Standardized Protocols for Ammonia Quantification and Reproducibility in Electrochemical Nitrogen Reduction
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical NRR Technology Background and Objectives
Electrochemical nitrogen reduction reaction (NRR) represents a groundbreaking approach to ammonia synthesis that operates under ambient conditions, offering a sustainable alternative to the energy-intensive Haber-Bosch process which currently dominates industrial ammonia production. The evolution of NRR technology traces back to early electrochemical studies in the 1980s, with significant acceleration in research occurring over the past decade as global focus on renewable energy and sustainable chemical production has intensified.
The fundamental principle of electrochemical NRR involves the reduction of atmospheric nitrogen (N₂) to ammonia (NH₃) using electricity, ideally derived from renewable sources, in the presence of water as a proton source. This process aims to achieve what nature accomplishes through nitrogenase enzymes in nitrogen-fixing bacteria, but through artificial electrocatalytic systems that can be scaled for industrial application.
Recent technological trends show a shift toward developing more efficient electrocatalysts, including transition metal-based materials, metal-nitrogen-carbon composites, and single-atom catalysts. These developments are driven by the need to overcome the inherent challenges of N₂ activation, including the high bond dissociation energy of the N≡N triple bond (941 kJ/mol) and competition with the hydrogen evolution reaction in aqueous media.
The primary technical objective in this field is to establish standardized protocols for ammonia quantification that ensure reproducibility across different research laboratories. Current literature reveals significant discrepancies in reported Faradaic efficiencies and production rates, largely attributed to inconsistent detection methodologies and potential contamination issues that can lead to false positives.
Secondary objectives include improving catalyst selectivity to minimize competing reactions, enhancing nitrogen reduction rates to commercially viable levels, and developing in-situ or operando characterization techniques that can provide real-time insights into reaction mechanisms and intermediate species formation.
The field is currently transitioning from proof-of-concept demonstrations toward more rigorous scientific validation and practical implementation considerations. This shift necessitates standardized testing protocols that can reliably distinguish true electrochemical nitrogen reduction from potential artifacts or contamination sources such as NOx reduction, catalyst decomposition, or ammonia impurities in feedstocks.
Achieving these objectives would represent a significant step toward decentralized, sustainable ammonia production that could revolutionize both the fertilizer industry and potentially enable ammonia's use as a carbon-free energy carrier. The environmental implications are substantial, as electrochemical NRR could dramatically reduce the carbon footprint of ammonia synthesis, which currently accounts for approximately 1-2% of global energy consumption and CO₂ emissions.
The fundamental principle of electrochemical NRR involves the reduction of atmospheric nitrogen (N₂) to ammonia (NH₃) using electricity, ideally derived from renewable sources, in the presence of water as a proton source. This process aims to achieve what nature accomplishes through nitrogenase enzymes in nitrogen-fixing bacteria, but through artificial electrocatalytic systems that can be scaled for industrial application.
Recent technological trends show a shift toward developing more efficient electrocatalysts, including transition metal-based materials, metal-nitrogen-carbon composites, and single-atom catalysts. These developments are driven by the need to overcome the inherent challenges of N₂ activation, including the high bond dissociation energy of the N≡N triple bond (941 kJ/mol) and competition with the hydrogen evolution reaction in aqueous media.
The primary technical objective in this field is to establish standardized protocols for ammonia quantification that ensure reproducibility across different research laboratories. Current literature reveals significant discrepancies in reported Faradaic efficiencies and production rates, largely attributed to inconsistent detection methodologies and potential contamination issues that can lead to false positives.
Secondary objectives include improving catalyst selectivity to minimize competing reactions, enhancing nitrogen reduction rates to commercially viable levels, and developing in-situ or operando characterization techniques that can provide real-time insights into reaction mechanisms and intermediate species formation.
The field is currently transitioning from proof-of-concept demonstrations toward more rigorous scientific validation and practical implementation considerations. This shift necessitates standardized testing protocols that can reliably distinguish true electrochemical nitrogen reduction from potential artifacts or contamination sources such as NOx reduction, catalyst decomposition, or ammonia impurities in feedstocks.
