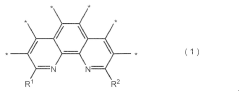
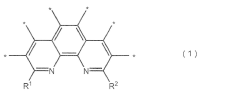
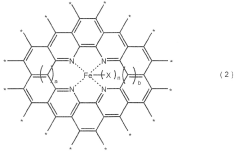
Stability Testing of Catalysts with One Thousand Hour Demonstration Metrics in Electrochemical Nitrogen Reduction
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Nitrogen Reduction Catalyst Evolution and Objectives
Electrochemical Nitrogen Reduction (ENR) technology has evolved significantly over the past decades, transitioning from theoretical concepts to practical applications with increasing efficiency. The journey began in the 1980s with rudimentary electrochemical cells showing minimal nitrogen conversion rates, progressing through various catalyst innovations that gradually improved reaction selectivity and efficiency.
The field experienced a pivotal shift around 2010 when researchers began systematically exploring transition metal-based catalysts, leading to the first reproducible demonstrations of ambient-condition nitrogen reduction. This period marked the transition from proof-of-concept to performance optimization, with catalytic activity becoming a central focus of research efforts worldwide.
Recent years have witnessed an acceleration in catalyst development, with novel materials including single-atom catalysts, 2D materials, and metal-organic frameworks demonstrating promising nitrogen reduction reaction (NRR) performance. However, while activity metrics have improved substantially, stability testing has remained largely confined to short-term demonstrations, typically ranging from several hours to days rather than the industrially relevant timeframes of months or years.
The current technological trajectory aims to bridge the gap between laboratory demonstrations and industrial implementation. This necessitates a fundamental shift from activity-focused research to stability-centered catalyst design. The one-thousand-hour demonstration metric represents a critical threshold that separates academic curiosity from commercial viability in electrochemical nitrogen reduction systems.
Primary objectives in this field now include developing catalysts that maintain consistent performance over extended operation periods while resisting common degradation mechanisms such as poisoning, leaching, and structural collapse. Additionally, researchers aim to establish standardized protocols for long-term stability assessment to enable meaningful comparisons between different catalyst systems.
Another crucial goal involves understanding the fundamental degradation mechanisms affecting ENR catalysts during prolonged operation. This includes investigating the impact of trace contaminants, intermediate species accumulation, and electrode-electrolyte interface evolution over time. Such knowledge is essential for designing next-generation catalysts with inherent resistance to degradation pathways.
The field is also moving toward integrating stability considerations earlier in the catalyst design process, rather than treating it as a secondary concern after activity optimization. This paradigm shift requires new computational models that can predict long-term stability alongside traditional activity metrics, potentially accelerating the discovery of industrially viable catalyst systems for sustainable ammonia production.
The field experienced a pivotal shift around 2010 when researchers began systematically exploring transition metal-based catalysts, leading to the first reproducible demonstrations of ambient-condition nitrogen reduction. This period marked the transition from proof-of-concept to performance optimization, with catalytic activity becoming a central focus of research efforts worldwide.
Recent years have witnessed an acceleration in catalyst development, with novel materials including single-atom catalysts, 2D materials, and metal-organic frameworks demonstrating promising nitrogen reduction reaction (NRR) performance. However, while activity metrics have improved substantially, stability testing has remained largely confined to short-term demonstrations, typically ranging from several hours to days rather than the industrially relevant timeframes of months or years.
The current technological trajectory aims to bridge the gap between laboratory demonstrations and industrial implementation. This necessitates a fundamental shift from activity-focused research to stability-centered catalyst design. The one-thousand-hour demonstration metric represents a critical threshold that separates academic curiosity from commercial viability in electrochemical nitrogen reduction systems.
Primary objectives in this field now include developing catalysts that maintain consistent performance over extended operation periods while resisting common degradation mechanisms such as poisoning, leaching, and structural collapse. Additionally, researchers aim to establish standardized protocols for long-term stability assessment to enable meaningful comparisons between different catalyst systems.
Another crucial goal involves understanding the fundamental degradation mechanisms affecting ENR catalysts during prolonged operation. This includes investigating the impact of trace contaminants, intermediate species accumulation, and electrode-electrolyte interface evolution over time. Such knowledge is essential for designing next-generation catalysts with inherent resistance to degradation pathways.
