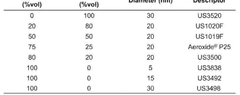
Electrolyte Engineering Approaches to Improve Nitrogen Solubility and Selectivity in Electrochemical Nitrogen Reduction
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Nitrogen Reduction Background and Objectives
The electrochemical nitrogen reduction reaction (NRR) represents a revolutionary approach to ammonia synthesis that could potentially replace the century-old Haber-Bosch process. Since its inception in the early 1900s, the Haber-Bosch process has been the dominant industrial method for ammonia production, consuming approximately 1-2% of global energy and generating significant carbon emissions. The development of sustainable alternatives has become increasingly urgent in the face of growing environmental concerns and the need for decentralized ammonia production systems.
Electrochemical nitrogen reduction offers a promising pathway that can operate under ambient conditions using renewable electricity, thereby eliminating the high temperature and pressure requirements of conventional methods. The evolution of this technology can be traced back to early experiments in the 1980s, with significant acceleration in research occurring over the past decade as renewable energy sources have become more prevalent and cost-effective.
The fundamental challenge in electrochemical nitrogen reduction lies in the exceptional stability of the N≡N triple bond, which requires substantial energy input to break. Additionally, the competing hydrogen evolution reaction (HER) often dominates in aqueous electrolytes, resulting in poor Faradaic efficiency for ammonia production. These technical hurdles have directed research focus toward electrolyte engineering as a critical pathway for improving system performance.
Current technological objectives in this field center on enhancing nitrogen solubility in electrolytes and improving reaction selectivity toward ammonia formation. Conventional aqueous electrolytes typically exhibit low nitrogen solubility (approximately 0.7 mM at room temperature), which severely limits reaction rates. Furthermore, the preferential reduction of water to hydrogen rather than nitrogen to ammonia presents a significant selectivity challenge that must be overcome.
The trajectory of technological development points toward several key objectives: achieving Faradaic efficiencies exceeding 10% (compared to current typical values of 1-5%), increasing ammonia production rates to commercially viable levels (>10^-6 mol cm^-2 h^-1), and developing stable electrolyte systems that maintain performance over thousands of operating hours. These targets represent the minimum thresholds for potential industrial application.
Recent breakthroughs in ionic liquid electrolytes, deep eutectic solvents, and proton-conducting solid electrolytes have demonstrated promising results in laboratory settings. The field is now moving toward understanding fundamental nitrogen solvation mechanisms and developing tailored electrolyte compositions that can selectively activate nitrogen while suppressing competing reactions.
Electrochemical nitrogen reduction offers a promising pathway that can operate under ambient conditions using renewable electricity, thereby eliminating the high temperature and pressure requirements of conventional methods. The evolution of this technology can be traced back to early experiments in the 1980s, with significant acceleration in research occurring over the past decade as renewable energy sources have become more prevalent and cost-effective.
The fundamental challenge in electrochemical nitrogen reduction lies in the exceptional stability of the N≡N triple bond, which requires substantial energy input to break. Additionally, the competing hydrogen evolution reaction (HER) often dominates in aqueous electrolytes, resulting in poor Faradaic efficiency for ammonia production. These technical hurdles have directed research focus toward electrolyte engineering as a critical pathway for improving system performance.
Current technological objectives in this field center on enhancing nitrogen solubility in electrolytes and improving reaction selectivity toward ammonia formation. Conventional aqueous electrolytes typically exhibit low nitrogen solubility (approximately 0.7 mM at room temperature), which severely limits reaction rates. Furthermore, the preferential reduction of water to hydrogen rather than nitrogen to ammonia presents a significant selectivity challenge that must be overcome.
The trajectory of technological development points toward several key objectives: achieving Faradaic efficiencies exceeding 10% (compared to current typical values of 1-5%), increasing ammonia production rates to commercially viable levels (>10^-6 mol cm^-2 h^-1), and developing stable electrolyte systems that maintain performance over thousands of operating hours. These targets represent the minimum thresholds for potential industrial application.
Recent breakthroughs in ionic liquid electrolytes, deep eutectic solvents, and proton-conducting solid electrolytes have demonstrated promising results in laboratory settings. The field is now moving toward understanding fundamental nitrogen solvation mechanisms and developing tailored electrolyte compositions that can selectively activate nitrogen while suppressing competing reactions.