Achieving these objectives would represent a significant step toward decentralized, sustainable ammonia production that could revolutionize both the fertilizer industry and potentially enable ammonia's use as a carbon-free energy carrier. The environmental implications are substantial, as electrochemical NRR could dramatically reduce the carbon footprint of ammonia synthesis, which currently accounts for approximately 1-2% of global energy consumption and CO₂ emissions.
Market Analysis for Ammonia Production Technologies
The global ammonia production market is currently dominated by the Haber-Bosch process, which accounts for approximately 80% of worldwide ammonia production. This century-old technology consumes 1-2% of global energy production and generates significant carbon emissions, estimated at 1.4% of global CO2 emissions. The market size for ammonia production reached $70 billion in 2022 and is projected to grow at a CAGR of 5.3% through 2030, driven primarily by agricultural demands for fertilizers.
Electrochemical nitrogen reduction reaction (NRR) represents a disruptive technology in this space, offering potential for decentralized, renewable-powered ammonia production with significantly lower environmental impact. However, market penetration remains minimal due to technical challenges, particularly in quantification reliability and reproducibility.
The market for standardized protocols and equipment for ammonia quantification in NRR research is emerging as a specialized segment. Current analytical equipment manufacturers like Thermo Fisher Scientific, Agilent Technologies, and Shimadzu Corporation are positioned to expand their offerings to address this need. The estimated market size for specialized ammonia detection and quantification equipment in research settings is approximately $300 million, with projected growth to $450 million by 2027.
Key market drivers include increasing research funding for sustainable ammonia production, with government initiatives in the EU, US, and China allocating over $2 billion collectively toward green ammonia technologies. Academic institutions and industrial R&D departments represent the primary customer segments, with over 200 research groups worldwide actively working on NRR technologies.
Market barriers include the high cost of precise analytical equipment, with specialized spectrophotometric systems ranging from $15,000 to $50,000, and advanced chromatography setups exceeding $100,000. This creates significant entry barriers for smaller research institutions and startups.
Regional analysis shows Asia-Pacific leading in research activity (42% of publications), followed by North America (28%) and Europe (24%). China specifically has demonstrated the most aggressive growth in patent filings related to NRR technologies, with a 300% increase over the past five years.
The market opportunity extends beyond research applications. As standardization improves reliability, commercial applications for distributed ammonia production could emerge, potentially creating a $5-7 billion market for small-scale electrochemical ammonia production systems by 2035, particularly in agricultural regions distant from traditional ammonia production facilities.
Electrochemical nitrogen reduction reaction (NRR) represents a disruptive technology in this space, offering potential for decentralized, renewable-powered ammonia production with significantly lower environmental impact. However, market penetration remains minimal due to technical challenges, particularly in quantification reliability and reproducibility.
The market for standardized protocols and equipment for ammonia quantification in NRR research is emerging as a specialized segment. Current analytical equipment manufacturers like Thermo Fisher Scientific, Agilent Technologies, and Shimadzu Corporation are positioned to expand their offerings to address this need. The estimated market size for specialized ammonia detection and quantification equipment in research settings is approximately $300 million, with projected growth to $450 million by 2027.
Key market drivers include increasing research funding for sustainable ammonia production, with government initiatives in the EU, US, and China allocating over $2 billion collectively toward green ammonia technologies. Academic institutions and industrial R&D departments represent the primary customer segments, with over 200 research groups worldwide actively working on NRR technologies.
Market barriers include the high cost of precise analytical equipment, with specialized spectrophotometric systems ranging from $15,000 to $50,000, and advanced chromatography setups exceeding $100,000. This creates significant entry barriers for smaller research institutions and startups.
Regional analysis shows Asia-Pacific leading in research activity (42% of publications), followed by North America (28%) and Europe (24%). China specifically has demonstrated the most aggressive growth in patent filings related to NRR technologies, with a 300% increase over the past five years.
