Design Principles for High Current Density Electrodes in Electrochemical Nitrogen Reduction
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Nitrogen Reduction Background and Objectives
Electrochemical nitrogen reduction (ENR) represents a revolutionary approach to ammonia synthesis that has gained significant attention over the past decade. Unlike the conventional Haber-Bosch process, which operates under harsh conditions of 400-500°C and 100-300 bar pressure while consuming approximately 1-2% of global energy production, ENR offers the potential for ammonia synthesis under ambient conditions with significantly reduced energy requirements. This technology evolution aligns with global sustainability goals and addresses the increasing demand for decentralized ammonia production systems.
The historical development of nitrogen fixation technologies began with the Haber-Bosch process in the early 20th century, which revolutionized agriculture and supported global population growth. However, its energy-intensive nature and centralized production model have prompted research into alternative approaches. Electrochemical methods emerged in the 1980s but gained substantial momentum only in the last decade, with breakthrough publications demonstrating the feasibility of ambient-condition nitrogen reduction.
Current technological trajectories indicate a shift toward electrode materials that can achieve higher current densities while maintaining selectivity for nitrogen reduction over competing reactions, particularly hydrogen evolution. This represents a critical technical objective for making ENR commercially viable. Research trends show increasing focus on nanostructured catalysts, single-atom catalysts, and hybrid materials that can efficiently activate the strong N≡N triple bond while suppressing side reactions.
The primary technical objectives in this field include developing electrodes capable of achieving industrial-relevant current densities (>300 mA/cm²) while maintaining Faradaic efficiency above 10% for ammonia production. Additionally, catalyst stability under operating conditions remains a significant challenge, with most reported materials showing performance degradation after several hours of operation. Addressing these limitations requires fundamental understanding of reaction mechanisms at the electrode-electrolyte interface.
Recent advances in operando characterization techniques and computational modeling have accelerated understanding of the molecular-level processes involved in electrochemical nitrogen reduction. These insights are driving more rational design approaches for high-performance electrodes, moving beyond traditional trial-and-error methodologies. The convergence of advanced materials science, electrochemistry, and computational tools is expected to enable breakthrough electrode designs in the coming years.
The ultimate goal of ENR technology development is to create economically viable systems that can be deployed at various scales, from distributed agricultural applications to grid-scale energy storage solutions utilizing excess renewable electricity. This aligns with broader trends toward decarbonization and electrification across industrial sectors.
The historical development of nitrogen fixation technologies began with the Haber-Bosch process in the early 20th century, which revolutionized agriculture and supported global population growth. However, its energy-intensive nature and centralized production model have prompted research into alternative approaches. Electrochemical methods emerged in the 1980s but gained substantial momentum only in the last decade, with breakthrough publications demonstrating the feasibility of ambient-condition nitrogen reduction.
Current technological trajectories indicate a shift toward electrode materials that can achieve higher current densities while maintaining selectivity for nitrogen reduction over competing reactions, particularly hydrogen evolution. This represents a critical technical objective for making ENR commercially viable. Research trends show increasing focus on nanostructured catalysts, single-atom catalysts, and hybrid materials that can efficiently activate the strong N≡N triple bond while suppressing side reactions.
The primary technical objectives in this field include developing electrodes capable of achieving industrial-relevant current densities (>300 mA/cm²) while maintaining Faradaic efficiency above 10% for ammonia production. Additionally, catalyst stability under operating conditions remains a significant challenge, with most reported materials showing performance degradation after several hours of operation. Addressing these limitations requires fundamental understanding of reaction mechanisms at the electrode-electrolyte interface.
Recent advances in operando characterization techniques and computational modeling have accelerated understanding of the molecular-level processes involved in electrochemical nitrogen reduction. These insights are driving more rational design approaches for high-performance electrodes, moving beyond traditional trial-and-error methodologies. The convergence of advanced materials science, electrochemistry, and computational tools is expected to enable breakthrough electrode designs in the coming years.
