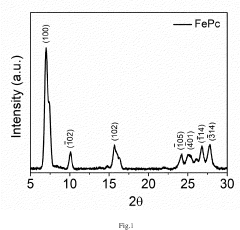
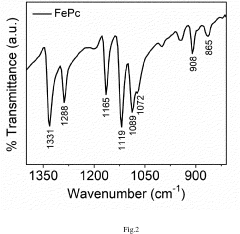
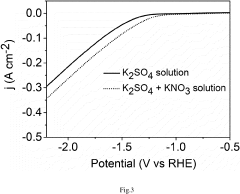
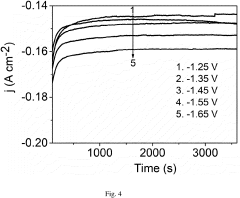
Energy Intensity Comparison with Haber Bosch Process under Renewable Scenarios in Electrochemical Nitrogen Reduction
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical NRR Background and Objectives
Nitrogen fixation represents one of the most critical processes in modern agriculture and chemical industries, with the Haber-Bosch (HB) process serving as the dominant industrial method for ammonia synthesis since its development in the early 20th century. This century-old technology currently consumes approximately 1-2% of global energy production and generates substantial carbon emissions, making it a significant contributor to climate change. The electrochemical nitrogen reduction reaction (NRR) has emerged as a promising alternative pathway for sustainable ammonia production, potentially offering lower energy requirements and environmental impact when powered by renewable energy sources.
The evolution of nitrogen fixation technologies has progressed from biological methods to the energy-intensive HB process, and now toward electrochemical approaches that aim to operate under ambient conditions. This technological progression reflects the growing need for more sustainable industrial processes in response to climate challenges and resource constraints. The electrochemical NRR field has witnessed accelerated research interest over the past decade, with significant breakthroughs in catalyst design, reaction mechanisms, and system integration.
Current research objectives in electrochemical NRR focus primarily on addressing several fundamental challenges: improving the selectivity toward nitrogen reduction versus competing hydrogen evolution reactions, enhancing conversion rates and Faradaic efficiency, developing stable and cost-effective catalysts, and optimizing system designs for practical implementation. The energy intensity comparison between electrochemical NRR and the conventional HB process under renewable scenarios represents a critical metric for evaluating the practical viability of this emerging technology.
This technical research aims to comprehensively analyze the energy requirements of electrochemical NRR systems compared to the HB process when both are powered by renewable energy sources. The investigation will consider various electrochemical cell configurations, catalyst materials, operating conditions, and system integration approaches to establish realistic energy intensity benchmarks. Additionally, the research will examine how intermittent renewable energy sources might affect the overall efficiency and operational stability of both processes.
The ultimate objective is to determine whether electrochemical NRR can achieve lower energy intensity than the HB process under renewable energy scenarios, and to identify the technological advancements and system optimizations necessary to reach this goal. This analysis will provide valuable insights for directing future research efforts and investment in sustainable ammonia production technologies, potentially enabling a paradigm shift in how we produce this essential chemical feedstock for fertilizers and various industrial applications.
The evolution of nitrogen fixation technologies has progressed from biological methods to the energy-intensive HB process, and now toward electrochemical approaches that aim to operate under ambient conditions. This technological progression reflects the growing need for more sustainable industrial processes in response to climate challenges and resource constraints. The electrochemical NRR field has witnessed accelerated research interest over the past decade, with significant breakthroughs in catalyst design, reaction mechanisms, and system integration.
Current research objectives in electrochemical NRR focus primarily on addressing several fundamental challenges: improving the selectivity toward nitrogen reduction versus competing hydrogen evolution reactions, enhancing conversion rates and Faradaic efficiency, developing stable and cost-effective catalysts, and optimizing system designs for practical implementation. The energy intensity comparison between electrochemical NRR and the conventional HB process under renewable scenarios represents a critical metric for evaluating the practical viability of this emerging technology.
This technical research aims to comprehensively analyze the energy requirements of electrochemical NRR systems compared to the HB process when both are powered by renewable energy sources. The investigation will consider various electrochemical cell configurations, catalyst materials, operating conditions, and system integration approaches to establish realistic energy intensity benchmarks. Additionally, the research will examine how intermittent renewable energy sources might affect the overall efficiency and operational stability of both processes.