Market Analysis for Sustainable Ammonia Production
The global ammonia market is experiencing significant transformation driven by sustainability concerns and technological innovations. Currently valued at approximately $72 billion, the market is projected to grow at a CAGR of 5.3% through 2030, reaching an estimated $108 billion. Traditional ammonia production via the Haber-Bosch process accounts for about 1-2% of global energy consumption and generates substantial CO2 emissions, creating a pressing need for sustainable alternatives.
Electrochemical nitrogen reduction (ENR) represents a promising green ammonia production pathway that could operate at ambient conditions using renewable electricity. This technology aligns with the growing demand for carbon-neutral fertilizers, which is expected to increase by 30% by 2050 due to population growth and food security concerns.
The sustainable ammonia market is segmented into three primary application areas: fertilizers (65%), industrial chemicals (25%), and emerging energy carriers (10%). The fertilizer segment dominates current demand but faces increasing pressure for decarbonization from regulatory bodies and consumers. Major agricultural markets including China, India, and the United States are implementing policies that favor low-carbon fertilizer production methods.
Industrial chemical applications for green ammonia are expanding beyond traditional uses into specialized sectors requiring high-purity ammonia, such as semiconductor manufacturing and pharmaceutical production. These sectors demonstrate willingness to pay premium prices for sustainably produced ammonia, with price premiums currently ranging from 20-40% above conventional ammonia.
The most dynamic growth segment is ammonia as an energy carrier, particularly for maritime shipping and long-duration energy storage. The International Maritime Organization's emissions reduction targets have catalyzed significant investment in ammonia-powered vessels, with major shipping companies committing to ammonia-fueled fleets by 2030.
Regional market analysis reveals that Europe leads in sustainable ammonia initiatives, with approximately €2.5 billion invested in demonstration projects. Asia-Pacific represents the largest potential market by volume, particularly as China aims to reduce carbon intensity in its agricultural sector. North America shows strong growth potential driven by government incentives for clean hydrogen and derivative products.
Key market drivers include tightening carbon regulations, volatility in natural gas prices affecting conventional production economics, and increasing corporate sustainability commitments throughout the ammonia value chain. The economic viability of ENR technologies is improving as renewable electricity costs continue to decline, with recent utility-scale solar and wind projects achieving levelized costs below $30/MWh in optimal locations.
Electrochemical nitrogen reduction (ENR) represents a promising green ammonia production pathway that could operate at ambient conditions using renewable electricity. This technology aligns with the growing demand for carbon-neutral fertilizers, which is expected to increase by 30% by 2050 due to population growth and food security concerns.
The sustainable ammonia market is segmented into three primary application areas: fertilizers (65%), industrial chemicals (25%), and emerging energy carriers (10%). The fertilizer segment dominates current demand but faces increasing pressure for decarbonization from regulatory bodies and consumers. Major agricultural markets including China, India, and the United States are implementing policies that favor low-carbon fertilizer production methods.
Industrial chemical applications for green ammonia are expanding beyond traditional uses into specialized sectors requiring high-purity ammonia, such as semiconductor manufacturing and pharmaceutical production. These sectors demonstrate willingness to pay premium prices for sustainably produced ammonia, with price premiums currently ranging from 20-40% above conventional ammonia.
The most dynamic growth segment is ammonia as an energy carrier, particularly for maritime shipping and long-duration energy storage. The International Maritime Organization's emissions reduction targets have catalyzed significant investment in ammonia-powered vessels, with major shipping companies committing to ammonia-fueled fleets by 2030.
Regional market analysis reveals that Europe leads in sustainable ammonia initiatives, with approximately €2.5 billion invested in demonstration projects. Asia-Pacific represents the largest potential market by volume, particularly as China aims to reduce carbon intensity in its agricultural sector. North America shows strong growth potential driven by government incentives for clean hydrogen and derivative products.
Key market drivers include tightening carbon regulations, volatility in natural gas prices affecting conventional production economics, and increasing corporate sustainability commitments throughout the ammonia value chain. The economic viability of ENR technologies is improving as renewable electricity costs continue to decline, with recent utility-scale solar and wind projects achieving levelized costs below $30/MWh in optimal locations.