The ultimate goal of ENR technology development is to create economically viable systems that can be deployed at various scales, from distributed agricultural applications to grid-scale energy storage solutions utilizing excess renewable electricity. This aligns with broader trends toward decarbonization and electrification across industrial sectors.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability concerns and technological innovations. Traditional ammonia production via the Haber-Bosch process consumes approximately 2% of global energy and generates substantial CO2 emissions. This creates a compelling market opportunity for electrochemical nitrogen reduction (ECNR) technologies that can produce ammonia at ambient conditions with renewable electricity.
The current global ammonia market is valued at over $70 billion, with an annual production exceeding 180 million tons. Industrial fertilizers represent the dominant application segment, accounting for roughly 80% of total consumption. However, emerging applications in energy storage, hydrogen carriers, and clean fuel are expanding market potential significantly.
Demand for sustainable ammonia production methods is accelerating due to stringent environmental regulations, carbon pricing mechanisms, and corporate sustainability commitments. The European Union's Carbon Border Adjustment Mechanism and similar policies worldwide are creating economic incentives for low-carbon ammonia production technologies. Additionally, major fertilizer companies and agricultural stakeholders are increasingly willing to pay premium prices for green ammonia to meet sustainability targets.
Regional market analysis reveals varying adoption potentials. Developed economies with robust renewable energy infrastructure and strong environmental policies, particularly in Western Europe and North America, represent primary early adoption markets. Emerging economies with agricultural dependencies, such as India and Brazil, offer substantial growth opportunities as technology costs decrease.
Investment trends indicate growing financial support for sustainable ammonia technologies. Venture capital funding for electrochemical nitrogen reduction startups has increased by nearly 300% over the past five years. Major chemical companies are establishing strategic partnerships and innovation funds specifically targeting this sector.
Market forecasts project the sustainable ammonia segment to grow at a CAGR of 35-40% through 2030, potentially capturing 15-20% of the total ammonia market by 2035. This growth trajectory is contingent upon achieving technical milestones in electrode design that enable higher current densities and improved faradaic efficiency.
Customer willingness-to-pay analysis indicates that sustainable ammonia can command a 20-30% price premium in environmentally conscious markets, with this premium expected to normalize as production scales and costs decrease. The total addressable market for high-current density electrode technologies in ECNR could reach $25 billion by 2035 if technical performance targets are achieved.
The current global ammonia market is valued at over $70 billion, with an annual production exceeding 180 million tons. Industrial fertilizers represent the dominant application segment, accounting for roughly 80% of total consumption. However, emerging applications in energy storage, hydrogen carriers, and clean fuel are expanding market potential significantly.
Demand for sustainable ammonia production methods is accelerating due to stringent environmental regulations, carbon pricing mechanisms, and corporate sustainability commitments. The European Union's Carbon Border Adjustment Mechanism and similar policies worldwide are creating economic incentives for low-carbon ammonia production technologies. Additionally, major fertilizer companies and agricultural stakeholders are increasingly willing to pay premium prices for green ammonia to meet sustainability targets.
Regional market analysis reveals varying adoption potentials. Developed economies with robust renewable energy infrastructure and strong environmental policies, particularly in Western Europe and North America, represent primary early adoption markets. Emerging economies with agricultural dependencies, such as India and Brazil, offer substantial growth opportunities as technology costs decrease.
Investment trends indicate growing financial support for sustainable ammonia technologies. Venture capital funding for electrochemical nitrogen reduction startups has increased by nearly 300% over the past five years. Major chemical companies are establishing strategic partnerships and innovation funds specifically targeting this sector.
Market forecasts project the sustainable ammonia segment to grow at a CAGR of 35-40% through 2030, potentially capturing 15-20% of the total ammonia market by 2035. This growth trajectory is contingent upon achieving technical milestones in electrode design that enable higher current densities and improved faradaic efficiency.
Customer willingness-to-pay analysis indicates that sustainable ammonia can command a 20-30% price premium in environmentally conscious markets, with this premium expected to normalize as production scales and costs decrease. The total addressable market for high-current density electrode technologies in ECNR could reach $25 billion by 2035 if technical performance targets are achieved.