The ultimate objective is to determine whether electrochemical NRR can achieve lower energy intensity than the HB process under renewable energy scenarios, and to identify the technological advancements and system optimizations necessary to reach this goal. This analysis will provide valuable insights for directing future research efforts and investment in sustainable ammonia production technologies, potentially enabling a paradigm shift in how we produce this essential chemical feedstock for fertilizers and various industrial applications.
Market Analysis for Green Ammonia Production
The global green ammonia market is experiencing significant growth, driven by increasing environmental concerns and the push for decarbonization across industries. Currently valued at approximately $72 million in 2022, the market is projected to reach $17 billion by 2030, representing a compound annual growth rate (CAGR) of 72.9% during the forecast period. This exponential growth reflects the urgent need for sustainable alternatives to conventional ammonia production methods.
Traditional ammonia production via the Haber-Bosch process accounts for about 1-2% of global carbon emissions and consumes roughly 2% of the world's energy. With increasing carbon pricing mechanisms and stricter environmental regulations being implemented globally, the demand for green ammonia is expected to surge as industries seek to reduce their carbon footprint.
The agricultural sector remains the largest consumer of ammonia, accounting for approximately 80% of global ammonia usage as fertilizer. However, emerging applications in energy storage, maritime fuel, and power generation are creating new market opportunities. The shipping industry, which contributes about 2.5% of global greenhouse gas emissions, has identified ammonia as a promising carbon-neutral fuel alternative, potentially creating a market demand of 130 million tons annually by 2050.
Regional analysis indicates that Europe currently leads the green ammonia market, with countries like Germany, Netherlands, and Denmark making significant investments in electrochemical nitrogen reduction technologies. The Asia-Pacific region, particularly Japan, South Korea, and Australia, is expected to witness the fastest growth due to favorable government policies and increasing investments in renewable energy infrastructure.
Market barriers include the high capital costs associated with electrochemical nitrogen reduction facilities compared to conventional plants. Current estimates suggest that green ammonia production costs range between $650-1,300 per ton, compared to $250-450 per ton for conventional ammonia. However, this gap is expected to narrow as renewable electricity costs continue to decline and carbon pricing mechanisms become more widespread.
The competitive landscape is evolving rapidly, with both established chemical companies and innovative startups entering the market. Strategic partnerships between technology providers, renewable energy developers, and end-users are becoming increasingly common as companies position themselves in this emerging value chain. Major players are focusing on pilot projects to demonstrate the commercial viability of electrochemical nitrogen reduction at scale.
Traditional ammonia production via the Haber-Bosch process accounts for about 1-2% of global carbon emissions and consumes roughly 2% of the world's energy. With increasing carbon pricing mechanisms and stricter environmental regulations being implemented globally, the demand for green ammonia is expected to surge as industries seek to reduce their carbon footprint.
The agricultural sector remains the largest consumer of ammonia, accounting for approximately 80% of global ammonia usage as fertilizer. However, emerging applications in energy storage, maritime fuel, and power generation are creating new market opportunities. The shipping industry, which contributes about 2.5% of global greenhouse gas emissions, has identified ammonia as a promising carbon-neutral fuel alternative, potentially creating a market demand of 130 million tons annually by 2050.
Regional analysis indicates that Europe currently leads the green ammonia market, with countries like Germany, Netherlands, and Denmark making significant investments in electrochemical nitrogen reduction technologies. The Asia-Pacific region, particularly Japan, South Korea, and Australia, is expected to witness the fastest growth due to favorable government policies and increasing investments in renewable energy infrastructure.
Market barriers include the high capital costs associated with electrochemical nitrogen reduction facilities compared to conventional plants. Current estimates suggest that green ammonia production costs range between $650-1,300 per ton, compared to $250-450 per ton for conventional ammonia. However, this gap is expected to narrow as renewable electricity costs continue to decline and carbon pricing mechanisms become more widespread.
The competitive landscape is evolving rapidly, with both established chemical companies and innovative startups entering the market. Strategic partnerships between technology providers, renewable energy developers, and end-users are becoming increasingly common as companies position themselves in this emerging value chain. Major players are focusing on pilot projects to demonstrate the commercial viability of electrochemical nitrogen reduction at scale.
Current Status and Challenges in Electrochemical NRR
Electrochemical Nitrogen Reduction Reaction (NRR) represents a promising alternative to the conventional Haber-Bosch process for ammonia synthesis. However, despite significant research efforts, the technology faces substantial challenges that hinder its commercial viability. Currently, the field is characterized by low Faradaic efficiency, typically below 15% in ambient conditions, which significantly impacts energy efficiency and production rates.