The market opportunity extends beyond research applications. As standardization improves reliability, commercial applications for distributed ammonia production could emerge, potentially creating a $5-7 billion market for small-scale electrochemical ammonia production systems by 2035, particularly in agricultural regions distant from traditional ammonia production facilities.
Current Challenges in Electrochemical Nitrogen Reduction
Electrochemical Nitrogen Reduction Reaction (NRR) represents a promising approach for sustainable ammonia synthesis under ambient conditions, offering an alternative to the energy-intensive Haber-Bosch process. However, the field faces significant challenges that hinder its advancement toward practical applications and commercial viability.
A primary challenge is the extremely low Faradaic efficiency (FE) typically achieved in NRR systems, often below 10%. This inefficiency stems from the competing hydrogen evolution reaction (HER), which is thermodynamically more favorable than nitrogen reduction. The similar reduction potentials of these reactions create a fundamental selectivity problem that researchers have struggled to overcome.
Ammonia yield rates in current systems remain orders of magnitude below what would be required for industrial relevance. Most reported catalysts produce NH3 at rates of 10^-10 to 10^-11 mol cm^-2 s^-1, whereas commercial viability would require at least 10^-8 mol cm^-2 s^-1. This performance gap represents one of the most significant barriers to practical implementation.
Reproducibility issues plague the field, with many reported high-performance catalysts failing validation in follow-up studies. This crisis of reproducibility stems partly from contamination problems, as trace nitrogen-containing compounds in electrolytes, membranes, or even laboratory air can be mistakenly measured as products of nitrogen reduction.
Quantification methodology presents another critical challenge. The most commonly used methods for ammonia detection—including the indophenol blue method, Nessler's reagent, and ion chromatography—each have limitations regarding sensitivity, selectivity, and reliability at the low concentrations typical of NRR experiments. The lack of standardized protocols for these measurements has led to inconsistent reporting across the literature.
Catalyst stability remains problematic, with many materials showing significant performance degradation after only hours of operation. This instability often results from surface poisoning, structural changes, or leaching of active components during the electrochemical process.
Mechanistic understanding of the NRR pathway is still incomplete, hampering rational catalyst design. The reaction involves multiple electron and proton transfer steps, with several possible intermediates and pathways whose energetics and kinetics remain poorly understood at the molecular level.
Scaling up NRR systems introduces additional challenges related to electrode architecture, electrolyte management, and system integration. Most research focuses on small-scale laboratory setups that may not translate directly to larger systems due to mass transport limitations, heat management issues, and engineering constraints.
A primary challenge is the extremely low Faradaic efficiency (FE) typically achieved in NRR systems, often below 10%. This inefficiency stems from the competing hydrogen evolution reaction (HER), which is thermodynamically more favorable than nitrogen reduction. The similar reduction potentials of these reactions create a fundamental selectivity problem that researchers have struggled to overcome.
Ammonia yield rates in current systems remain orders of magnitude below what would be required for industrial relevance. Most reported catalysts produce NH3 at rates of 10^-10 to 10^-11 mol cm^-2 s^-1, whereas commercial viability would require at least 10^-8 mol cm^-2 s^-1. This performance gap represents one of the most significant barriers to practical implementation.
Reproducibility issues plague the field, with many reported high-performance catalysts failing validation in follow-up studies. This crisis of reproducibility stems partly from contamination problems, as trace nitrogen-containing compounds in electrolytes, membranes, or even laboratory air can be mistakenly measured as products of nitrogen reduction.
Quantification methodology presents another critical challenge. The most commonly used methods for ammonia detection—including the indophenol blue method, Nessler's reagent, and ion chromatography—each have limitations regarding sensitivity, selectivity, and reliability at the low concentrations typical of NRR experiments. The lack of standardized protocols for these measurements has led to inconsistent reporting across the literature.
Catalyst stability remains problematic, with many materials showing significant performance degradation after only hours of operation. This instability often results from surface poisoning, structural changes, or leaching of active components during the electrochemical process.
Mechanistic understanding of the NRR pathway is still incomplete, hampering rational catalyst design. The reaction involves multiple electron and proton transfer steps, with several possible intermediates and pathways whose energetics and kinetics remain poorly understood at the molecular level.