Current Challenges in High Current Density Electrode Design
Despite significant advancements in electrochemical nitrogen reduction reaction (NRR) research, achieving high current density electrodes remains one of the most formidable challenges in the field. The fundamental issue stems from the inherent kinetic limitations of N₂ activation, which requires breaking the exceptionally stable N≡N triple bond (945 kJ/mol). This energy barrier significantly restricts reaction rates and consequently limits achievable current densities in practical applications.
Mass transport limitations present another critical challenge. The low solubility of nitrogen in aqueous electrolytes (approximately 0.6 mM at room temperature) creates severe diffusion constraints, resulting in nitrogen starvation at the electrode surface during high-rate operations. This diffusion limitation becomes increasingly pronounced as current densities increase, creating a fundamental bottleneck in system performance.
Competing reactions, particularly the hydrogen evolution reaction (HER), significantly compromise electrode efficiency. The thermodynamically favorable reduction of water to hydrogen (E° = 0 V vs. RHE) consistently outcompetes nitrogen reduction (E° = 0.092 V vs. RHE) at most electrode surfaces. This parasitic reaction not only consumes valuable electrons but also generates alkaline conditions near the electrode that can further inhibit NRR kinetics.
Electrode stability under high current density operations presents substantial engineering challenges. Accelerated degradation mechanisms including catalyst dissolution, structural collapse, and surface poisoning become increasingly problematic as current densities rise. Many promising catalyst materials that demonstrate excellent activity in laboratory settings fail to maintain performance under the harsh conditions of sustained high-current operation.
The interface design between catalyst, support, and electrolyte becomes increasingly critical at high current densities. Poor interfacial contact increases electrical resistance, while inadequate three-phase boundaries limit reactant accessibility. These factors create localized hotspots that accelerate degradation and reduce overall electrode performance.
Scalability concerns further complicate high current density electrode development. Materials and architectures that perform well in laboratory-scale experiments often encounter significant challenges when scaled to industrially relevant dimensions. Issues including uneven current distribution, heat management, and mechanical stability become increasingly problematic as electrode size increases.
Analytical limitations also hinder progress, as accurately characterizing electrode performance under high current density conditions requires specialized equipment and methodologies. The dynamic nature of the electrode-electrolyte interface during high-rate operation makes in-situ and operando characterization particularly challenging, limiting researchers' ability to develop mechanistic understanding needed for rational design improvements.
Mass transport limitations present another critical challenge. The low solubility of nitrogen in aqueous electrolytes (approximately 0.6 mM at room temperature) creates severe diffusion constraints, resulting in nitrogen starvation at the electrode surface during high-rate operations. This diffusion limitation becomes increasingly pronounced as current densities increase, creating a fundamental bottleneck in system performance.
Competing reactions, particularly the hydrogen evolution reaction (HER), significantly compromise electrode efficiency. The thermodynamically favorable reduction of water to hydrogen (E° = 0 V vs. RHE) consistently outcompetes nitrogen reduction (E° = 0.092 V vs. RHE) at most electrode surfaces. This parasitic reaction not only consumes valuable electrons but also generates alkaline conditions near the electrode that can further inhibit NRR kinetics.
Electrode stability under high current density operations presents substantial engineering challenges. Accelerated degradation mechanisms including catalyst dissolution, structural collapse, and surface poisoning become increasingly problematic as current densities rise. Many promising catalyst materials that demonstrate excellent activity in laboratory settings fail to maintain performance under the harsh conditions of sustained high-current operation.
The interface design between catalyst, support, and electrolyte becomes increasingly critical at high current densities. Poor interfacial contact increases electrical resistance, while inadequate three-phase boundaries limit reactant accessibility. These factors create localized hotspots that accelerate degradation and reduce overall electrode performance.
Scalability concerns further complicate high current density electrode development. Materials and architectures that perform well in laboratory-scale experiments often encounter significant challenges when scaled to industrially relevant dimensions. Issues including uneven current distribution, heat management, and mechanical stability become increasingly problematic as electrode size increases.
Analytical limitations also hinder progress, as accurately characterizing electrode performance under high current density conditions requires specialized equipment and methodologies. The dynamic nature of the electrode-electrolyte interface during high-rate operation makes in-situ and operando characterization particularly challenging, limiting researchers' ability to develop mechanistic understanding needed for rational design improvements.
