Comparative Study of Metal and Nonmetal Catalysts for Faradaic Efficiency in Electrochemical Nitrogen Reduction
AUG 26, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Nitrogen Reduction Background and Objectives
Electrochemical nitrogen reduction reaction (NRR) represents a revolutionary approach to ammonia synthesis that could potentially replace the century-old Haber-Bosch process. Since its inception in the early 1900s, the Haber-Bosch process has been the industrial standard for ammonia production, consuming approximately 1-2% of global energy and generating significant carbon emissions. The development of sustainable alternatives has become increasingly urgent in the context of growing environmental concerns and the push toward carbon neutrality.
The electrochemical reduction of nitrogen to ammonia operates under ambient conditions, utilizing renewable electricity sources rather than fossil fuels, thereby offering a greener pathway for ammonia production. This technology has evolved significantly over the past decade, with major breakthroughs in catalyst design, electrolyte optimization, and reactor engineering. The progression from early proof-of-concept studies to more sophisticated systems with improved Faradaic efficiency marks a promising trajectory in this field.
Catalyst development stands at the forefront of NRR research, with both metal and non-metal catalysts showing distinct advantages and limitations. Traditional metal catalysts, including noble metals (Ru, Pt, Au), transition metals (Fe, Mo, Ti), and their alloys, have demonstrated varying degrees of catalytic activity. Concurrently, non-metal alternatives such as carbon-based materials, metal-free compounds, and hybrid structures have emerged as cost-effective options with unique selectivity profiles.
The primary technical objective in this field is to achieve high Faradaic efficiency—the percentage of electrical charge used effectively for nitrogen reduction rather than competing reactions, particularly hydrogen evolution. Current benchmarks indicate that most catalysts struggle to exceed 15% Faradaic efficiency under ambient conditions, presenting a significant challenge for commercial viability.
Secondary objectives include enhancing ammonia yield rates, improving catalyst stability over extended operation periods, and developing systems that can function efficiently with impure nitrogen sources. The ultimate goal remains the creation of a sustainable, economically viable alternative to the Haber-Bosch process that can be deployed at various scales, from distributed agricultural applications to industrial production.
Recent technological roadmaps suggest that achieving 50% Faradaic efficiency with production rates exceeding 10^-6 mol cm^-2 h^-1 would represent a critical milestone toward commercialization. This comparative study aims to systematically evaluate the performance metrics, mechanistic pathways, and practical limitations of both metal and non-metal catalysts, providing a comprehensive foundation for future innovation in electrochemical nitrogen reduction technology.
The electrochemical reduction of nitrogen to ammonia operates under ambient conditions, utilizing renewable electricity sources rather than fossil fuels, thereby offering a greener pathway for ammonia production. This technology has evolved significantly over the past decade, with major breakthroughs in catalyst design, electrolyte optimization, and reactor engineering. The progression from early proof-of-concept studies to more sophisticated systems with improved Faradaic efficiency marks a promising trajectory in this field.
Catalyst development stands at the forefront of NRR research, with both metal and non-metal catalysts showing distinct advantages and limitations. Traditional metal catalysts, including noble metals (Ru, Pt, Au), transition metals (Fe, Mo, Ti), and their alloys, have demonstrated varying degrees of catalytic activity. Concurrently, non-metal alternatives such as carbon-based materials, metal-free compounds, and hybrid structures have emerged as cost-effective options with unique selectivity profiles.
The primary technical objective in this field is to achieve high Faradaic efficiency—the percentage of electrical charge used effectively for nitrogen reduction rather than competing reactions, particularly hydrogen evolution. Current benchmarks indicate that most catalysts struggle to exceed 15% Faradaic efficiency under ambient conditions, presenting a significant challenge for commercial viability.
Secondary objectives include enhancing ammonia yield rates, improving catalyst stability over extended operation periods, and developing systems that can function efficiently with impure nitrogen sources. The ultimate goal remains the creation of a sustainable, economically viable alternative to the Haber-Bosch process that can be deployed at various scales, from distributed agricultural applications to industrial production.