The field is also moving toward integrating stability considerations earlier in the catalyst design process, rather than treating it as a secondary concern after activity optimization. This paradigm shift requires new computational models that can predict long-term stability alongside traditional activity metrics, potentially accelerating the discovery of industrially viable catalyst systems for sustainable ammonia production.
Market Analysis for Sustainable Ammonia Production Technologies
The global ammonia market is experiencing significant transformation driven by sustainability concerns, with the market value projected to reach $70 billion by 2025. Traditional ammonia production via the Haber-Bosch process consumes approximately 2% of global energy and generates substantial CO2 emissions. This creates a compelling market opportunity for sustainable alternatives such as electrochemical nitrogen reduction (ECNR), which could potentially operate using renewable electricity and produce zero direct emissions.
Market segmentation reveals three primary sectors interested in sustainable ammonia production: agriculture (fertilizer production), energy storage, and industrial applications. The agricultural sector represents the largest market share at approximately 80% of current ammonia consumption, with growing demand for "green fertilizers" that command premium pricing in environmentally conscious markets.
The energy sector presents an emerging opportunity, with ammonia increasingly viewed as a promising hydrogen carrier and carbon-free fuel. Several pilot projects in Japan, Australia, and Europe are exploring ammonia as a shipping fuel and for power generation, potentially creating a market valued at $30 billion by 2030.
Industrial applications, including mining explosives and refrigeration, constitute smaller but stable market segments that are increasingly seeking sustainable alternatives to meet corporate environmental targets and regulatory requirements.
Regional analysis indicates that Europe leads in sustainable ammonia development due to stringent carbon regulations and substantial renewable energy investments. The Asia-Pacific region, particularly China, Japan, and South Korea, shows the fastest growth trajectory driven by energy security concerns and industrial decarbonization policies.
Market barriers include the significant cost differential between conventional and sustainable ammonia production methods. Current electrochemical nitrogen reduction processes remain 2-3 times more expensive than conventional methods, though this gap is narrowing with technological improvements and increasing carbon pricing mechanisms.
Customer willingness to pay premiums for sustainable ammonia varies by sector, with consumer-facing industries and companies with strong sustainability commitments showing greater acceptance of price premiums ranging from 15-30%.
The competitive landscape features both established industrial gas companies (Air Liquide, Linde) investing in sustainable ammonia technologies and specialized startups focused exclusively on electrochemical nitrogen reduction. Venture capital investment in this sector has grown by 45% annually since 2018, indicating strong market confidence in the technology's commercial potential.
Market segmentation reveals three primary sectors interested in sustainable ammonia production: agriculture (fertilizer production), energy storage, and industrial applications. The agricultural sector represents the largest market share at approximately 80% of current ammonia consumption, with growing demand for "green fertilizers" that command premium pricing in environmentally conscious markets.
The energy sector presents an emerging opportunity, with ammonia increasingly viewed as a promising hydrogen carrier and carbon-free fuel. Several pilot projects in Japan, Australia, and Europe are exploring ammonia as a shipping fuel and for power generation, potentially creating a market valued at $30 billion by 2030.
Industrial applications, including mining explosives and refrigeration, constitute smaller but stable market segments that are increasingly seeking sustainable alternatives to meet corporate environmental targets and regulatory requirements.
Regional analysis indicates that Europe leads in sustainable ammonia development due to stringent carbon regulations and substantial renewable energy investments. The Asia-Pacific region, particularly China, Japan, and South Korea, shows the fastest growth trajectory driven by energy security concerns and industrial decarbonization policies.
Market barriers include the significant cost differential between conventional and sustainable ammonia production methods. Current electrochemical nitrogen reduction processes remain 2-3 times more expensive than conventional methods, though this gap is narrowing with technological improvements and increasing carbon pricing mechanisms.
Customer willingness to pay premiums for sustainable ammonia varies by sector, with consumer-facing industries and companies with strong sustainability commitments showing greater acceptance of price premiums ranging from 15-30%.
The competitive landscape features both established industrial gas companies (Air Liquide, Linde) investing in sustainable ammonia technologies and specialized startups focused exclusively on electrochemical nitrogen reduction. Venture capital investment in this sector has grown by 45% annually since 2018, indicating strong market confidence in the technology's commercial potential.