Scaling up NRR systems introduces additional challenges related to electrode architecture, electrolyte management, and system integration. Most research focuses on small-scale laboratory setups that may not translate directly to larger systems due to mass transport limitations, heat management issues, and engineering constraints.
Standardized Protocols for Ammonia Detection
01 Electrochemical methods for nitrogen reduction to ammonia
Various electrochemical approaches for reducing nitrogen to ammonia under ambient conditions. These methods typically involve specialized catalysts, electrode materials, and reaction conditions to facilitate the nitrogen reduction reaction (NRR). The electrochemical processes offer advantages such as operation at room temperature and atmospheric pressure, making them more energy-efficient compared to traditional ammonia synthesis methods.- Electrochemical methods for nitrogen reduction to ammonia: Electrochemical processes can be used to reduce nitrogen to ammonia under ambient conditions. These methods typically involve catalysts, electrodes, and specific reaction conditions to facilitate the nitrogen reduction reaction (NRR). The electrochemical approach offers advantages such as operation at room temperature and atmospheric pressure, making it more energy-efficient compared to traditional ammonia synthesis methods.
- Ammonia quantification techniques for NRR evaluation: Various analytical methods are employed to accurately quantify the ammonia produced during electrochemical nitrogen reduction. These include spectrophotometric methods (such as indophenol blue method), ion chromatography, nuclear magnetic resonance spectroscopy, and colorimetric assays. Precise quantification is crucial for determining the efficiency and performance of the nitrogen reduction reaction and ensuring reliable research outcomes.
- Catalyst materials for enhanced NRR performance: Different catalyst materials significantly impact the efficiency and selectivity of electrochemical nitrogen reduction. Metal-based catalysts (including noble metals, transition metals, and their alloys), metal oxides, nitrides, and carbon-based materials have been investigated. The development of advanced catalysts with high nitrogen adsorption capability, appropriate binding energy, and stability is essential for improving ammonia yield rates and Faradaic efficiency.
- Reproducibility challenges in electrochemical nitrogen reduction: Ensuring reproducibility in electrochemical nitrogen reduction experiments presents significant challenges. Factors affecting reproducibility include contamination from nitrogen-containing compounds, ammonia from the environment, catalyst preparation methods, electrode surface conditions, and experimental setup variations. Standardized protocols, rigorous control experiments, and systematic reporting of experimental conditions are necessary to address these challenges and validate research findings.
- Reactor design and system optimization for NRR: The design of electrochemical reactors and optimization of reaction systems play crucial roles in ammonia synthesis efficiency. Factors such as electrode configuration, electrolyte composition, membrane selection, gas diffusion layers, and operating parameters (pH, temperature, pressure, applied potential) significantly influence the nitrogen reduction performance. Advanced reactor designs aim to enhance mass transfer, increase active surface area, and improve overall system efficiency.