Recent technological roadmaps suggest that achieving 50% Faradaic efficiency with production rates exceeding 10^-6 mol cm^-2 h^-1 would represent a critical milestone toward commercialization. This comparative study aims to systematically evaluate the performance metrics, mechanistic pathways, and practical limitations of both metal and non-metal catalysts, providing a comprehensive foundation for future innovation in electrochemical nitrogen reduction technology.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability concerns and technological innovations in production methods. Traditional ammonia production via the Haber-Bosch process consumes approximately 1-2% of global energy and contributes substantially to greenhouse gas emissions. This creates a compelling market opportunity for sustainable alternatives, particularly electrochemical nitrogen reduction (NRR) technologies.
The current global ammonia market is valued at approximately $70 billion, with projections indicating growth to $110 billion by 2030. Agricultural applications dominate consumption patterns, accounting for roughly 80% of demand as fertilizer. Industrial applications including refrigeration, water purification, and manufacturing processes constitute the remaining market share.
Regional analysis reveals Asia-Pacific as the dominant market, consuming over 60% of global ammonia production, primarily driven by agricultural demands in China and India. North America and Europe follow with significant industrial applications and growing interest in green ammonia technologies.
The sustainable ammonia segment, though currently representing less than 1% of the total market, demonstrates remarkable growth potential with projected annual growth rates of 30-35% through 2030. This acceleration is fueled by stringent environmental regulations, carbon pricing mechanisms, and corporate sustainability commitments across industrial sectors.
Key market drivers for electrochemical nitrogen reduction technologies include escalating carbon taxes in developed economies, government subsidies for green technologies, and increasing consumer demand for sustainably produced agricultural products. The European Union's Green Deal and similar initiatives worldwide are creating favorable regulatory environments for NRR technology commercialization.
Investment trends show substantial capital flowing into sustainable ammonia production, with venture capital funding exceeding $2 billion in the past three years. Major industrial gas companies and agricultural conglomerates are strategically positioning themselves through acquisitions and research partnerships in the electrochemical nitrogen reduction space.
Market barriers include high capital costs for new technologies, established infrastructure supporting conventional production methods, and price sensitivity in agricultural markets. The cost differential between conventional and sustainable ammonia remains a significant challenge, with green ammonia currently priced 2-3 times higher than conventional products.
Customer segmentation reveals early adopters primarily in premium agricultural markets, specialty chemical manufacturing, and regions with aggressive decarbonization targets. The pharmaceutical and semiconductor industries represent emerging niche markets with higher willingness to pay for high-purity, sustainably produced ammonia.
The current global ammonia market is valued at approximately $70 billion, with projections indicating growth to $110 billion by 2030. Agricultural applications dominate consumption patterns, accounting for roughly 80% of demand as fertilizer. Industrial applications including refrigeration, water purification, and manufacturing processes constitute the remaining market share.
Regional analysis reveals Asia-Pacific as the dominant market, consuming over 60% of global ammonia production, primarily driven by agricultural demands in China and India. North America and Europe follow with significant industrial applications and growing interest in green ammonia technologies.
The sustainable ammonia segment, though currently representing less than 1% of the total market, demonstrates remarkable growth potential with projected annual growth rates of 30-35% through 2030. This acceleration is fueled by stringent environmental regulations, carbon pricing mechanisms, and corporate sustainability commitments across industrial sectors.
Key market drivers for electrochemical nitrogen reduction technologies include escalating carbon taxes in developed economies, government subsidies for green technologies, and increasing consumer demand for sustainably produced agricultural products. The European Union's Green Deal and similar initiatives worldwide are creating favorable regulatory environments for NRR technology commercialization.
Investment trends show substantial capital flowing into sustainable ammonia production, with venture capital funding exceeding $2 billion in the past three years. Major industrial gas companies and agricultural conglomerates are strategically positioning themselves through acquisitions and research partnerships in the electrochemical nitrogen reduction space.