Current Challenges in Long-Duration Catalyst Stability Testing
Despite significant advancements in electrochemical nitrogen reduction reaction (NRR) catalysts, long-term stability testing remains one of the most formidable challenges in the field. Current protocols for thousand-hour demonstration metrics face several critical limitations that impede reliable assessment and commercial viability of these catalysts.
The primary challenge lies in maintaining consistent testing conditions over extended periods. Environmental factors such as temperature fluctuations, humidity variations, and atmospheric contaminants can significantly influence catalyst performance during long-duration tests. Even minor deviations in these parameters can lead to misleading stability data, making it difficult to distinguish between actual catalyst degradation and testing artifacts.
Electrode poisoning presents another significant obstacle, particularly in aqueous electrolytes where competing reactions like hydrogen evolution can dominate. Over prolonged testing periods, catalyst active sites become progressively blocked by reaction intermediates or contaminants, resulting in performance deterioration that may be misinterpreted as inherent catalyst instability rather than a reversible fouling process.
Standardization deficiencies further complicate stability assessment. The field lacks universally accepted protocols for long-duration testing, with variations in electrolyte composition, potential control methods, and performance metrics making cross-study comparisons nearly impossible. This absence of standardization hinders meaningful benchmarking of catalyst stability across different research groups and technologies.
Technical limitations of testing equipment also pose substantial challenges. Potentiostats must maintain precise potential control over thousands of hours without drift, while gas analysis systems require consistent sensitivity and calibration throughout the testing period. Many laboratories lack specialized equipment designed specifically for such extended operations, leading to data collection interruptions and inconsistencies.
Product quantification represents a particularly difficult challenge in NRR stability testing. The extremely low concentrations of ammonia produced necessitate highly sensitive detection methods that remain reliable over extended periods. Current analytical techniques often struggle with detection limits and interference from contaminants that accumulate during long-duration experiments.
Accelerated aging protocols, which have proven valuable in other electrochemical systems, remain underdeveloped for NRR catalysts. The field lacks validated methods to predict thousand-hour stability from shorter tests, forcing researchers to conduct full-duration experiments that consume significant time and resources.
These challenges collectively highlight the urgent need for advanced testing methodologies, standardized protocols, and specialized equipment designed specifically for long-duration stability assessment of electrochemical nitrogen reduction catalysts. Addressing these limitations is crucial for advancing NRR technology toward practical applications and commercial viability.
The primary challenge lies in maintaining consistent testing conditions over extended periods. Environmental factors such as temperature fluctuations, humidity variations, and atmospheric contaminants can significantly influence catalyst performance during long-duration tests. Even minor deviations in these parameters can lead to misleading stability data, making it difficult to distinguish between actual catalyst degradation and testing artifacts.
Electrode poisoning presents another significant obstacle, particularly in aqueous electrolytes where competing reactions like hydrogen evolution can dominate. Over prolonged testing periods, catalyst active sites become progressively blocked by reaction intermediates or contaminants, resulting in performance deterioration that may be misinterpreted as inherent catalyst instability rather than a reversible fouling process.
Standardization deficiencies further complicate stability assessment. The field lacks universally accepted protocols for long-duration testing, with variations in electrolyte composition, potential control methods, and performance metrics making cross-study comparisons nearly impossible. This absence of standardization hinders meaningful benchmarking of catalyst stability across different research groups and technologies.
Technical limitations of testing equipment also pose substantial challenges. Potentiostats must maintain precise potential control over thousands of hours without drift, while gas analysis systems require consistent sensitivity and calibration throughout the testing period. Many laboratories lack specialized equipment designed specifically for such extended operations, leading to data collection interruptions and inconsistencies.
Product quantification represents a particularly difficult challenge in NRR stability testing. The extremely low concentrations of ammonia produced necessitate highly sensitive detection methods that remain reliable over extended periods. Current analytical techniques often struggle with detection limits and interference from contaminants that accumulate during long-duration experiments.
Accelerated aging protocols, which have proven valuable in other electrochemical systems, remain underdeveloped for NRR catalysts. The field lacks validated methods to predict thousand-hour stability from shorter tests, forcing researchers to conduct full-duration experiments that consume significant time and resources.
These challenges collectively highlight the urgent need for advanced testing methodologies, standardized protocols, and specialized equipment designed specifically for long-duration stability assessment of electrochemical nitrogen reduction catalysts. Addressing these limitations is crucial for advancing NRR technology toward practical applications and commercial viability.
