected challenges requires interdisciplinary approaches combining advanced materials science, electrochemistry, and engineering to develop next-generation electrode architectures specifically optimized for nitrogen reduction pathways.
Current Electrode Morphology Optimization Strategies
01 Electrode morphology optimization for nitrogen adsorption
The morphology of electrodes can be optimized to enhance nitrogen adsorption capabilities. This involves designing specific surface structures, pore distributions, and surface area characteristics that maximize the interaction with nitrogen molecules. Various manufacturing techniques can be employed to create electrodes with controlled porosity, surface roughness, and active sites that facilitate nitrogen adsorption processes. These optimized morphologies improve the overall efficiency of nitrogen-related electrochemical reactions.- Electrode morphology optimization for nitrogen adsorption: The morphology of electrodes can be optimized to enhance nitrogen adsorption capabilities. Various structural designs, such as porous architectures, nanostructured surfaces, and hierarchical arrangements, can significantly increase the surface area available for nitrogen adsorption. These optimized morphologies provide more active sites for nitrogen molecules to interact with the electrode surface, improving the overall efficiency of nitrogen-related electrochemical processes.
- Nitrogen reduction reaction (NRR) catalytic pathways: Different catalytic pathways can be employed for the electrochemical reduction of nitrogen. These pathways involve various reaction mechanisms, including associative and dissociative mechanisms, which determine how nitrogen molecules are activated and reduced on the electrode surface. Understanding these reduction pathways is crucial for designing efficient catalysts that can overcome the kinetic barriers associated with breaking the strong N≡N triple bond and facilitating the formation of desired nitrogen-containing products.
- Surface modification techniques for enhanced nitrogen interaction: Various surface modification techniques can be applied to electrodes to enhance their interaction with nitrogen molecules. These include doping with heteroatoms, creating defects, introducing functional groups, and depositing active metal sites. Such modifications alter the electronic structure of the electrode surface, creating favorable binding sites for nitrogen adsorption and activation, which ultimately improves the efficiency of nitrogen reduction processes.
- Characterization methods for nitrogen adsorption properties: Various analytical techniques can be used to characterize the nitrogen adsorption properties of electrode materials. These include Brunauer-Emmett-Teller (BET) surface area analysis, temperature-programmed desorption (TPD), X-ray photoelectron spectroscopy (XPS), and in-situ spectroscopic methods. These techniques provide valuable information about the adsorption capacity, binding energy, adsorption sites, and the nature of the interaction between nitrogen molecules and the electrode surface.
- Electrode materials for selective nitrogen conversion: Various electrode materials can be designed for selective nitrogen conversion processes. These include transition metal-based catalysts, metal oxides, nitrides, carbon-based materials, and composite structures. The selection of appropriate electrode materials is crucial for achieving high selectivity and efficiency in nitrogen reduction reactions, as different materials provide unique active sites and electronic structures that influence the adsorption and activation of nitrogen molecules.
02 Nitrogen reduction reaction (NRR) catalytic pathways
Different catalytic pathways can be employed for nitrogen reduction reactions, particularly for ammonia synthesis. These pathways involve specific reaction mechanisms where nitrogen molecules are adsorbed onto catalyst surfaces and undergo sequential reduction steps. The efficiency of these pathways depends on the catalyst composition, electronic structure, and the presence of specific active sites. Understanding these reduction pathways is crucial for developing more efficient catalysts for nitrogen fixation and ammonia production.Expand Specific Solutions03 Surface modification techniques for enhanced nitrogen interactions
Various surface modification techniques can be applied to electrodes to enhance their interaction with nitrogen molecules. These include doping with heteroatoms, creating defects, introducing functional groups, and depositing active metal nanoparticles. Such modifications alter the electronic properties of the electrode surface, creating favorable binding sites for nitrogen adsorption and activation. These techniques can significantly improve the performance of electrodes in nitrogen-related applications such as sensing, separation, and catalytic conversion.Expand Specific Solutions04 Porous electrode structures for nitrogen applications