Market barriers include high capital costs for new technologies, established infrastructure supporting conventional production methods, and price sensitivity in agricultural markets. The cost differential between conventional and sustainable ammonia remains a significant challenge, with green ammonia currently priced 2-3 times higher than conventional products.
Customer segmentation reveals early adopters primarily in premium agricultural markets, specialty chemical manufacturing, and regions with aggressive decarbonization targets. The pharmaceutical and semiconductor industries represent emerging niche markets with higher willingness to pay for high-purity, sustainably produced ammonia.
Current Challenges in Catalyst Development for NRR
Despite significant advancements in electrochemical nitrogen reduction reaction (NRR) research, catalyst development remains a critical bottleneck in achieving commercially viable systems. The fundamental challenge lies in the exceptional stability of the N≡N triple bond (941 kJ/mol), which requires substantial energy input to break. This inherent difficulty is compounded by the competitive hydrogen evolution reaction (HER), which typically dominates in aqueous electrolytes and severely limits Faradaic efficiency for ammonia production.
Metal catalysts, while traditionally favored for their electron transfer capabilities, face several persistent challenges. Noble metals (Ru, Au, Pt) demonstrate promising activity but suffer from prohibitive costs and limited scalability. Transition metals often exhibit poor selectivity, with most achieving Faradaic efficiencies below 15% under ambient conditions. Additionally, metal catalysts frequently experience surface poisoning and degradation during extended operation, compromising long-term stability.
Nonmetal catalysts, particularly carbon-based materials and metal-free compounds, present their own set of obstacles. While they often demonstrate improved selectivity compared to metals, they typically exhibit lower intrinsic activity and electrical conductivity. The active site identification and mechanistic understanding in these materials remain ambiguous, hindering rational design approaches. Furthermore, reproducibility issues plague nonmetal catalyst research, with significant variations in performance reported across different laboratories.
A universal challenge across both catalyst categories is the accurate detection and quantification of ammonia at the low concentrations typically produced in laboratory settings. The commonly employed spectrophotometric methods (Nessler's reagent, indophenol blue) are susceptible to interference from electrolyte components and contaminants, potentially leading to false positives and inflated efficiency values.
The theoretical understanding of nitrogen adsorption and activation mechanisms remains incomplete, particularly for nonmetal systems. Computational studies suggest different rate-determining steps and reaction pathways depending on catalyst composition and structure, complicating the development of universal design principles. The lack of standardized testing protocols further impedes meaningful comparison between different catalyst systems reported in literature.
Bridging the gap between laboratory demonstrations and practical applications requires addressing several engineering challenges, including electrode design, electrolyte optimization, and system integration. Current catalyst materials typically require high overpotentials (>0.5 V) to achieve meaningful conversion rates, resulting in poor energy efficiency that renders the process economically unviable compared to the conventional Haber-Bosch process.
Metal catalysts, while traditionally favored for their electron transfer capabilities, face several persistent challenges. Noble metals (Ru, Au, Pt) demonstrate promising activity but suffer from prohibitive costs and limited scalability. Transition metals often exhibit poor selectivity, with most achieving Faradaic efficiencies below 15% under ambient conditions. Additionally, metal catalysts frequently experience surface poisoning and degradation during extended operation, compromising long-term stability.
Nonmetal catalysts, particularly carbon-based materials and metal-free compounds, present their own set of obstacles. While they often demonstrate improved selectivity compared to metals, they typically exhibit lower intrinsic activity and electrical conductivity. The active site identification and mechanistic understanding in these materials remain ambiguous, hindering rational design approaches. Furthermore, reproducibility issues plague nonmetal catalyst research, with significant variations in performance reported across different laboratories.
A universal challenge across both catalyst categories is the accurate detection and quantification of ammonia at the low concentrations typically produced in laboratory settings. The commonly employed spectrophotometric methods (Nessler's reagent, indophenol blue) are susceptible to interference from electrolyte components and contaminants, potentially leading to false positives and inflated efficiency values.