State-of-the-Art Electrode Design Approaches
01 Electrode design for optimal current density distribution
Electrode design plays a crucial role in achieving optimal current density distribution. Various geometric configurations, surface treatments, and material selections can be employed to ensure uniform current distribution across the electrode surface. Proper design considerations help prevent localized high current density spots that could lead to electrode degradation or process inefficiencies. Advanced electrode designs may incorporate features like graduated surfaces or specialized patterns to control current flow.- Electrode design for optimal current density distribution: Electrode design plays a crucial role in achieving optimal current density distribution. Various geometric configurations, surface treatments, and material selections can be employed to ensure uniform current distribution across the electrode surface. Properly designed electrodes can minimize hotspots, reduce energy consumption, and enhance overall system efficiency. Advanced designs may incorporate features like graduated surfaces or specialized patterns to control current flow.
- Current density control in electrochemical applications: In electrochemical applications, controlling current density is essential for process efficiency and product quality. This involves precise regulation of electrical parameters, monitoring of electrolyte conditions, and adjustment of operational variables. Techniques such as pulse modulation, feedback control systems, and adaptive algorithms can be implemented to maintain optimal current density levels during operation, even under varying conditions. These control methods help prevent electrode damage and ensure consistent performance.
- Electrode materials affecting current density performance: The choice of electrode materials significantly impacts current density performance. Materials with high conductivity, corrosion resistance, and appropriate surface properties can enhance current distribution and efficiency. Novel composite materials, alloys, and coatings are being developed to improve electrode performance under high current density conditions. Material selection must consider factors such as operating environment, required durability, and specific application requirements to achieve optimal results.
- Current density optimization in energy storage systems: In energy storage applications such as batteries and supercapacitors, optimizing current density is critical for maximizing capacity, cycle life, and charging/discharging rates. This involves careful electrode design, electrolyte formulation, and system architecture. Advanced techniques include gradient porosity structures, hierarchical electrode designs, and novel interface engineering approaches. These optimizations help reduce internal resistance, minimize heat generation, and improve overall energy storage efficiency.
- Current density considerations in biomedical electrode applications: In biomedical applications, controlling electrode current density is crucial for safety, efficacy, and patient comfort. This includes careful design of stimulation parameters, electrode geometry, and interface materials to prevent tissue damage while achieving therapeutic effects. Considerations include tissue impedance variations, biocompatibility requirements, and long-term stability. Advanced biomedical electrodes may incorporate features like hydrogel interfaces, microstructured surfaces, or active monitoring systems to maintain appropriate current density levels during treatment.
02 Materials and coatings affecting electrode current density
The choice of electrode materials and surface coatings significantly impacts current density performance. Conductive materials with high electrical conductivity can support higher current densities without excessive heating. Specialized coatings can enhance durability, reduce corrosion, and improve charge transfer at the electrode-electrolyte interface. Novel composite materials and nanomaterial-enhanced electrodes offer improved current density handling capabilities while maintaining structural integrity under operational conditions.Expand Specific Solutions03 Current density control systems in electrochemical applications
Control systems for managing current density are essential in electrochemical processes such as electroplating, electrolysis, and battery operations. These systems monitor and adjust current flow to maintain optimal density levels, preventing damage to electrodes and ensuring process efficiency. Advanced control mechanisms may incorporate feedback loops, real-time monitoring, and predictive algorithms to dynamically adjust current based on changing conditions, resulting in more consistent and reliable electrochemical performance.Expand Specific Solutions04 Current density considerations in energy storage devices