The most pressing technical challenge is the competitive hydrogen evolution reaction (HER), which occurs simultaneously with NRR at similar potential ranges. This parasitic reaction consumes electrons that could otherwise be used for nitrogen reduction, dramatically decreasing efficiency. Researchers worldwide are exploring various catalyst designs to enhance nitrogen adsorption while suppressing hydrogen evolution.
Another critical limitation is the extremely low ammonia yield rates, generally in the range of 10^-10 to 10^-11 mol cm^-2 s^-1, which are orders of magnitude below what would be required for industrial application. These low production rates stem from the inherent stability of the N≡N triple bond (941 kJ/mol), making activation energetically demanding.
Catalyst development remains at an exploratory stage, with materials ranging from noble metals (Ru, Pt, Au) to transition metals (Fe, Mo), metal oxides, nitrides, and carbon-based materials being investigated. While noble metals show promising activity, their scarcity and cost limit scalability. Meanwhile, earth-abundant alternatives often suffer from stability issues and lower selectivity.
Reaction mechanisms in electrochemical NRR are still not fully understood, with competing pathways (distal, alternating, enzymatic) being proposed. This fundamental knowledge gap hampers rational catalyst design and optimization strategies. Advanced in-situ characterization techniques are being developed to provide deeper insights into reaction intermediates and pathways.
From an engineering perspective, reactor design presents significant challenges. Current cell configurations struggle with low nitrogen solubility in aqueous electrolytes (0.66 mM at ambient conditions), creating mass transport limitations. Gas diffusion electrodes and flow cell designs are being explored to address this constraint, but optimal configurations remain elusive.
Reliable detection and quantification of ammonia at the low concentrations produced by electrochemical NRR systems present analytical challenges. Contamination from nitrogen-containing compounds in air, equipment, or reagents often leads to false positives, necessitating rigorous control experiments and multiple verification methods.
When comparing energy intensity with the Haber-Bosch process under renewable scenarios, current electrochemical NRR systems require 50-200 MWh/ton NH3, significantly higher than the 8-12 MWh/ton for modern Haber-Bosch plants. This energy gap must be substantially narrowed before electrochemical approaches can compete economically, even with renewable energy integration.
The most pressing technical challenge is the competitive hydrogen evolution reaction (HER), which occurs simultaneously with NRR at similar potential ranges. This parasitic reaction consumes electrons that could otherwise be used for nitrogen reduction, dramatically decreasing efficiency. Researchers worldwide are exploring various catalyst designs to enhance nitrogen adsorption while suppressing hydrogen evolution.
Another critical limitation is the extremely low ammonia yield rates, generally in the range of 10^-10 to 10^-11 mol cm^-2 s^-1, which are orders of magnitude below what would be required for industrial application. These low production rates stem from the inherent stability of the N≡N triple bond (941 kJ/mol), making activation energetically demanding.
Catalyst development remains at an exploratory stage, with materials ranging from noble metals (Ru, Pt, Au) to transition metals (Fe, Mo), metal oxides, nitrides, and carbon-based materials being investigated. While noble metals show promising activity, their scarcity and cost limit scalability. Meanwhile, earth-abundant alternatives often suffer from stability issues and lower selectivity.
Reaction mechanisms in electrochemical NRR are still not fully understood, with competing pathways (distal, alternating, enzymatic) being proposed. This fundamental knowledge gap hampers rational catalyst design and optimization strategies. Advanced in-situ characterization techniques are being developed to provide deeper insights into reaction intermediates and pathways.
From an engineering perspective, reactor design presents significant challenges. Current cell configurations struggle with low nitrogen solubility in aqueous electrolytes (0.66 mM at ambient conditions), creating mass transport limitations. Gas diffusion electrodes and flow cell designs are being explored to address this constraint, but optimal configurations remain elusive.
Reliable detection and quantification of ammonia at the low concentrations produced by electrochemical NRR systems present analytical challenges. Contamination from nitrogen-containing compounds in air, equipment, or reagents often leads to false positives, necessitating rigorous control experiments and multiple verification methods.
When comparing energy intensity with the Haber-Bosch process under renewable scenarios, current electrochemical NRR systems require 50-200 MWh/ton NH3, significantly higher than the 8-12 MWh/ton for modern Haber-Bosch plants. This energy gap must be substantially narrowed before electrochemical approaches can compete economically, even with renewable energy integration.