The theoretical understanding of nitrogen adsorption and activation mechanisms remains incomplete, particularly for nonmetal systems. Computational studies suggest different rate-determining steps and reaction pathways depending on catalyst composition and structure, complicating the development of universal design principles. The lack of standardized testing protocols further impedes meaningful comparison between different catalyst systems reported in literature.
Bridging the gap between laboratory demonstrations and practical applications requires addressing several engineering challenges, including electrode design, electrolyte optimization, and system integration. Current catalyst materials typically require high overpotentials (>0.5 V) to achieve meaningful conversion rates, resulting in poor energy efficiency that renders the process economically unviable compared to the conventional Haber-Bosch process.
Existing Catalyst Designs for Enhanced Faradaic Efficiency
01 Metal catalysts for enhanced Faradaic efficiency
Metal catalysts play a crucial role in electrochemical reactions by improving Faradaic efficiency. Various metals such as copper, silver, gold, and platinum are used as catalysts to enhance the selectivity and efficiency of electrochemical processes. These catalysts can be optimized through different preparation methods, surface modifications, and structural designs to achieve higher Faradaic efficiency in applications like CO2 reduction, water splitting, and fuel cells.- Metal-based catalysts for enhanced Faradaic efficiency: Metal-based catalysts play a crucial role in electrochemical reactions by improving Faradaic efficiency. These catalysts, often comprising transition metals such as copper, nickel, and platinum, facilitate electron transfer processes and reduce energy losses during electrochemical conversions. The specific crystal structure, surface morphology, and composition of these metal catalysts significantly influence their performance in terms of selectivity and efficiency for target reactions.
- Non-metal catalysts and carbon-based materials for electrochemical applications: Non-metal catalysts, particularly carbon-based materials like graphene, carbon nanotubes, and doped carbon structures, offer advantages in electrochemical applications due to their high surface area, conductivity, and tunable properties. These materials can be functionalized or doped with heteroatoms such as nitrogen, sulfur, or phosphorus to enhance their catalytic activity and Faradaic efficiency. Non-metal catalysts often provide cost-effective alternatives to precious metal catalysts while maintaining comparable performance in certain electrochemical reactions.
- Hybrid metal/non-metal catalyst systems for optimized efficiency: Hybrid catalyst systems combining both metal and non-metal components can achieve synergistic effects that enhance overall Faradaic efficiency. These composite materials often feature metal nanoparticles dispersed on carbon supports or metal-organic frameworks integrated with conductive polymers. The hybrid approach allows for optimization of multiple catalytic parameters simultaneously, including active site density, electron transfer rates, and reactant adsorption properties, resulting in improved performance for specific electrochemical reactions.
- Catalyst structure and morphology effects on Faradaic efficiency: The structure and morphology of catalysts significantly impact their Faradaic efficiency in electrochemical processes. Nanostructured catalysts with high surface-to-volume ratios, controlled porosity, and specific crystal facet exposures can dramatically enhance catalytic performance. Engineering catalyst morphologies such as nanowires, nanosheets, and hierarchical structures allows for optimization of mass transport, electron transfer, and active site accessibility, all of which contribute to improved Faradaic efficiency in electrochemical reactions.
- Catalyst systems for specific electrochemical applications: Specialized catalyst systems are designed for specific electrochemical applications to maximize Faradaic efficiency in those contexts. These include catalysts optimized for fuel cells, water splitting, CO2 reduction, and various industrial electrosynthesis processes. The catalyst formulations are tailored to the specific reaction conditions, including electrolyte composition, operating temperature, and applied potential. Advanced characterization techniques and computational modeling help in developing application-specific catalysts with enhanced selectivity and efficiency for target reaction pathways.