Established Methodologies for 1000-Hour Catalyst Stability Assessment
01 Thermal stability enhancement of catalysts
Various methods can be employed to enhance the thermal stability of catalysts, which is crucial for maintaining catalytic activity at high temperatures. These methods include the incorporation of stabilizing agents, the use of specific support materials, and the development of heat-resistant catalyst structures. Enhanced thermal stability prevents sintering and agglomeration of catalyst particles, thereby extending the catalyst's operational lifetime and maintaining its efficiency in high-temperature reactions.- Thermal stability enhancement of catalysts: Various methods can be employed to enhance the thermal stability of catalysts, which is crucial for maintaining catalytic activity at high temperatures. These methods include the incorporation of stabilizing agents, controlled calcination processes, and the use of specific support materials that can withstand thermal stress. Enhanced thermal stability prevents sintering and agglomeration of catalyst particles, thereby extending the catalyst's operational lifetime in high-temperature applications.
- Chemical resistance improvement for catalysts: Improving the chemical resistance of catalysts involves protecting them from deactivation due to poisoning, coking, or chemical attack by reactants or byproducts. This can be achieved through surface modification techniques, protective coatings, or by incorporating specific elements that enhance resistance to chemical degradation. Catalysts with improved chemical resistance maintain their activity and selectivity for longer periods, even in harsh chemical environments.
- Support material selection for catalyst stability: The choice of support material significantly impacts catalyst stability. High surface area materials with appropriate porosity, mechanical strength, and chemical inertness can enhance catalyst performance and longevity. Materials such as alumina, silica, zeolites, and carbon-based supports can be tailored to specific catalytic applications. The interaction between the active catalyst component and the support material plays a crucial role in determining overall stability and activity.
- Stabilization through metal-support interactions: Metal-support interactions can be engineered to enhance catalyst stability. Strong interactions between the active metal component and the support can prevent metal particle migration and sintering. This can be achieved through specific preparation methods, such as controlled impregnation techniques, the use of anchoring groups, or the creation of core-shell structures. Optimized metal-support interactions lead to more stable catalysts with maintained dispersion of active sites.
- Regeneration and reactivation methods for catalysts: Developing effective regeneration and reactivation methods is essential for extending catalyst lifetime and maintaining stability over multiple cycles. These methods include controlled oxidation to remove carbonaceous deposits, washing procedures to eliminate poisons, and redispersion techniques to restore active metal surface area. Proper regeneration protocols can significantly enhance the economic viability of catalytic processes by reducing the frequency of complete catalyst replacement.
02 Chemical resistance improvement for catalysts
Improving the chemical resistance of catalysts involves protecting them from deactivation due to poisoning, coking, or chemical attack by reactants or byproducts. This can be achieved through the addition of protective coatings, the incorporation of specific elements that enhance resistance to chemical degradation, or the development of catalyst compositions that are inherently resistant to chemical attack. Enhanced chemical resistance ensures longer catalyst life and consistent performance in harsh chemical environments.Expand Specific Solutions03 Mechanical stability of catalyst structures
The mechanical stability of catalysts is essential for applications involving physical stress, such as fluid catalytic cracking or automotive catalytic converters. Techniques to improve mechanical stability include optimizing the catalyst support structure, controlling the porosity and density of the catalyst material, and developing robust catalyst shapes that resist attrition, crushing, and erosion. Enhanced mechanical stability prevents catalyst breakdown and loss during operation, maintaining catalytic efficiency over extended periods.Expand Specific Solutions04 Stabilization through support material selection
The choice of support material significantly impacts catalyst stability. Support materials can be selected or modified to enhance thermal, chemical, and mechanical stability of the active catalyst components. Materials such as alumina, silica, zeolites, and carbon-based supports can be engineered with specific properties to prevent catalyst sintering, provide protection against chemical attack, and maintain structural integrity. The interaction between the catalyst and support material can also be optimized to enhance overall stability and performance.Expand Specific Solutions05 Stabilization additives and promoters
Various additives and promoters can be incorporated into catalyst formulations to enhance stability. These include rare earth metals, alkaline earth compounds, and specific metal oxides that can prevent sintering, inhibit poisoning, or maintain catalyst structure. Some additives work by forming protective layers around active sites, while others modify the electronic properties of the catalyst to resist deactivation. The strategic use of stabilizing additives can significantly extend catalyst lifetime and maintain activity under challenging operational conditions.Expand Specific Solutions
Leading Research Groups and Industrial Players in NRR Catalysis
The electrochemical nitrogen reduction field is currently in an early development stage, with market size still limited but showing significant growth potential due to increasing interest in sustainable ammonia production. The technology remains at a pre-commercial maturity level, with stability testing over 1000 hours representing a critical benchmark for practical applications. Key players include established petrochemical corporations like China Petroleum & Chemical Corp. (Sinopec) and Shell, alongside specialized catalyst developers such as Clariant International and CDTi Advanced Materials. Academic institutions including EPFL and Nanyang Technological University are driving fundamental research, while industrial players like Wanhua Chemical and Idemitsu Kosan are exploring commercial applications. The competitive landscape features collaboration between research institutions and industry to overcome significant technical challenges in catalyst stability and efficiency.