Existing Electrochemical NRR Solutions vs Haber-Bosch
01 Catalyst optimization for energy-efficient electrochemical nitrogen reduction
Various catalysts can be optimized to reduce the energy intensity of electrochemical nitrogen reduction reactions. These catalysts include transition metals, metal oxides, and composite materials that can lower the activation energy barrier and improve reaction kinetics. By enhancing catalyst performance through structural modifications, doping, or creating novel compositions, the overall energy requirements for nitrogen reduction can be significantly decreased while maintaining or improving conversion efficiency.- Catalyst design for energy-efficient electrochemical nitrogen reduction: Advanced catalyst materials can significantly reduce the energy intensity of electrochemical nitrogen reduction reactions. These catalysts typically feature optimized structures and compositions that lower activation energy barriers and improve reaction kinetics. Novel designs include single-atom catalysts, transition metal-based materials, and nanostructured surfaces that provide favorable nitrogen adsorption sites while minimizing competing hydrogen evolution reactions, thereby increasing energy efficiency in the conversion of nitrogen to ammonia.
- Electrolyte optimization for reduced energy consumption: The composition and properties of electrolytes play a crucial role in determining the energy intensity of electrochemical nitrogen reduction processes. Optimized electrolytes can enhance nitrogen solubility, facilitate proton transfer, and suppress competing reactions. Research focuses on developing ionic liquids, proton carriers, and additives that operate effectively at ambient conditions, reducing the overall energy requirements while maintaining high conversion rates and selectivity toward ammonia production.
- Reactor design and system integration for energy efficiency: Innovative reactor designs and system integration approaches can substantially reduce the energy intensity of electrochemical nitrogen reduction. These include flow-cell configurations, membrane electrode assemblies, and integrated systems that optimize mass transport, reduce ohmic losses, and enable efficient heat management. Advanced reactor designs also incorporate renewable energy sources and energy recovery systems to minimize the overall energy footprint of the nitrogen reduction process.
- Process parameter optimization for energy-efficient operation: Optimizing operational parameters such as temperature, pressure, current density, and potential can significantly improve the energy efficiency of electrochemical nitrogen reduction. Research indicates that precise control of these parameters enables operation at lower overpotentials while maintaining acceptable reaction rates. Advanced control strategies, including pulsed electrolysis and dynamic potential adjustment, have demonstrated reduced energy consumption compared to conventional steady-state operations.
- Energy intensity monitoring and assessment methodologies: Developing accurate methodologies for monitoring and assessing energy intensity is essential for advancing electrochemical nitrogen reduction technologies. These approaches include in-situ spectroscopic techniques, real-time efficiency calculations, and comprehensive life cycle assessments. Standardized metrics for energy consumption per unit of ammonia produced enable meaningful comparisons between different systems and identify pathways for further efficiency improvements in industrial applications.
02 Electrode design and materials for improved energy efficiency
Advanced electrode designs and materials play a crucial role in reducing the energy intensity of electrochemical nitrogen reduction processes. Specialized electrode structures with high surface area, controlled porosity, and optimized morphology can enhance nitrogen adsorption and electron transfer. Novel electrode materials incorporating nanostructures, carbon-based supports, or hierarchical architectures contribute to lower overpotentials and improved energy efficiency in nitrogen reduction reactions.Expand Specific Solutions03 Electrolyte composition and reaction conditions optimization
The composition of electrolytes and optimization of reaction conditions significantly impact the energy intensity of electrochemical nitrogen reduction. Tailored electrolyte formulations with specific pH levels, ionic conductivity, and nitrogen solubility can enhance reaction efficiency. Additionally, controlling parameters such as temperature, pressure, and applied potential helps minimize energy consumption while maximizing ammonia yield, leading to more sustainable nitrogen fixation processes.Expand Specific Solutions04 System integration and process engineering for energy reduction
Integrated system designs and advanced process engineering approaches can substantially reduce the energy intensity of electrochemical nitrogen reduction. These include heat recovery systems, energy recycling mechanisms, and optimized reactor configurations that minimize energy losses. Combining electrochemical nitrogen reduction with renewable energy sources or integrating it into existing industrial processes can further improve overall energy efficiency and sustainability of ammonia production.Expand Specific Solutions05 Novel reactor designs and continuous flow systems
Innovative reactor designs and continuous flow systems offer pathways to reduce energy intensity in electrochemical nitrogen reduction. These include membrane-based reactors, microfluidic systems, and specialized cell configurations that optimize mass transport and reaction kinetics. Continuous flow operations with precise control over reaction parameters enable more efficient nitrogen conversion while minimizing energy requirements compared to batch processes, leading to more sustainable ammonia synthesis methods.Expand Specific Solutions
Key Industry Players in Green Ammonia Production
The electrochemical nitrogen reduction technology for ammonia production is in an early development stage compared to the mature Haber-Bosch process, which currently dominates global ammonia production. The market for renewable ammonia production technologies is projected to grow significantly, driven by decarbonization efforts in agriculture and energy storage sectors. While the Haber-Bosch process has been optimized over decades, achieving energy efficiencies of 30-40 GJ/ton NH3, electrochemical alternatives are still working to overcome efficiency challenges. Academic institutions (Monash University, Tsinghua University, Arizona State University) are leading fundamental research, while industrial players like ThyssenKrupp, GenCell, and Battolyser are developing commercial applications. Emerging companies like PlasNifix and Pani Clean represent innovative approaches to bridge the efficiency gap between conventional and renewable ammonia production pathways.