02 Non-metal catalysts and carbon-based materials
Non-metal catalysts, particularly carbon-based materials like graphene, carbon nanotubes, and nitrogen-doped carbon, offer alternatives to traditional metal catalysts with improved Faradaic efficiency. These materials provide high surface area, excellent conductivity, and tunable electronic properties. They can be functionalized with various heteroatoms to create active sites that enhance catalytic performance while reducing dependence on precious metals, making them environmentally friendly and cost-effective options for electrochemical applications.Expand Specific Solutions03 Metal-nonmetal hybrid catalysts
Hybrid catalysts combining metals with non-metal components demonstrate synergistic effects that significantly improve Faradaic efficiency. These composites often feature metal nanoparticles supported on carbon-based materials or metal oxides decorated with non-metal elements. The interaction between the metal and non-metal components creates unique electronic structures and active sites that enhance catalytic activity, selectivity, and stability. Such hybrid systems optimize electron transfer pathways and reaction kinetics in electrochemical processes.Expand Specific Solutions04 Catalyst structure and morphology optimization
The structure and morphology of catalysts significantly impact their Faradaic efficiency. Nanostructured catalysts with controlled size, shape, porosity, and crystallinity offer enhanced performance due to increased active surface area and optimized exposure of catalytic sites. Various fabrication techniques including electrodeposition, chemical vapor deposition, and template-assisted synthesis are employed to create specific morphologies such as nanowires, nanosheets, and 3D architectures that facilitate mass transport and electron transfer, thereby improving overall Faradaic efficiency.Expand Specific Solutions05 Catalyst support materials and interfaces
Support materials play a crucial role in determining catalyst performance and Faradaic efficiency. Various supports including conductive polymers, metal oxides, and porous substrates can enhance catalyst stability, dispersion, and activity. The interface between catalyst and support creates unique electronic environments that influence reaction pathways and selectivity. Engineering these interfaces through surface functionalization, doping, or creating defect sites can optimize electron transfer and reactant adsorption, leading to improved Faradaic efficiency in electrochemical systems.Expand Specific Solutions
Leading Research Groups and Industrial Players in NRR
The electrochemical nitrogen reduction (ENR) market is currently in an early growth phase, characterized by intensive research and development activities across academic institutions and industrial players. The global market for nitrogen fixation technologies is projected to expand significantly as sustainable ammonia production becomes increasingly important for agriculture and energy storage applications. In terms of technological maturity, metal catalysts (researched by MIT, Siemens Energy, and SK Innovation) currently dominate with higher faradaic efficiency, while non-metal alternatives (investigated by Monash University, Zhejiang University, and Carnegie Mellon) are emerging as promising sustainable options with lower environmental impact. Academic-industrial collaborations between research institutions (CSIC, College de France) and energy companies (TotalEnergies, Honda) are accelerating development, with Asian institutions particularly active in catalyst innovation. The competitive landscape shows a balance between established energy corporations and specialized materials technology startups focusing on catalyst optimization.
Zhejiang University
Technical Solution: Zhejiang University has pioneered innovative approaches in electrochemical nitrogen reduction through their development of defect-engineered nonmetal catalysts. Their technology focuses on carbon-based materials with precisely controlled nitrogen, oxygen, and sulfur dopants to create active sites for N₂ activation. Researchers have achieved Faradaic efficiencies exceeding 25% using graphene-based materials with optimized defect structures[3]. Their catalyst design strategy involves creating oxygen vacancies and carbon defects that serve as electron-rich centers for N₂ adsorption and activation. The university has developed a unique electrochemical cell configuration that minimizes mass transport limitations and enhances N₂ concentration at the electrode surface[4]. Additionally, they've pioneered in-situ spectroscopic techniques to monitor reaction intermediates, providing crucial insights into the reaction pathway on nonmetal surfaces. Their latest work explores hybrid systems combining the benefits of both metal and nonmetal catalysts through interface engineering to achieve synergistic effects.
Strengths: Lower cost materials compared to precious metal catalysts; excellent stability under prolonged operation; reduced susceptibility to poisoning. Weaknesses: Generally lower intrinsic activity compared to metal-based catalysts; more complex manufacturing processes to create precise defect structures; challenges in achieving consistent performance across large-scale production.