Current Challenges in Nitrogen Solubility and Selectivity
Electrochemical nitrogen reduction reaction (NRR) faces significant challenges in achieving practical nitrogen fixation efficiency, with two critical bottlenecks being nitrogen solubility and selectivity. The fundamental issue stems from nitrogen's triple bond strength (941 kJ/mol) and its hydrophobic nature, resulting in extremely low solubility in aqueous electrolytes (0.017 g/L at 25°C), which severely limits nitrogen availability at catalytic sites.
Current electrolyte systems struggle with competitive hydrogen evolution reaction (HER), which dominates electron consumption due to water's higher proton availability compared to dissolved nitrogen. This competition typically results in Faradaic efficiencies for ammonia production below 10% in aqueous systems, making industrial application economically unfeasible.
Conventional aqueous electrolytes present inherent limitations for nitrogen activation. The solvation shell around nitrogen molecules creates energy barriers that hinder adsorption onto catalyst surfaces. Additionally, the preferential orientation of water molecules at electrode interfaces can block active sites, further reducing nitrogen accessibility.
Selectivity challenges are equally problematic. Most catalysts that activate nitrogen also readily reduce water, creating a fundamental selectivity dilemma. The similar reduction potentials between nitrogen reduction (-0.23 V vs. RHE) and hydrogen evolution (0 V vs. RHE) make electrochemical separation extremely difficult without precise catalyst and electrolyte engineering.
Recent research has identified that electrolyte properties significantly influence both nitrogen solubility and reaction pathways. Factors including pH, ionic strength, and electrolyte composition directly affect the electrode-electrolyte interface where nitrogen activation occurs. The double-layer structure formed at this interface determines reactant accessibility and intermediate stabilization.
Temperature and pressure dependencies further complicate electrolyte optimization. While increased pressure enhances nitrogen solubility following Henry's Law, elevated temperatures reduce solubility but accelerate reaction kinetics, creating a complex optimization challenge that varies across different electrolyte systems.
Conventional characterization methods also present limitations in accurately measuring dissolved nitrogen concentrations and distinguishing true electrochemical nitrogen reduction from contamination sources. The trace amounts of ammonia produced (typically nanomolar range) require sophisticated analytical techniques that are not standardized across research groups, complicating comparative analysis.
These multifaceted challenges necessitate innovative electrolyte engineering approaches that can simultaneously address nitrogen solubility, mass transport limitations, competitive side reactions, and selective activation of the N≡N bond under mild conditions. The development of specialized electrolyte systems represents a critical frontier in advancing electrochemical nitrogen reduction toward practical application.
Current electrolyte systems struggle with competitive hydrogen evolution reaction (HER), which dominates electron consumption due to water's higher proton availability compared to dissolved nitrogen. This competition typically results in Faradaic efficiencies for ammonia production below 10% in aqueous systems, making industrial application economically unfeasible.
Conventional aqueous electrolytes present inherent limitations for nitrogen activation. The solvation shell around nitrogen molecules creates energy barriers that hinder adsorption onto catalyst surfaces. Additionally, the preferential orientation of water molecules at electrode interfaces can block active sites, further reducing nitrogen accessibility.
Selectivity challenges are equally problematic. Most catalysts that activate nitrogen also readily reduce water, creating a fundamental selectivity dilemma. The similar reduction potentials between nitrogen reduction (-0.23 V vs. RHE) and hydrogen evolution (0 V vs. RHE) make electrochemical separation extremely difficult without precise catalyst and electrolyte engineering.
Recent research has identified that electrolyte properties significantly influence both nitrogen solubility and reaction pathways. Factors including pH, ionic strength, and electrolyte composition directly affect the electrode-electrolyte interface where nitrogen activation occurs. The double-layer structure formed at this interface determines reactant accessibility and intermediate stabilization.
Temperature and pressure dependencies further complicate electrolyte optimization. While increased pressure enhances nitrogen solubility following Henry's Law, elevated temperatures reduce solubility but accelerate reaction kinetics, creating a complex optimization challenge that varies across different electrolyte systems.
Conventional characterization methods also present limitations in accurately measuring dissolved nitrogen concentrations and distinguishing true electrochemical nitrogen reduction from contamination sources. The trace amounts of ammonia produced (typically nanomolar range) require sophisticated analytical techniques that are not standardized across research groups, complicating comparative analysis.