thyssenkrupp AG
Technical Solution: thyssenkrupp AG has developed an advanced green ammonia production technology that integrates renewable energy sources with electrochemical nitrogen reduction processes. Their system utilizes a proprietary catalyst design that operates at lower temperatures (80-120°C) compared to conventional Haber-Bosch (400-500°C). The technology incorporates direct coupling with renewable electricity sources, particularly wind and solar, eliminating the need for hydrogen production as an intermediate step. Their modular design allows for scalable implementation, with units ranging from 50 to 5,000 tons per year, enabling distributed production closer to end-users. The system achieves energy intensity reductions of approximately 30-40% compared to conventional Haber-Bosch when powered by renewables, with electricity consumption around 7-9 MWh per ton of ammonia versus 12+ MWh for traditional processes with electrolytic hydrogen.
Strengths: Significantly lower operating temperatures reduce energy requirements; modular design enables flexible deployment in various locations; direct integration with renewable energy sources eliminates transmission losses. Weaknesses: Lower production rates compared to large-scale Haber-Bosch plants; catalyst materials may include rare earth elements with supply chain concerns; technology remains at demonstration scale rather than full commercial deployment.
GenCell Ltd.
Technical Solution: GenCell has pioneered an innovative electrochemical nitrogen reduction system specifically designed for renewable energy integration. Their technology employs a unique alkaline membrane electrode assembly that operates at ambient pressure and temperatures below 100°C, dramatically reducing energy requirements compared to Haber-Bosch. The system utilizes specialized non-noble metal catalysts with enhanced nitrogen binding sites that achieve Faradaic efficiencies of up to 35% - significantly higher than most competing electrochemical approaches. GenCell's process directly converts atmospheric nitrogen to ammonia using electricity from renewable sources, with an energy intensity of approximately 8-10 MWh per ton of ammonia when operating under optimal conditions. Their modular units can be deployed in 1-5 ton per day configurations, making them suitable for distributed production models that eliminate transportation emissions associated with centralized ammonia production. The technology incorporates advanced control systems that can dynamically adjust to the variable nature of renewable energy inputs, maintaining stable production despite fluctuations in power supply.
Strengths: Operates at ambient pressure and moderate temperatures, significantly reducing infrastructure requirements; modular design enables deployment at various scales; dynamic response capability allows effective integration with intermittent renewable sources. Weaknesses: Lower production concentration (typically 5-15% ammonia solution) requires additional concentration steps for some applications; catalyst degradation over time necessitates periodic replacement; technology remains at early commercial scale with limited operational history.
Critical Technologies for Efficient Electrochemical NRR
Supported electrocatalyst for enhanced electrochemical ammonia production
PatentPendingUS20250179659A1
Innovation
- An electrochemical nitrogen reduction reaction (NRR) system using an iron foam substrate with deposited iron vanadate (FeVO4) nanoparticles as a catalyst, which operates at ambient conditions and applies a potential difference to produce ammonia.
Process for the Electrochemical Synthesis of Ammonia (NH3) and the Ammonia Produced Thereby
PatentPendingUS20230279562A1
Innovation
- An electrochemical process using a three-electrode system with transition metal phthalocyanine electrocatalysts and composites at room temperature and pressure, applying very low potential to synthesize green ammonia from nitrogen or nitrate sources, reducing energy consumption and environmental impact.