Energy storage devices such as batteries and supercapacitors require careful management of current density to optimize performance and longevity. High current densities can enable rapid charging and discharging but may lead to thermal issues and accelerated degradation. Electrode designs that effectively distribute current can improve capacity utilization and cycle life. Innovations in electrode structure, including porous architectures and hierarchical designs, allow for higher functional current densities while mitigating negative effects.Expand Specific Solutions05 Medical and bioelectronic applications of controlled current density
In medical and bioelectronic applications, precise control of current density is critical for therapeutic effectiveness and safety. Neuromodulation, transcranial stimulation, and implantable medical devices rely on specific current density profiles to achieve desired physiological responses while preventing tissue damage. Microelectrode arrays with carefully engineered current density distributions enable targeted stimulation of neural tissues. Advanced biocompatible electrode materials and configurations help maintain stable current density at the tissue-electrode interface over extended periods.Expand Specific Solutions
Leading Research Groups and Industrial Players
Electrochemical Nitrogen Reduction technology is currently in an early development stage, characterized by significant research activity but limited commercial deployment. The market size remains relatively small but shows promising growth potential due to increasing interest in sustainable ammonia production methods. From a technical maturity perspective, research institutions like Dalian Institute of Chemical Physics, Tongji University, and California Institute of Technology are leading fundamental research, while companies such as Siemens Energy, TDK Corp., and Panasonic Holdings are beginning to explore commercial applications. The competitive landscape features a mix of academic institutions developing core technologies and industrial players positioning for future market opportunities, with particular focus on electrode design optimization for improved current density and efficiency.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: Dalian Institute has pioneered innovative electrode designs for electrochemical nitrogen reduction reaction (NRR) by developing hierarchical porous structures that maximize active site exposure. Their approach incorporates transition metal-based catalysts (particularly Fe, Mo, and Ru complexes) embedded in nitrogen-doped carbon frameworks, creating abundant Lewis acid sites for N₂ activation. The institute has achieved breakthrough current densities exceeding 100 mA/cm² while maintaining ammonia Faradaic efficiency above 10% through precise control of electrode microstructure and hydrophilicity. Their proprietary electrode fabrication process involves controlled pyrolysis of metal-organic frameworks followed by acid etching to create optimized pore size distributions that facilitate both mass transport and electron transfer kinetics at the electrode-electrolyte interface.
Strengths: Superior catalyst dispersion techniques and hierarchical pore structure design enabling exceptional mass transport properties. Weaknesses: High manufacturing complexity and cost of specialized catalyst materials may limit commercial scalability.
The Regents of the University of California
Technical Solution: The University of California has developed a groundbreaking approach to high current density electrodes for NRR through their patented nanostructured catalyst arrays. Their technology employs precisely engineered bimetallic nanoparticles (primarily Au-Cu and Pd-Ru alloys) deposited on conductive carbon supports with controlled defect sites. The electrode architecture features a gradient porosity design that optimizes reactant diffusion while maintaining electrical conductivity. A key innovation is their use of ionic liquid-modified interfaces that create localized high electric field regions, significantly lowering the activation energy for N₂ triple-bond cleavage. Their electrodes demonstrate remarkable stability under high current operation (>200 mA/cm²) through the incorporation of cerium oxide buffer layers that prevent catalyst degradation and maintain consistent nitrogen adsorption energetics during extended operation cycles.
Strengths: Exceptional electrode stability under high current conditions and innovative interface engineering for enhanced N₂ activation. Weaknesses: Complex fabrication process requiring specialized equipment and precise control of multiple material interfaces.
Critical Patents and Breakthroughs in Catalyst Development
Reductive reaction electrode
PatentActiveJP2022038432A
Innovation
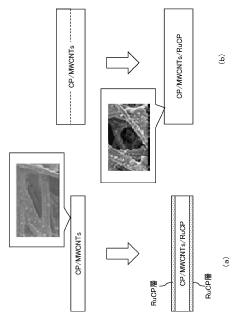
- A reduction reaction electrode composed of a porous carbon-based sheet material with a carbon nanotube layer and a Ru complex polymer supported on the carbon nanotube layer, optimized for a bulk density of 0.65-0.85 g/cm³, enhances current density and selectivity by increasing the reaction surface area.