Porous electrode structures offer advantages for nitrogen adsorption and reduction applications due to their high surface area and abundant active sites. These structures can be designed with hierarchical porosity, including micropores, mesopores, and macropores, to facilitate mass transport and provide accessible reaction sites. Various materials including carbon-based materials, metal oxides, and metal-organic frameworks can be fabricated into porous electrodes with tailored properties for specific nitrogen-related applications.Expand Specific Solutions05 Characterization methods for nitrogen adsorption properties
Various analytical techniques can be used to characterize the nitrogen adsorption properties of electrode materials. These include Brunauer-Emmett-Teller (BET) surface area analysis, temperature-programmed desorption (TPD), X-ray photoelectron spectroscopy (XPS), and electrochemical impedance spectroscopy (EIS). These methods provide insights into the adsorption capacity, binding energy, surface chemistry, and kinetics of nitrogen interactions with electrode surfaces, which are essential for understanding and optimizing electrode performance in nitrogen-related applications.Expand Specific Solutions
Leading Research Groups and Companies in Electrochemical Nitrogen Reduction
The electrochemical nitrogen reduction field is currently in an early development stage, with market size still limited but growing rapidly due to increasing interest in sustainable ammonia production. Research institutions like Dalian Institute of Chemical Physics, Arizona State University, and Tsinghua University are leading fundamental research on electrode morphology effects, while companies including Covestro, Siemens AG, and Industrie De Nora are beginning to explore commercial applications. The technology remains at low maturity (TRL 3-4), with academic institutions dominating patent filings. Key players are focusing on catalyst design optimization and understanding nitrogen adsorption mechanisms, with international collaboration between research centers and industrial partners accelerating development toward practical electrochemical nitrogen fixation systems.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed advanced electrode architectures for electrochemical nitrogen reduction reaction (NRR). Their approach focuses on hierarchical porous structures with optimized micro/meso/macro-porosity to enhance nitrogen adsorption and activation. DICP researchers have engineered single-atom catalysts dispersed on carbon supports with precisely controlled morphology, achieving nitrogen fixation rates up to 29.6 μg h−1 mg−1cat under ambient conditions. Their electrodes incorporate defect-rich surfaces and oxygen vacancies that serve as active sites for N2 adsorption, significantly lowering the energy barrier for the rate-determining step. DICP has also pioneered the development of hollow nanostructured electrodes that provide confined reaction spaces to concentrate reactants and facilitate the electron transfer process, resulting in improved Faradaic efficiency for ammonia production compared to conventional flat electrodes.
Strengths: Superior control over hierarchical porosity enabling enhanced mass transport and reactant accessibility; expertise in single-atom catalyst design providing maximum atom utilization efficiency. Weaknesses: Scalability challenges for complex nanostructured electrodes; potential stability issues during long-term operation under industrial conditions.
The Regents of the University of California
Technical Solution: The University of California has pioneered electrode designs for nitrogen reduction featuring biomimetic approaches inspired by nitrogenase enzymes. Their technology incorporates molybdenum-based active sites embedded in precisely engineered carbon matrices with controlled hydrophilicity gradients to optimize nitrogen access while managing proton and electron transfer. UC researchers have developed electrodes with dual-function components: outer layers optimized for nitrogen adsorption and inner structures designed for efficient electron transport. Their approach includes the strategic introduction of oxygen-containing functional groups at specific densities (0.5-2.0 at%) to create favorable binding sites for N2 molecules. The electrode architecture features hierarchical porosity with macropores (>50 nm) for mass transport and mesopores (2-50 nm) that serve as reaction chambers, achieving ammonia production rates of 18.5 μg h−1 mg−1cat with Faradaic efficiencies approaching 10% under ambient conditions, representing significant improvements over conventional flat electrode designs.
Strengths: Sophisticated biomimetic design principles; excellent balance between nitrogen adsorption and electron transfer properties. Weaknesses: High manufacturing complexity potentially increasing production costs; challenges in maintaining consistent performance across varying operating conditions.
Key Mechanisms of Nitrogen Adsorption on Different Electrode Surfaces
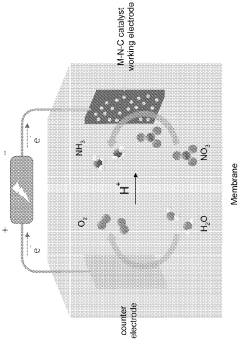
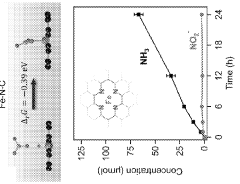
Electrocatalytic conversion of nitrates and nitrites to ammonia
PatentWO2023192228A1
Innovation
- The use of an electrochemical cell with an atomically dispersed transition metal-nitrogen-carbon (M-N-C) catalyst, specifically FeMo-based catalysts, to electrocatalytically convert nitrates to ammonia, leveraging distinct reaction pathways and synergizing Mo and Fe sites for enhanced selectivity and stability.