02 Quantification techniques for ammonia detection
Analytical methods for accurate quantification of ammonia produced during electrochemical nitrogen reduction. These include spectrophotometric methods (such as indophenol blue method), ion chromatography, nuclear magnetic resonance spectroscopy, and electrochemical sensing techniques. Precise quantification is crucial for determining the efficiency and performance of nitrogen reduction reaction systems.Expand Specific Solutions03 Catalyst design for improved NRR performance
Development of advanced catalysts to enhance the efficiency and selectivity of electrochemical nitrogen reduction. These catalysts include metal-based materials, metal oxides, nitrides, single-atom catalysts, and carbon-based materials with specific structural modifications. Catalyst design focuses on increasing active sites, optimizing binding energies, and improving electron transfer to achieve higher ammonia yields.Expand Specific Solutions04 Reproducibility protocols and standardization
Methodologies and protocols to ensure reproducibility in electrochemical nitrogen reduction experiments. These include standardized testing conditions, control experiments to eliminate contamination sources, benchmark procedures, and reporting guidelines. Addressing reproducibility challenges is essential for validating research findings and enabling meaningful comparisons between different studies in the field.Expand Specific Solutions05 System design and operational parameters
Engineering aspects of electrochemical nitrogen reduction systems, including cell configuration, electrode design, electrolyte composition, and operational parameters. Optimization of these factors significantly impacts ammonia yield, Faradaic efficiency, and system stability. Considerations include membrane selection, gas diffusion layer design, electrolyte pH, applied potential, and temperature control to maximize performance.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrochemical nitrogen reduction field for ammonia quantification standardization is currently in an early growth phase, with market size expanding as green ammonia production gains traction in sustainable agriculture and energy storage applications. The technology remains in developmental stages with moderate maturity, as evidenced by the diverse institutional involvement. Academic institutions like Fudan University, Monash University, and Colorado State University are leading fundamental research, while companies such as Huawei Technologies, Janssen Pharmaceutica, and Codexis are exploring commercial applications. Research organizations including Korea Institute of Energy Research and KIST are bridging the gap between academic discoveries and industrial implementation. The competitive landscape shows strong international collaboration with participants from Asia, North America, Europe, and Australia working to establish reliable protocols that can accelerate technology commercialization and standardization.
Monash University
Technical Solution: Monash University has developed comprehensive standardized protocols for ammonia quantification in electrochemical nitrogen reduction reactions (NRR), focusing on eliminating false positives and ensuring reproducibility. Their approach includes rigorous control experiments with isotope labeling (15N2) to verify the nitrogen source, combined with multiple complementary detection methods such as nuclear magnetic resonance (NMR) spectroscopy, ion chromatography, and colorimetric methods (indophenol blue). The university has established a multi-step verification framework that includes catalyst pre-cleaning procedures to remove nitrogen-containing contaminants, quantification of background ammonia levels, and statistical validation across multiple experimental runs. Their protocols specifically address common contamination sources including air, reagents, and membrane materials that can lead to overestimation of NRR performance.
Strengths: Comprehensive multi-technique verification approach provides higher confidence in results; isotope labeling methodology offers definitive proof of nitrogen source; established clear guidelines for background correction. Weaknesses: Isotope labeling techniques require specialized equipment not available in all laboratories; protocols may be time-intensive and costly for routine implementation; some standardized methods may not be sensitive enough for ultra-low ammonia concentrations.
Korea Institute of Energy Research
Technical Solution: The Korea Institute of Energy Research (KIER) has pioneered standardized protocols for electrochemical nitrogen reduction with a focus on in-situ ammonia quantification techniques. Their approach integrates real-time monitoring systems using ion-selective electrodes and spectroelectrochemical methods that allow for continuous measurement during the NRR process. KIER has developed specialized electrochemical cells with isolated compartments to prevent cross-contamination and ammonia loss during quantification. Their protocols emphasize reproducibility through automated testing platforms that control environmental variables including temperature, pressure, and electrolyte purity. The institute has also established calibration standards specifically for electrochemical systems, accounting for matrix effects in complex electrolytes. KIER's methodology includes systematic procedures for electrode preparation, electrolyte purification, and gas feed purification to minimize nitrogen-containing impurities that could lead to false positive results in ammonia detection.
Strengths: In-situ monitoring capabilities provide real-time performance data; automated systems ensure consistent testing conditions across experiments; specialized cell designs minimize contamination. Weaknesses: Custom equipment requirements may limit adoption by other research groups; protocols may be optimized for specific catalyst types and might require modification for novel materials; higher implementation costs compared to conventional batch testing methods.
Key Innovations in Electrochemical NRR Reproducibility
A method of oxidising an inorganic amine to nitrate
PatentWO2023201394A1
Innovation
- A method involving the use of a titanium dioxide photocatalyst contacted with a phosphorous-based species, such as phosphate, to photocatalytically oxidize inorganic amines to nitrates at neutral or acidic pH values, minimizing metal cations and achieving high conversion rates.