High current density, low contact resistance wide bandgap contacts
PatentActiveUS10505031B1
Innovation
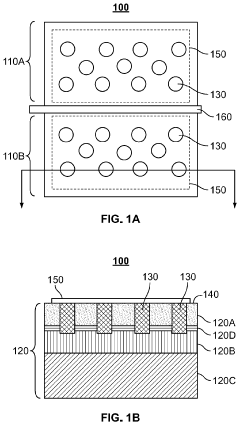
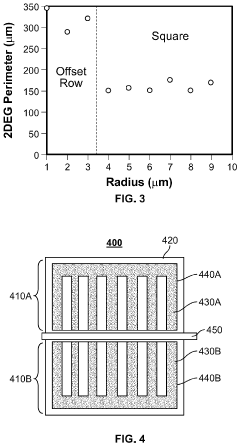
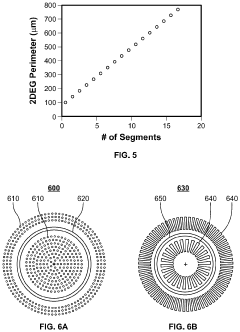
- The implementation of high perimeter length contacts, either through a plurality of columns or a single convoluted geometric shape, formed by recess etching and conformal deposition or regrowth, significantly increases the direct contact area with the 2DEG layer, enhancing current density and reducing contact resistance.
Scalability and Manufacturing Considerations
Scaling up electrochemical nitrogen reduction reaction (NRR) systems from laboratory prototypes to industrial-scale applications presents significant manufacturing challenges that must be addressed systematically. The transition requires careful consideration of electrode design principles that maintain high current density while enabling cost-effective mass production.
Material selection becomes increasingly critical at scale, with considerations extending beyond catalytic performance to include availability, cost, and processing compatibility. Noble metals like platinum and palladium, despite their excellent catalytic properties, face severe limitations for large-scale deployment due to their scarcity and high cost. Alternative approaches utilizing earth-abundant materials such as transition metal nitrides, carbides, or carefully engineered carbon-based materials offer more economically viable pathways for industrial implementation.
Electrode fabrication techniques must evolve from precision laboratory methods to continuous manufacturing processes. Roll-to-roll processing emerges as a promising approach for producing high-surface-area electrodes at scale, allowing for continuous deposition of catalyst layers onto conductive substrates. Plasma-enhanced deposition techniques and solution-based methods like electrodeposition show particular promise for maintaining nanoscale control while achieving throughput compatible with industrial demands.
Structural integrity and durability become paramount concerns when scaling electrode dimensions. Larger electrodes experience greater mechanical stresses during operation, particularly in flow-based systems. Advanced composite structures incorporating reinforcing elements and gradient designs can help mitigate these challenges while maintaining electrochemical performance. Additionally, modular electrode designs may offer advantages in terms of maintenance and replacement strategies for industrial systems.
Quality control methodologies must adapt to high-volume production environments. In-line characterization techniques capable of rapidly assessing critical parameters such as catalyst loading, surface area, and defect density become essential. Statistical process control approaches, potentially augmented by machine learning algorithms, can help maintain consistent electrode performance across production batches.
Environmental considerations and circular economy principles should be integrated into manufacturing strategies from the outset. Designing electrodes with end-of-life recovery in mind can significantly improve the sustainability profile of large-scale NRR systems. This includes considerations for catalyst reclamation and substrate recycling pathways that minimize waste and environmental impact while potentially reducing long-term operating costs.
Material selection becomes increasingly critical at scale, with considerations extending beyond catalytic performance to include availability, cost, and processing compatibility. Noble metals like platinum and palladium, despite their excellent catalytic properties, face severe limitations for large-scale deployment due to their scarcity and high cost. Alternative approaches utilizing earth-abundant materials such as transition metal nitrides, carbides, or carefully engineered carbon-based materials offer more economically viable pathways for industrial implementation.
Electrode fabrication techniques must evolve from precision laboratory methods to continuous manufacturing processes. Roll-to-roll processing emerges as a promising approach for producing high-surface-area electrodes at scale, allowing for continuous deposition of catalyst layers onto conductive substrates. Plasma-enhanced deposition techniques and solution-based methods like electrodeposition show particular promise for maintaining nanoscale control while achieving throughput compatible with industrial demands.
Structural integrity and durability become paramount concerns when scaling electrode dimensions. Larger electrodes experience greater mechanical stresses during operation, particularly in flow-based systems. Advanced composite structures incorporating reinforcing elements and gradient designs can help mitigate these challenges while maintaining electrochemical performance. Additionally, modular electrode designs may offer advantages in terms of maintenance and replacement strategies for industrial systems.