China Petroleum & Chemical Corp.
Technical Solution: Sinopec has developed a comprehensive stability testing framework for NRR catalysts focused on industrial-scale implementation requirements. Their approach utilizes specialized high-pressure electrochemical cells capable of operating continuously for 1000+ hours under elevated nitrogen pressures (up to 10 MPa) to enhance reaction rates and better simulate potential industrial conditions. Sinopec's methodology incorporates multi-scale testing, beginning with accelerated screening protocols followed by progressively longer stability evaluations culminating in the 1000-hour demonstration. Their testing platform includes integrated online gas chromatography and ion chromatography systems for continuous monitoring of both desired products (ammonia) and potential degradation products or contaminants. Sinopec researchers have developed specialized catalyst preparation techniques that enhance long-term stability, including controlled encapsulation methods and hierarchical support structures that minimize catalyst leaching and agglomeration during extended operation. Their protocol includes systematic evaluation of electrolyte composition effects on long-term stability, with particular focus on anion effects and trace contaminant impacts.
Strengths: Their industrial focus provides practical insights into scale-up challenges for NRR catalysts. The high-pressure testing capabilities offer unique evaluation conditions not commonly available in academic settings. Weaknesses: Their testing protocols may prioritize industrial relevance over fundamental mechanistic understanding, potentially limiting insights into degradation pathways.
École Polytechnique Fédérale de Lausanne
Technical Solution: EPFL has developed a comprehensive stability testing protocol for electrochemical nitrogen reduction reaction (NRR) catalysts that incorporates both accelerated stress tests and long-term durability evaluations. Their approach utilizes in-situ spectroscopic techniques (X-ray absorption spectroscopy and Raman spectroscopy) to monitor catalyst structural changes during the 1000-hour demonstration period. EPFL's methodology includes periodic electrochemical impedance spectroscopy measurements to track resistance changes and identify degradation mechanisms. They've engineered novel catalyst supports with enhanced corrosion resistance specifically for NRR environments, utilizing carbon-based materials doped with nitrogen and phosphorus to improve stability. Their testing protocol incorporates multiple potential cycling regimes that simulate real-world operational conditions while monitoring ammonia production rates and faradaic efficiency throughout the entire 1000-hour testing period.
Strengths: Advanced in-situ characterization capabilities allow for real-time monitoring of catalyst degradation mechanisms. Their multi-parameter approach provides comprehensive understanding of failure modes. Weaknesses: Their testing protocols require sophisticated equipment that may not be accessible to all research groups, potentially limiting widespread adoption of their stability assessment standards.
Critical Patents and Breakthroughs in Durable NRR Catalysts
An electrocatalytic composition and cathode for the nitrogen reduction reaction
PatentActiveAU2019295418B2
Innovation
- Development of an electrocatalytic composition with metallic clusters (Ru, Fe, Rh, Ir, Mo) dispersed on a semiconductive crystalline support material with specific conduction band minimum energy properties for enhanced nitrogen reduction reaction.
- Design of a cathode structure incorporating a semiconductive crystalline support material where at least 80 mass% has a conduction band minimum energy below -0.3V vs. NHE, optimizing electron transfer properties for nitrogen reduction.
- Integration of specific metal clusters (Ru, Fe, Rh, Ir, Mo) with precisely controlled electronic properties of the support material to create an efficient interface for N₂ activation and reduction.