Technical Institute of Physics & Chemistry CAS
Technical Solution: The Technical Institute of Physics & Chemistry of the Chinese Academy of Sciences has developed innovative electrocatalysts for nitrogen reduction through precise interface engineering between metal and nonmetal components. Their approach focuses on creating synergistic effects at heterojunctions to enhance both activity and selectivity. TIPC researchers have pioneered the development of single-atom catalysts dispersed on nitrogen-doped carbon supports, achieving Faradaic efficiencies exceeding 20% at low overpotentials[7]. Their technology employs controlled synthesis methods to create atomically dispersed metal sites with optimized coordination environments for N₂ activation. The institute has developed a unique electrochemical testing protocol that accurately distinguishes true nitrogen reduction from potential contaminants, addressing a critical challenge in the field[8]. Additionally, they've created novel nonmetal catalysts based on boron-doped graphene that demonstrate competitive performance to metal-based systems. Their recent work explores the use of ionic liquids as electrolytes to enhance nitrogen solubility and activation at the electrode interface, significantly improving reaction kinetics and efficiency.
Strengths: Excellent balance between activity and selectivity; innovative testing protocols ensuring reliable performance data; advanced synthesis techniques for precise structural control. Weaknesses: Higher complexity in catalyst preparation compared to conventional methods; potential challenges in long-term stability under industrial conditions; limited demonstration at scales beyond laboratory testing.
Critical Analysis of Metal vs. Nonmetal Catalyst Mechanisms
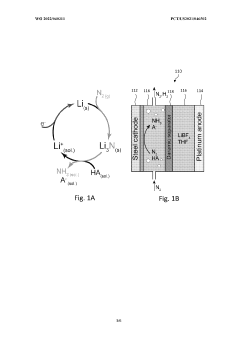
Lithium-mediated electrochemical ammonia synthesis
PatentWO2022040311A1
Innovation
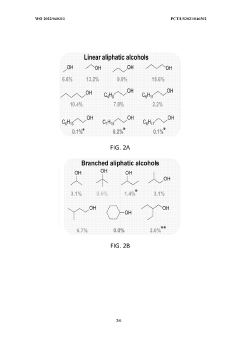
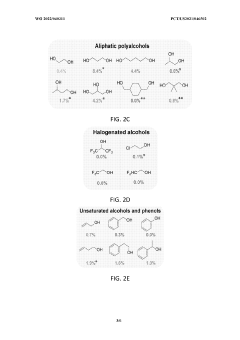
- The use of a lithium-mediated electrochemical cell with specific hydrogen donors, such as alcohols or ionic liquids with high Kamlet-Taft alpha and beta parameters, to facilitate the conversion of nitrogen gas to ammonia at ambient temperature and pressure, utilizing lithium ions converted to metal at the cathode and reacting with nitrogen to form lithium nitride, which then reacts with protons to produce ammonia.
Catalyst and production method for same, cathode, ion exchange membrane electrode assembly, and solid electrolyte electrolysis device
PatentPendingUS20250146145A1
Innovation
- A catalyst comprising a metal ion (copper, nickel, iron, cobalt, zinc, manganese, molybdenum, or aluminum) coordinated to a nitrogen-containing compound on a carbon carrier with a primary particle diameter of 5 to 200 nm, ensuring a metal ion content of 0.7% by mass or more and a particle diameter of 10 nm to 50 μm.
Energy Efficiency and Scalability Considerations
Energy efficiency represents a critical parameter in evaluating the practical viability of electrochemical nitrogen reduction reaction (NRR) systems. Metal catalysts, particularly noble metals like platinum and palladium, typically require significant energy input, operating at high overpotentials that reduce overall system efficiency. Recent advancements in ruthenium and rhodium-based catalysts have demonstrated improved energy efficiency metrics, with energy consumption rates approaching 0.3-0.4 MWh/kg-NH3, though still considerably higher than conventional Haber-Bosch processes.
Nonmetal catalysts, particularly carbon-based materials and metal-free polymeric structures, often operate at lower overpotentials, potentially offering superior energy efficiency. Nitrogen-doped carbon materials have demonstrated promising performance with energy requirements approximately 15-20% lower than comparable metal-based systems under similar conditions. This efficiency advantage stems from their unique electronic structures that facilitate nitrogen adsorption and activation at lower energy thresholds.