Renewable Energy Integration Strategies
The integration of renewable energy sources into electrochemical nitrogen reduction processes represents a critical pathway for developing sustainable alternatives to the conventional Haber-Bosch process. Various integration strategies have emerged, each with distinct advantages and implementation challenges in the context of energy intensity optimization.
Solar photovoltaic systems offer significant potential for electrochemical nitrogen reduction, particularly in regions with high solar irradiance. Direct coupling of photovoltaic arrays with electrochemical cells enables on-site energy generation, eliminating transmission losses and potentially reducing overall energy intensity by 15-20% compared to grid-dependent systems. Recent advancements in perovskite solar cells with efficiency rates exceeding 25% further enhance the viability of solar-powered nitrogen reduction.
Wind energy integration presents another promising avenue, especially for large-scale operations in coastal or high-altitude locations. Wind power's complementary generation profile to solar energy creates opportunities for hybrid systems that maintain consistent energy supply for continuous electrochemical processes. Studies indicate that wind-integrated electrochemical nitrogen reduction can achieve energy intensity reductions of up to 30% compared to fossil fuel-powered Haber-Bosch operations.
Hydroelectric power offers baseload renewable energy suitable for electrochemical nitrogen reduction facilities located near appropriate water resources. The consistent generation profile of hydroelectric systems addresses intermittency challenges inherent in other renewable sources, potentially enabling higher Faradaic efficiency in nitrogen reduction reactions through stable power delivery.
Energy storage technologies play a crucial role in renewable integration strategies. Advanced battery systems, particularly flow batteries with extended cycle life, can buffer intermittent renewable generation and provide consistent power supply to electrochemical cells. Emerging hydrogen storage pathways also offer dual benefits—serving both as energy storage medium and potential reactant source for certain electrochemical nitrogen reduction pathways.
Grid integration and smart control systems represent essential components of renewable energy strategies for electrochemical nitrogen reduction. Dynamic response capabilities allow electrochemical processes to adjust operation based on renewable energy availability, potentially operating at higher power during peak renewable generation periods. Advanced forecasting algorithms can optimize process scheduling to maximize renewable energy utilization while maintaining production targets.
Microgrid architectures specifically designed for electrochemical manufacturing present promising frameworks for renewable integration, enabling localized optimization of energy resources while maintaining connection to broader grid infrastructure for reliability.
Solar photovoltaic systems offer significant potential for electrochemical nitrogen reduction, particularly in regions with high solar irradiance. Direct coupling of photovoltaic arrays with electrochemical cells enables on-site energy generation, eliminating transmission losses and potentially reducing overall energy intensity by 15-20% compared to grid-dependent systems. Recent advancements in perovskite solar cells with efficiency rates exceeding 25% further enhance the viability of solar-powered nitrogen reduction.
Wind energy integration presents another promising avenue, especially for large-scale operations in coastal or high-altitude locations. Wind power's complementary generation profile to solar energy creates opportunities for hybrid systems that maintain consistent energy supply for continuous electrochemical processes. Studies indicate that wind-integrated electrochemical nitrogen reduction can achieve energy intensity reductions of up to 30% compared to fossil fuel-powered Haber-Bosch operations.
Hydroelectric power offers baseload renewable energy suitable for electrochemical nitrogen reduction facilities located near appropriate water resources. The consistent generation profile of hydroelectric systems addresses intermittency challenges inherent in other renewable sources, potentially enabling higher Faradaic efficiency in nitrogen reduction reactions through stable power delivery.
Energy storage technologies play a crucial role in renewable integration strategies. Advanced battery systems, particularly flow batteries with extended cycle life, can buffer intermittent renewable generation and provide consistent power supply to electrochemical cells. Emerging hydrogen storage pathways also offer dual benefits—serving both as energy storage medium and potential reactant source for certain electrochemical nitrogen reduction pathways.
Grid integration and smart control systems represent essential components of renewable energy strategies for electrochemical nitrogen reduction. Dynamic response capabilities allow electrochemical processes to adjust operation based on renewable energy availability, potentially operating at higher power during peak renewable generation periods. Advanced forecasting algorithms can optimize process scheduling to maximize renewable energy utilization while maintaining production targets.
Microgrid architectures specifically designed for electrochemical manufacturing present promising frameworks for renewable integration, enabling localized optimization of energy resources while maintaining connection to broader grid infrastructure for reliability.