Catalyst for carbon dioxide electroreduction reaction or nitrogen electroreduction reaction, method for producing catalyst for carbon dioxide electroreduction reaction or nitrogen electroreduction reaction, and electrode for carbon dioxide electroreduction reaction or nitrogen electroreduction reaction
PatentInactiveJP2021115501A
Innovation
- Incorporating Fe-N4 structures with a high active point density of 3.0 × 10^-5 to 1.0 × 10^-4 Mol Sites / g in nitrogen-containing carbon materials, enhanced by a heat treatment process involving a zinc phenanthroline complex and transition metal particles, to improve catalytic activity.
Standardization and Benchmarking Protocols for NRR Catalyst Evaluation
The standardization and benchmarking of catalyst evaluation protocols for the electrochemical nitrogen reduction reaction (NRR) represents a critical challenge in advancing this promising technology. Current research in NRR catalyst development suffers from significant inconsistencies in testing methodologies, making cross-study comparisons nearly impossible and hindering scientific progress.
A comprehensive standardization framework must address multiple parameters simultaneously. Testing conditions including electrolyte composition, pH levels, temperature, and pressure need precise specification, as these variables dramatically influence catalyst performance. The electrochemical cell configuration—whether H-cell, flow cell, or membrane electrode assembly—must be standardized to ensure reproducible results across different research groups.
Analytical methods for ammonia quantification require particular attention, as false positives and contamination have plagued the field. Protocols should mandate multiple orthogonal detection techniques (e.g., indophenol blue method, ion chromatography, and NMR spectroscopy) to verify ammonia production. Background ammonia measurements and isotope labeling experiments using 15N2 should become mandatory components of any rigorous evaluation protocol.
Stability metrics present perhaps the most significant standardization challenge. The proposed one-thousand-hour demonstration represents an ambitious but necessary benchmark for practical application. Standardized protocols should define specific current density requirements during long-term testing, acceptable performance degradation thresholds, and intermittent characterization intervals to track catalyst structural changes.
Faradaic efficiency and ammonia production rate calculations must follow consistent methodologies. The field should adopt standardized units (e.g., μgNH3 cm-2 h-1 or μmol NH3 cm-2 h-1) and normalization approaches (by geometric area, electrochemically active surface area, or catalyst mass) to facilitate meaningful comparisons between different catalysts.
Control experiments must be rigorously defined, including N2-free tests, open-circuit measurements, and electrolyte-only controls to eliminate false positives. Additionally, catalyst characterization techniques before and after stability testing should be standardized to provide insights into degradation mechanisms and inform future catalyst design.
Implementation of these standardized protocols will require collaborative efforts from academic institutions, industry partners, and standards organizations. The development of reference catalysts with well-documented performance metrics would provide valuable benchmarks against which new catalysts could be evaluated, accelerating progress toward commercially viable electrochemical nitrogen reduction technologies.
A comprehensive standardization framework must address multiple parameters simultaneously. Testing conditions including electrolyte composition, pH levels, temperature, and pressure need precise specification, as these variables dramatically influence catalyst performance. The electrochemical cell configuration—whether H-cell, flow cell, or membrane electrode assembly—must be standardized to ensure reproducible results across different research groups.
Analytical methods for ammonia quantification require particular attention, as false positives and contamination have plagued the field. Protocols should mandate multiple orthogonal detection techniques (e.g., indophenol blue method, ion chromatography, and NMR spectroscopy) to verify ammonia production. Background ammonia measurements and isotope labeling experiments using 15N2 should become mandatory components of any rigorous evaluation protocol.
Stability metrics present perhaps the most significant standardization challenge. The proposed one-thousand-hour demonstration represents an ambitious but necessary benchmark for practical application. Standardized protocols should define specific current density requirements during long-term testing, acceptable performance degradation thresholds, and intermittent characterization intervals to track catalyst structural changes.
Faradaic efficiency and ammonia production rate calculations must follow consistent methodologies. The field should adopt standardized units (e.g., μgNH3 cm-2 h-1 or μmol NH3 cm-2 h-1) and normalization approaches (by geometric area, electrochemically active surface area, or catalyst mass) to facilitate meaningful comparisons between different catalysts.
Control experiments must be rigorously defined, including N2-free tests, open-circuit measurements, and electrolyte-only controls to eliminate false positives. Additionally, catalyst characterization techniques before and after stability testing should be standardized to provide insights into degradation mechanisms and inform future catalyst design.