The scalability landscape presents divergent challenges between metal and nonmetal catalysts. Metal-based systems benefit from established manufacturing protocols and integration pathways with existing electrochemical infrastructure. However, precious metal catalysts face significant scalability constraints due to limited global reserves and high material costs, with platinum group metals experiencing price volatility that impacts large-scale deployment economics.
Nonmetal catalysts offer compelling scalability advantages through abundant precursor materials and potentially simpler synthesis routes. Carbon-based catalysts derived from biomass or waste materials present particularly attractive scaling economics. However, these systems currently face challenges in maintaining performance consistency across production batches and demonstrating long-term operational stability at industrial scales.
Reactor design considerations significantly impact both energy efficiency and scalability. Flow-cell configurations have demonstrated superior performance for both catalyst types compared to traditional H-cell arrangements, with enhanced mass transport properties reducing energy losses. Metal catalysts typically require more sophisticated electrode architectures to maximize active site utilization, while nonmetal catalysts often permit simpler electrode designs but may require larger surface areas to achieve equivalent production rates.
Future development pathways for improving energy efficiency include hybrid catalyst systems that combine the selectivity advantages of certain metals with the efficiency benefits of nonmetal supports. Emerging research on single-atom catalysts dispersed on carbon matrices represents a promising approach to maximize atomic efficiency while minimizing precious metal loading. Additionally, integration with renewable energy sources presents opportunities to address intermittency challenges while potentially achieving carbon-neutral ammonia production.
Nonmetal catalysts, particularly carbon-based materials and metal-free polymeric structures, often operate at lower overpotentials, potentially offering superior energy efficiency. Nitrogen-doped carbon materials have demonstrated promising performance with energy requirements approximately 15-20% lower than comparable metal-based systems under similar conditions. This efficiency advantage stems from their unique electronic structures that facilitate nitrogen adsorption and activation at lower energy thresholds.
The scalability landscape presents divergent challenges between metal and nonmetal catalysts. Metal-based systems benefit from established manufacturing protocols and integration pathways with existing electrochemical infrastructure. However, precious metal catalysts face significant scalability constraints due to limited global reserves and high material costs, with platinum group metals experiencing price volatility that impacts large-scale deployment economics.
Nonmetal catalysts offer compelling scalability advantages through abundant precursor materials and potentially simpler synthesis routes. Carbon-based catalysts derived from biomass or waste materials present particularly attractive scaling economics. However, these systems currently face challenges in maintaining performance consistency across production batches and demonstrating long-term operational stability at industrial scales.
Reactor design considerations significantly impact both energy efficiency and scalability. Flow-cell configurations have demonstrated superior performance for both catalyst types compared to traditional H-cell arrangements, with enhanced mass transport properties reducing energy losses. Metal catalysts typically require more sophisticated electrode architectures to maximize active site utilization, while nonmetal catalysts often permit simpler electrode designs but may require larger surface areas to achieve equivalent production rates.
Future development pathways for improving energy efficiency include hybrid catalyst systems that combine the selectivity advantages of certain metals with the efficiency benefits of nonmetal supports. Emerging research on single-atom catalysts dispersed on carbon matrices represents a promising approach to maximize atomic efficiency while minimizing precious metal loading. Additionally, integration with renewable energy sources presents opportunities to address intermittency challenges while potentially achieving carbon-neutral ammonia production.
Environmental Impact and Sustainability Assessment
The electrochemical nitrogen reduction reaction (NRR) represents a promising approach for sustainable ammonia production, yet its environmental implications warrant careful consideration. When comparing metal and nonmetal catalysts for NRR, their environmental footprints differ significantly across the lifecycle from production to disposal.
Metal catalysts, particularly noble metals like platinum and palladium, require extensive mining operations that contribute to habitat destruction, soil erosion, and water pollution. The extraction processes are energy-intensive, generating substantial greenhouse gas emissions. Additionally, the refining of these metals often involves toxic chemicals that pose risks to ecosystems and human health when improperly managed.