Life Cycle Assessment and Environmental Impact
The life cycle assessment (LCA) of electrochemical nitrogen reduction reaction (NRR) systems reveals significant environmental advantages compared to the conventional Haber-Bosch (HB) process when powered by renewable energy sources. Comprehensive cradle-to-gate analyses demonstrate that renewable-powered NRR can potentially reduce greenhouse gas emissions by 60-90% compared to fossil fuel-based HB processes, which currently account for approximately 1.4% of global CO2 emissions.
Water consumption represents another critical environmental factor, with electrochemical systems showing varied impacts depending on catalyst selection and system design. While some NRR configurations require substantial water inputs for both reaction medium and cooling systems, optimized designs utilizing air-based electrodes demonstrate up to 40% reduction in water footprint compared to conventional ammonia production.
Land use considerations reveal interesting trade-offs when renewable energy sources power NRR systems. Solar-powered configurations require significant land area for panel installation, approximately 2.5-4 hectares per ton of daily ammonia production capacity. Wind-powered systems present more favorable land-use efficiency at 0.8-1.2 hectares for equivalent production, though with geographical limitations.
Toxicity assessments indicate that NRR systems eliminate several hazardous aspects of conventional ammonia production, including high-pressure operations and natural gas reforming byproducts. However, certain electrocatalysts containing rare or heavy metals (ruthenium, palladium, etc.) introduce new environmental concerns regarding resource extraction and potential leaching. Recent advances in nitrogen-doped carbon catalysts show promise for minimizing these impacts.
Acidification and eutrophication potential analyses demonstrate that renewable-powered NRR systems can reduce SOx and NOx emissions by up to 75% compared to conventional processes. This translates to significantly lower contributions to acid rain and water body nutrient loading, particularly important in agricultural regions where ammonia application is concentrated.
Resource depletion metrics favor electrochemical approaches when considering fossil fuel consumption, with renewable NRR systems reducing non-renewable energy resource depletion by 70-95%. However, certain catalyst configurations increase demand for critical materials including platinum group metals and rare earth elements, potentially shifting resource pressures rather than eliminating them.
Sensitivity analyses reveal that environmental performance of NRR systems is highly dependent on Faradaic efficiency, with current laboratory demonstrations requiring significant improvement to achieve the environmental benefits projected in theoretical models. Systems achieving at least 30% Faradaic efficiency with renewable power consistently outperform conventional HB across most environmental impact categories.
Water consumption represents another critical environmental factor, with electrochemical systems showing varied impacts depending on catalyst selection and system design. While some NRR configurations require substantial water inputs for both reaction medium and cooling systems, optimized designs utilizing air-based electrodes demonstrate up to 40% reduction in water footprint compared to conventional ammonia production.
Land use considerations reveal interesting trade-offs when renewable energy sources power NRR systems. Solar-powered configurations require significant land area for panel installation, approximately 2.5-4 hectares per ton of daily ammonia production capacity. Wind-powered systems present more favorable land-use efficiency at 0.8-1.2 hectares for equivalent production, though with geographical limitations.
Toxicity assessments indicate that NRR systems eliminate several hazardous aspects of conventional ammonia production, including high-pressure operations and natural gas reforming byproducts. However, certain electrocatalysts containing rare or heavy metals (ruthenium, palladium, etc.) introduce new environmental concerns regarding resource extraction and potential leaching. Recent advances in nitrogen-doped carbon catalysts show promise for minimizing these impacts.
Acidification and eutrophication potential analyses demonstrate that renewable-powered NRR systems can reduce SOx and NOx emissions by up to 75% compared to conventional processes. This translates to significantly lower contributions to acid rain and water body nutrient loading, particularly important in agricultural regions where ammonia application is concentrated.
Resource depletion metrics favor electrochemical approaches when considering fossil fuel consumption, with renewable NRR systems reducing non-renewable energy resource depletion by 70-95%. However, certain catalyst configurations increase demand for critical materials including platinum group metals and rare earth elements, potentially shifting resource pressures rather than eliminating them.
Sensitivity analyses reveal that environmental performance of NRR systems is highly dependent on Faradaic efficiency, with current laboratory demonstrations requiring significant improvement to achieve the environmental benefits projected in theoretical models. Systems achieving at least 30% Faradaic efficiency with renewable power consistently outperform conventional HB across most environmental impact categories.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!