Electrochemical method for reducing molecular oxygen
PatentInactiveEP2379782A1
Innovation
- The use of nitrogen-doped carbon nanotubes with a proportion of pyridinic and quaternary nitrogen allows for the direct electrochemical reduction of molecular oxygen to doubly negatively charged oxygen ions without intermediate hydrogen peroxide formation, using a voltage to transfer four electrons, thereby preventing hydrogen peroxide formation and maximizing the yield of desired oxygen ions.
Environmental Impact and Sustainability of Electrochemical Nitrogen Reduction
Electrochemical Nitrogen Reduction (ENR) technology represents a promising alternative to the conventional Haber-Bosch process for ammonia production, offering significant environmental benefits. Unlike the energy-intensive Haber-Bosch process that operates under harsh conditions (400-500°C, 150-300 bar) and consumes 1-2% of global energy while generating substantial CO2 emissions, ENR operates at ambient conditions with potentially zero carbon emissions when powered by renewable energy sources.
The environmental impact of ENR technology is primarily determined by the electrode materials and their morphology. Precious metal-based electrodes, while effective, raise sustainability concerns due to their scarcity and environmental impact during mining and processing. Recent research has focused on developing earth-abundant alternatives such as transition metal nitrides, oxides, and carbon-based materials that demonstrate comparable performance with reduced environmental footprint.
Electrode morphology significantly influences the sustainability profile of ENR systems. Nanostructured electrodes with high surface area-to-volume ratios enhance nitrogen adsorption and reduction efficiency, potentially reducing energy requirements. However, the synthesis of these complex morphologies often involves energy-intensive processes and hazardous chemicals, creating a sustainability trade-off that must be carefully evaluated through comprehensive life cycle assessments.
Water consumption represents another critical environmental consideration for ENR technology. The competing hydrogen evolution reaction consumes water and reduces faradaic efficiency. Electrode morphologies that selectively favor nitrogen reduction over hydrogen evolution not only improve ammonia yield but also enhance water use efficiency, a crucial factor in regions facing water scarcity.
The long-term durability of electrodes directly impacts the sustainability of ENR systems. Electrodes with stable morphologies that resist degradation during operation minimize the need for frequent replacement, reducing material consumption and waste generation. Research indicates that certain hierarchical structures and composite materials demonstrate superior stability while maintaining high catalytic activity.
From a circular economy perspective, the recyclability of electrode materials presents both challenges and opportunities. Complex nanostructured electrodes may be difficult to recycle effectively, while simpler morphologies using abundant materials offer better end-of-life management options. Developing electrode designs that facilitate material recovery and reuse will be essential for maximizing the sustainability benefits of ENR technology.
In conclusion, optimizing electrode morphology for ENR represents a multifaceted sustainability challenge that extends beyond performance metrics to include material selection, synthesis methods, operational efficiency, and end-of-life considerations. Addressing these factors holistically will be crucial for realizing the full environmental potential of this promising technology.
The environmental impact of ENR technology is primarily determined by the electrode materials and their morphology. Precious metal-based electrodes, while effective, raise sustainability concerns due to their scarcity and environmental impact during mining and processing. Recent research has focused on developing earth-abundant alternatives such as transition metal nitrides, oxides, and carbon-based materials that demonstrate comparable performance with reduced environmental footprint.
Electrode morphology significantly influences the sustainability profile of ENR systems. Nanostructured electrodes with high surface area-to-volume ratios enhance nitrogen adsorption and reduction efficiency, potentially reducing energy requirements. However, the synthesis of these complex morphologies often involves energy-intensive processes and hazardous chemicals, creating a sustainability trade-off that must be carefully evaluated through comprehensive life cycle assessments.
Water consumption represents another critical environmental consideration for ENR technology. The competing hydrogen evolution reaction consumes water and reduces faradaic efficiency. Electrode morphologies that selectively favor nitrogen reduction over hydrogen evolution not only improve ammonia yield but also enhance water use efficiency, a crucial factor in regions facing water scarcity.
The long-term durability of electrodes directly impacts the sustainability of ENR systems. Electrodes with stable morphologies that resist degradation during operation minimize the need for frequent replacement, reducing material consumption and waste generation. Research indicates that certain hierarchical structures and composite materials demonstrate superior stability while maintaining high catalytic activity.
From a circular economy perspective, the recyclability of electrode materials presents both challenges and opportunities. Complex nanostructured electrodes may be difficult to recycle effectively, while simpler morphologies using abundant materials offer better end-of-life management options. Developing electrode designs that facilitate material recovery and reuse will be essential for maximizing the sustainability benefits of ENR technology.