In contrast, nonmetal catalysts such as carbon-based materials, nitrogen-doped graphene, and metal-free polymers generally exhibit lower environmental impacts during production. Many can be synthesized from renewable or waste resources, aligning with circular economy principles. Their production typically requires less energy and generates fewer toxic byproducts compared to metal catalyst manufacturing.
The operational phase presents another dimension for environmental assessment. Metal catalysts often demonstrate higher initial Faradaic efficiency but may suffer from stability issues requiring more frequent replacement. This turnover increases the cumulative environmental burden. Nonmetal alternatives, while sometimes less efficient initially, frequently offer superior durability and resistance to poisoning, potentially reducing long-term resource consumption.
Water usage represents a critical sustainability metric for NRR systems. Metal catalyst systems may require higher purity water inputs to prevent catalyst poisoning, necessitating additional purification processes with associated energy demands. Nonmetal catalysts often demonstrate greater tolerance to impurities, potentially enabling operation with less intensively treated water sources.
End-of-life considerations further differentiate these catalyst classes. Many precious metal catalysts can be recovered and recycled, though the processes are energy-intensive. Nonmetal catalysts may present fewer recycling opportunities but often pose reduced disposal hazards and lower toxicity concerns.
Carbon footprint analysis reveals that while metal catalysts may offer higher immediate efficiency, their embodied carbon from production often exceeds that of nonmetal alternatives. When considering the entire lifecycle, nonmetal catalysts frequently demonstrate superior sustainability profiles despite potentially lower Faradaic efficiency, particularly when derived from waste streams or renewable feedstocks.
The environmental assessment must also consider potential unintended consequences, such as ammonia leakage during operation or the release of catalyst particles into wastewater streams, which could contribute to eutrophication or introduce novel environmental contaminants.
Metal catalysts, particularly noble metals like platinum and palladium, require extensive mining operations that contribute to habitat destruction, soil erosion, and water pollution. The extraction processes are energy-intensive, generating substantial greenhouse gas emissions. Additionally, the refining of these metals often involves toxic chemicals that pose risks to ecosystems and human health when improperly managed.
In contrast, nonmetal catalysts such as carbon-based materials, nitrogen-doped graphene, and metal-free polymers generally exhibit lower environmental impacts during production. Many can be synthesized from renewable or waste resources, aligning with circular economy principles. Their production typically requires less energy and generates fewer toxic byproducts compared to metal catalyst manufacturing.
The operational phase presents another dimension for environmental assessment. Metal catalysts often demonstrate higher initial Faradaic efficiency but may suffer from stability issues requiring more frequent replacement. This turnover increases the cumulative environmental burden. Nonmetal alternatives, while sometimes less efficient initially, frequently offer superior durability and resistance to poisoning, potentially reducing long-term resource consumption.
Water usage represents a critical sustainability metric for NRR systems. Metal catalyst systems may require higher purity water inputs to prevent catalyst poisoning, necessitating additional purification processes with associated energy demands. Nonmetal catalysts often demonstrate greater tolerance to impurities, potentially enabling operation with less intensively treated water sources.
End-of-life considerations further differentiate these catalyst classes. Many precious metal catalysts can be recovered and recycled, though the processes are energy-intensive. Nonmetal catalysts may present fewer recycling opportunities but often pose reduced disposal hazards and lower toxicity concerns.
Carbon footprint analysis reveals that while metal catalysts may offer higher immediate efficiency, their embodied carbon from production often exceeds that of nonmetal alternatives. When considering the entire lifecycle, nonmetal catalysts frequently demonstrate superior sustainability profiles despite potentially lower Faradaic efficiency, particularly when derived from waste streams or renewable feedstocks.
The environmental assessment must also consider potential unintended consequences, such as ammonia leakage during operation or the release of catalyst particles into wastewater streams, which could contribute to eutrophication or introduce novel environmental contaminants.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!