Environmental Impact Assessment of Electrochemical NRR
The environmental implications of electrochemical nitrogen reduction reaction (NRR) technologies extend far beyond their technical capabilities. When properly implemented, these systems offer significant potential for reducing greenhouse gas emissions compared to conventional ammonia production methods. The Haber-Bosch process, which currently dominates industrial ammonia synthesis, consumes approximately 1-2% of global energy and generates substantial CO2 emissions. In contrast, electrochemical NRR powered by renewable electricity presents a pathway toward carbon-neutral ammonia production.
However, comprehensive life cycle assessments (LCAs) of electrochemical NRR systems reveal complex environmental trade-offs. While operational emissions may decrease, the environmental footprint associated with catalyst materials—particularly those utilizing precious metals or rare earth elements—must be carefully evaluated. Mining, refining, and processing these materials can generate significant upstream environmental impacts, including habitat disruption, water pollution, and energy consumption.
Water usage represents another critical environmental consideration for electrochemical NRR implementation. These systems require ultrapure water to maintain efficiency and prevent catalyst poisoning. The purification processes necessary to achieve this water quality standard are energy-intensive and may strain local water resources in water-scarce regions.
The standardization of ammonia quantification protocols directly influences environmental impact assessments by ensuring accurate measurement of conversion efficiencies. Overestimated production rates in research literature have historically led to overly optimistic environmental benefit projections. More rigorous and standardized quantification methods enable more realistic environmental impact modeling and prevent greenwashing in technology development.
Electrolyte selection and management present additional environmental considerations. Many NRR systems utilize specialized electrolytes containing potentially hazardous components that require proper handling and disposal protocols. The environmental fate of these materials must be incorporated into comprehensive impact assessments.
Scaling considerations further complicate environmental evaluations. Laboratory-scale demonstrations often fail to account for the additional resource requirements and efficiency losses that occur during industrial implementation. Standardized protocols that address scale-dependent factors are essential for accurate environmental impact projections.
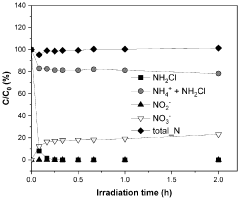
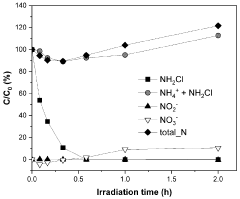
The potential for unintended nitrogen emissions during electrochemical NRR operation must also be monitored. Incomplete conversion or side reactions may produce NOx compounds or other reactive nitrogen species that contribute to air pollution and ecosystem eutrophication if released. Standardized protocols that quantify all nitrogen-containing products, not just ammonia, are necessary for complete environmental assessment.
However, comprehensive life cycle assessments (LCAs) of electrochemical NRR systems reveal complex environmental trade-offs. While operational emissions may decrease, the environmental footprint associated with catalyst materials—particularly those utilizing precious metals or rare earth elements—must be carefully evaluated. Mining, refining, and processing these materials can generate significant upstream environmental impacts, including habitat disruption, water pollution, and energy consumption.
Water usage represents another critical environmental consideration for electrochemical NRR implementation. These systems require ultrapure water to maintain efficiency and prevent catalyst poisoning. The purification processes necessary to achieve this water quality standard are energy-intensive and may strain local water resources in water-scarce regions.
The standardization of ammonia quantification protocols directly influences environmental impact assessments by ensuring accurate measurement of conversion efficiencies. Overestimated production rates in research literature have historically led to overly optimistic environmental benefit projections. More rigorous and standardized quantification methods enable more realistic environmental impact modeling and prevent greenwashing in technology development.
Electrolyte selection and management present additional environmental considerations. Many NRR systems utilize specialized electrolytes containing potentially hazardous components that require proper handling and disposal protocols. The environmental fate of these materials must be incorporated into comprehensive impact assessments.
Scaling considerations further complicate environmental evaluations. Laboratory-scale demonstrations often fail to account for the additional resource requirements and efficiency losses that occur during industrial implementation. Standardized protocols that address scale-dependent factors are essential for accurate environmental impact projections.