In conclusion, optimizing electrode morphology for ENR represents a multifaceted sustainability challenge that extends beyond performance metrics to include material selection, synthesis methods, operational efficiency, and end-of-life considerations. Addressing these factors holistically will be crucial for realizing the full environmental potential of this promising technology.
Scalability and Industrial Implementation Considerations
Scaling up electrochemical nitrogen reduction reaction (NRR) technologies from laboratory demonstrations to industrial applications presents significant challenges that must be addressed systematically. The electrode morphology, which has proven critical for nitrogen adsorption and reduction pathways at the laboratory scale, faces additional complexities when considered for large-scale implementation.
The transition from small-scale electrodes to industrial-sized systems requires careful engineering to maintain the beneficial morphological features that enhance nitrogen adsorption. Surface area-to-volume ratios typically decrease with scaling, potentially reducing catalytic efficiency. Industrial implementations must therefore incorporate advanced manufacturing techniques such as precision 3D printing, controlled electrodeposition, or template-assisted synthesis to preserve nanoscale features across larger electrode surfaces.
Material stability becomes increasingly critical at industrial scales where electrodes must maintain performance over thousands of operational hours. Morphological degradation through mechanisms such as surface reconstruction, particle agglomeration, or pore blockage can significantly impact long-term nitrogen reduction efficiency. Implementing protective coatings or developing self-healing electrode structures may provide viable solutions to these durability challenges.
Economic considerations heavily influence industrial viability. While complex nanostructured electrodes with optimized morphologies demonstrate superior performance in laboratories, their production costs may be prohibitively expensive for large-scale applications. A balance must be struck between performance and manufacturability, potentially through hierarchical structures that combine inexpensive bulk materials with strategically placed high-performance catalytic sites.
Process integration presents another crucial consideration. Industrial NRR systems must operate within existing chemical production infrastructure, requiring electrodes designed for compatibility with standard equipment. Factors such as pressure tolerance, flow dynamics, and electrical connectivity must be addressed during scale-up, often necessitating compromises in morphological design to accommodate practical constraints.
Energy efficiency at scale directly impacts operational costs and environmental footprint. Electrode morphologies that minimize parasitic reactions (particularly hydrogen evolution) become even more valuable in industrial settings. Advanced flow field designs that optimize nitrogen transport to active sites while minimizing mass transfer limitations can significantly improve overall system efficiency.
Standardization and quality control protocols must be established to ensure consistent electrode morphology across production batches. Variations in surface features can lead to unpredictable performance in industrial settings, undermining process reliability. Non-destructive characterization techniques capable of rapidly assessing morphological parameters will be essential for quality assurance in scaled manufacturing.
The transition from small-scale electrodes to industrial-sized systems requires careful engineering to maintain the beneficial morphological features that enhance nitrogen adsorption. Surface area-to-volume ratios typically decrease with scaling, potentially reducing catalytic efficiency. Industrial implementations must therefore incorporate advanced manufacturing techniques such as precision 3D printing, controlled electrodeposition, or template-assisted synthesis to preserve nanoscale features across larger electrode surfaces.
Material stability becomes increasingly critical at industrial scales where electrodes must maintain performance over thousands of operational hours. Morphological degradation through mechanisms such as surface reconstruction, particle agglomeration, or pore blockage can significantly impact long-term nitrogen reduction efficiency. Implementing protective coatings or developing self-healing electrode structures may provide viable solutions to these durability challenges.
Economic considerations heavily influence industrial viability. While complex nanostructured electrodes with optimized morphologies demonstrate superior performance in laboratories, their production costs may be prohibitively expensive for large-scale applications. A balance must be struck between performance and manufacturability, potentially through hierarchical structures that combine inexpensive bulk materials with strategically placed high-performance catalytic sites.
Process integration presents another crucial consideration. Industrial NRR systems must operate within existing chemical production infrastructure, requiring electrodes designed for compatibility with standard equipment. Factors such as pressure tolerance, flow dynamics, and electrical connectivity must be addressed during scale-up, often necessitating compromises in morphological design to accommodate practical constraints.
Energy efficiency at scale directly impacts operational costs and environmental footprint. Electrode morphologies that minimize parasitic reactions (particularly hydrogen evolution) become even more valuable in industrial settings. Advanced flow field designs that optimize nitrogen transport to active sites while minimizing mass transfer limitations can significantly improve overall system efficiency.
Standardization and quality control protocols must be established to ensure consistent electrode morphology across production batches. Variations in surface features can lead to unpredictable performance in industrial settings, undermining process reliability. Non-destructive characterization techniques capable of rapidly assessing morphological parameters will be essential for quality assurance in scaled manufacturing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!