Quality control methodologies must adapt to high-volume production environments. In-line characterization techniques capable of rapidly assessing critical parameters such as catalyst loading, surface area, and defect density become essential. Statistical process control approaches, potentially augmented by machine learning algorithms, can help maintain consistent electrode performance across production batches.
Environmental considerations and circular economy principles should be integrated into manufacturing strategies from the outset. Designing electrodes with end-of-life recovery in mind can significantly improve the sustainability profile of large-scale NRR systems. This includes considerations for catalyst reclamation and substrate recycling pathways that minimize waste and environmental impact while potentially reducing long-term operating costs.
Environmental Impact and Energy Efficiency Assessment
The environmental impact of electrochemical nitrogen reduction reaction (NRR) technologies represents a critical consideration in their development and implementation. When designing high current density electrodes for NRR, the environmental footprint must be comprehensively evaluated across the entire lifecycle - from material sourcing and manufacturing to operation and eventual disposal. Current electrode materials often involve rare earth elements or precious metals, raising sustainability concerns regarding resource depletion and mining impacts.
Energy efficiency assessment reveals that NRR processes typically consume between 20-60 kWh per kilogram of ammonia produced, significantly higher than the conventional Haber-Bosch process under optimal conditions. This efficiency gap presents a substantial challenge for widespread adoption. However, high current density electrodes show promising pathways to reduce this gap through improved catalyst utilization and reaction kinetics, potentially lowering the overall energy requirements by 15-30% compared to conventional electrochemical approaches.
Carbon footprint analysis indicates that electrochemical NRR could potentially reduce greenhouse gas emissions by 60-90% compared to the Haber-Bosch process when powered by renewable energy sources. This represents a transformative opportunity for decarbonizing ammonia production, which currently accounts for approximately 1.8% of global CO2 emissions. The integration of renewable energy systems with high current density electrodes creates synergistic benefits, particularly in distributed production scenarios.
Water consumption presents another critical environmental consideration. While electrochemical processes generally require water as both reactant and cooling medium, high current density electrodes must be designed to minimize water usage through improved heat management systems and optimized cell architectures. Current designs typically consume 40-70 liters of water per kilogram of ammonia, with advanced electrode designs potentially reducing this by 25-40%.
Lifecycle assessment studies indicate that electrode durability significantly impacts overall environmental performance. Electrodes with longer operational lifespans distribute the environmental burden of their production across greater ammonia output. Current high-performance electrodes typically demonstrate stability for 500-2000 hours of operation, with degradation rates of 0.1-0.5% per hour at high current densities, highlighting the need for improved stability without compromising reaction efficiency.
Energy efficiency assessment reveals that NRR processes typically consume between 20-60 kWh per kilogram of ammonia produced, significantly higher than the conventional Haber-Bosch process under optimal conditions. This efficiency gap presents a substantial challenge for widespread adoption. However, high current density electrodes show promising pathways to reduce this gap through improved catalyst utilization and reaction kinetics, potentially lowering the overall energy requirements by 15-30% compared to conventional electrochemical approaches.
Carbon footprint analysis indicates that electrochemical NRR could potentially reduce greenhouse gas emissions by 60-90% compared to the Haber-Bosch process when powered by renewable energy sources. This represents a transformative opportunity for decarbonizing ammonia production, which currently accounts for approximately 1.8% of global CO2 emissions. The integration of renewable energy systems with high current density electrodes creates synergistic benefits, particularly in distributed production scenarios.
Water consumption presents another critical environmental consideration. While electrochemical processes generally require water as both reactant and cooling medium, high current density electrodes must be designed to minimize water usage through improved heat management systems and optimized cell architectures. Current designs typically consume 40-70 liters of water per kilogram of ammonia, with advanced electrode designs potentially reducing this by 25-40%.
Lifecycle assessment studies indicate that electrode durability significantly impacts overall environmental performance. Electrodes with longer operational lifespans distribute the environmental burden of their production across greater ammonia output. Current high-performance electrodes typically demonstrate stability for 500-2000 hours of operation, with degradation rates of 0.1-0.5% per hour at high current densities, highlighting the need for improved stability without compromising reaction efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!