The potential for unintended nitrogen emissions during electrochemical NRR operation must also be monitored. Incomplete conversion or side reactions may produce NOx compounds or other reactive nitrogen species that contribute to air pollution and ecosystem eutrophication if released. Standardized protocols that quantify all nitrogen-containing products, not just ammonia, are necessary for complete environmental assessment.
Benchmarking Criteria for NRR Performance Evaluation
Establishing reliable benchmarking criteria for Nitrogen Reduction Reaction (NRR) performance evaluation is essential for advancing electrochemical nitrogen reduction technology. Current research in this field suffers from inconsistent reporting standards, making cross-study comparisons challenging and hindering technological progress.
A comprehensive benchmarking framework must include standardized metrics for catalyst performance assessment. Faradaic Efficiency (FE) and ammonia yield rate should be reported with clearly defined calculation methodologies. The scientific community increasingly recognizes that partial current density toward ammonia production provides a more meaningful performance indicator than overall current density.
Experimental conditions must be rigorously standardized and reported. This includes electrode preparation protocols, electrolyte composition, applied potential (with conversion to RHE scale), temperature, pressure, and gas flow rates. The electrochemical cell configuration should be thoroughly documented with dimensions, materials, and membrane specifications when applicable.
Control experiments represent a critical component of reliable benchmarking. Isotope labeling studies using 15N2 are becoming the gold standard for confirming nitrogen origin. Ar-purged control experiments and open-circuit potential tests should be mandatory to rule out contamination sources. Catalyst stability testing protocols should specify minimum operation times (typically >10 hours) with consistent performance reporting intervals.
Analytical validation requires multiple independent ammonia detection methods. The indophenol blue method, ion chromatography, and NMR spectroscopy should be calibrated with standard curves covering the expected concentration range. Detection limits must be clearly stated, and statistical analysis of replicate measurements should be included to establish confidence intervals.
Reproducibility standards should mandate multiple experimental runs with freshly prepared catalysts and electrolytes. Inter-laboratory validation is increasingly valued, with detailed protocols enabling external verification. Raw data sharing through repositories is becoming an expected practice for high-impact publications.
Economic viability metrics are gaining importance in benchmarking criteria. Energy efficiency calculations, catalyst cost analysis, and theoretical maximum production rates provide context for practical applications. Lifecycle assessment considerations are emerging as additional evaluation criteria for sustainable technology development.
A comprehensive benchmarking framework must include standardized metrics for catalyst performance assessment. Faradaic Efficiency (FE) and ammonia yield rate should be reported with clearly defined calculation methodologies. The scientific community increasingly recognizes that partial current density toward ammonia production provides a more meaningful performance indicator than overall current density.
Experimental conditions must be rigorously standardized and reported. This includes electrode preparation protocols, electrolyte composition, applied potential (with conversion to RHE scale), temperature, pressure, and gas flow rates. The electrochemical cell configuration should be thoroughly documented with dimensions, materials, and membrane specifications when applicable.
Control experiments represent a critical component of reliable benchmarking. Isotope labeling studies using 15N2 are becoming the gold standard for confirming nitrogen origin. Ar-purged control experiments and open-circuit potential tests should be mandatory to rule out contamination sources. Catalyst stability testing protocols should specify minimum operation times (typically >10 hours) with consistent performance reporting intervals.
Analytical validation requires multiple independent ammonia detection methods. The indophenol blue method, ion chromatography, and NMR spectroscopy should be calibrated with standard curves covering the expected concentration range. Detection limits must be clearly stated, and statistical analysis of replicate measurements should be included to establish confidence intervals.
Reproducibility standards should mandate multiple experimental runs with freshly prepared catalysts and electrolytes. Inter-laboratory validation is increasingly valued, with detailed protocols enabling external verification. Raw data sharing through repositories is becoming an expected practice for high-impact publications.
Economic viability metrics are gaining importance in benchmarking criteria. Energy efficiency calculations, catalyst cost analysis, and theoretical maximum production rates provide context for practical applications. Lifecycle assessment considerations are emerging as additional evaluation criteria for sustainable technology development.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!