Implementation of these standardized protocols will require collaborative efforts from academic institutions, industry partners, and standards organizations. The development of reference catalysts with well-documented performance metrics would provide valuable benchmarks against which new catalysts could be evaluated, accelerating progress toward commercially viable electrochemical nitrogen reduction technologies.
Environmental Impact and Sustainability Metrics of NRR Technologies
The environmental impact of electrochemical nitrogen reduction reaction (NRR) technologies must be comprehensively evaluated against conventional nitrogen fixation methods, particularly the Haber-Bosch process which currently consumes approximately 1-2% of global energy and generates significant carbon emissions. Long-term stability testing of catalysts provides critical data for assessing the true environmental footprint of NRR technologies.
Lifecycle assessment (LCA) studies indicate that electrochemical NRR systems could potentially reduce greenhouse gas emissions by 30-60% compared to conventional methods when powered by renewable energy sources. However, these projections depend heavily on catalyst stability over extended operational periods, as demonstrated in thousand-hour testing protocols. Catalysts exhibiting performance degradation require more frequent replacement, significantly increasing material consumption and waste generation.
Water consumption represents another critical sustainability metric for NRR technologies. While electrochemical processes generally require less water than conventional methods, the quality of wastewater discharge and potential contamination with catalyst materials must be monitored during long-duration stability tests. Studies show that stable catalysts with minimal leaching can reduce wastewater treatment requirements by up to 40%.
Land use efficiency of NRR technologies also presents advantages over conventional fertilizer production facilities. Distributed electrochemical ammonia production systems could potentially reduce transportation emissions by 15-25% through localized production. However, this benefit is only realized when catalysts demonstrate consistent performance over thousand-hour operational periods under real-world conditions.
Resource efficiency metrics from extended stability testing reveal that precious metal-based catalysts, despite their high initial activity, often present sustainability challenges due to material scarcity and environmental impacts from mining. Recent thousand-hour demonstrations with earth-abundant metal nitrides and carbon-based catalysts show promising stability profiles with significantly lower environmental footprints, reducing critical raw material dependency by up to 70%.
Energy efficiency remains the dominant factor in environmental impact assessments. Thousand-hour stability tests reveal that maintaining high Faradaic efficiency throughout the catalyst lifetime is essential for realizing the projected environmental benefits. Catalysts demonstrating less than 10% performance degradation over thousand-hour operations can maintain energy advantages over conventional processes, while those with more significant degradation may ultimately offer no net environmental benefit despite promising initial performance.
Lifecycle assessment (LCA) studies indicate that electrochemical NRR systems could potentially reduce greenhouse gas emissions by 30-60% compared to conventional methods when powered by renewable energy sources. However, these projections depend heavily on catalyst stability over extended operational periods, as demonstrated in thousand-hour testing protocols. Catalysts exhibiting performance degradation require more frequent replacement, significantly increasing material consumption and waste generation.
Water consumption represents another critical sustainability metric for NRR technologies. While electrochemical processes generally require less water than conventional methods, the quality of wastewater discharge and potential contamination with catalyst materials must be monitored during long-duration stability tests. Studies show that stable catalysts with minimal leaching can reduce wastewater treatment requirements by up to 40%.
Land use efficiency of NRR technologies also presents advantages over conventional fertilizer production facilities. Distributed electrochemical ammonia production systems could potentially reduce transportation emissions by 15-25% through localized production. However, this benefit is only realized when catalysts demonstrate consistent performance over thousand-hour operational periods under real-world conditions.
Resource efficiency metrics from extended stability testing reveal that precious metal-based catalysts, despite their high initial activity, often present sustainability challenges due to material scarcity and environmental impacts from mining. Recent thousand-hour demonstrations with earth-abundant metal nitrides and carbon-based catalysts show promising stability profiles with significantly lower environmental footprints, reducing critical raw material dependency by up to 70%.
Energy efficiency remains the dominant factor in environmental impact assessments. Thousand-hour stability tests reveal that maintaining high Faradaic efficiency throughout the catalyst lifetime is essential for realizing the projected environmental benefits. Catalysts demonstrating less than 10% performance degradation over thousand-hour operations can maintain energy advantages over conventional processes, while those with more significant degradation may ultimately offer no net environmental benefit despite promising initial performance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!