These multifaceted challenges necessitate innovative electrolyte engineering approaches that can simultaneously address nitrogen solubility, mass transport limitations, competitive side reactions, and selective activation of the N≡N bond under mild conditions. The development of specialized electrolyte systems represents a critical frontier in advancing electrochemical nitrogen reduction toward practical application.
Current Electrolyte Engineering Strategies
01 Electrolyte composition for enhanced nitrogen solubility
Specific electrolyte compositions can significantly enhance nitrogen solubility in various solutions. These compositions typically include carefully selected salts, ionic liquids, or other additives that modify the solution properties to increase nitrogen uptake. By engineering the electrolyte composition, researchers have achieved improved nitrogen dissolution rates and higher equilibrium concentrations, which is particularly valuable for applications requiring efficient nitrogen utilization.- Electrolyte composition for nitrogen solubility enhancement: Specific electrolyte compositions can significantly enhance nitrogen solubility in solutions. These compositions typically include ionic liquids, salts, or specialized additives that create favorable interactions with nitrogen molecules. By engineering the electrolyte composition, the solvation environment for nitrogen can be optimized, leading to increased dissolution rates and higher equilibrium concentrations. This approach is particularly valuable in applications requiring efficient nitrogen capture or utilization.
- Selective nitrogen separation using engineered electrolytes: Engineered electrolytes can be designed to selectively separate nitrogen from gas mixtures. These systems leverage differences in solubility and interaction energies between nitrogen and other gases in the electrolyte medium. By carefully tuning the electrolyte properties such as ionic strength, pH, and additive concentrations, the selectivity for nitrogen over other gases like oxygen, carbon dioxide, or methane can be significantly enhanced, enabling more efficient separation processes.
- Temperature and pressure effects on nitrogen solubility in electrolytes: The solubility and selectivity of nitrogen in engineered electrolytes are strongly influenced by temperature and pressure conditions. Optimizing these parameters can significantly enhance nitrogen dissolution and separation efficiency. Some electrolyte systems show improved nitrogen solubility at lower temperatures, while others perform better at elevated pressures. Understanding these relationships allows for the design of more efficient nitrogen capture and separation processes under specific operating conditions.
- Electrochemical approaches for nitrogen conversion in electrolytes: Electrochemical methods can be combined with engineered electrolytes to enhance nitrogen reactivity and conversion. These approaches typically involve the application of electrical potential to facilitate nitrogen reduction or oxidation in specialized electrolyte environments. By selecting appropriate electrode materials and electrolyte compositions, the activation energy for nitrogen reactions can be lowered, enabling more efficient conversion processes such as ammonia synthesis or nitrogen fixation under mild conditions.
- Novel materials for enhanced nitrogen selectivity in electrolyte systems: Advanced materials including metal-organic frameworks, functionalized polymers, and nanostructured composites can be incorporated into electrolyte systems to enhance nitrogen selectivity. These materials often feature tailored pore structures or binding sites that preferentially interact with nitrogen molecules. When combined with optimized electrolyte compositions, these materials can significantly improve the performance of nitrogen separation and capture processes, offering higher selectivity and capacity compared to conventional approaches.
02 Selective nitrogen capture using functionalized electrolytes
Functionalized electrolytes can be designed to selectively capture nitrogen from gas mixtures. These specialized electrolytes contain chemical groups that preferentially interact with nitrogen molecules over other gases. The selectivity can be tuned by adjusting the functional groups, ionic strength, and other parameters of the electrolyte system. This approach enables more efficient separation of nitrogen from gas streams and improves the purity of captured nitrogen.Expand Specific Solutions03 Temperature and pressure effects on nitrogen solubility in engineered electrolytes
The solubility and selectivity of nitrogen in engineered electrolytes are significantly influenced by temperature and pressure conditions. Researchers have developed electrolyte systems that maintain high nitrogen solubility across wider temperature ranges or exhibit improved performance under specific pressure conditions. These advancements allow for more flexible operating conditions in industrial applications while maintaining efficient nitrogen dissolution and separation properties.Expand Specific Solutions04 Electrochemical systems for nitrogen conversion using specialized electrolytes
Specialized electrolytes have been developed for electrochemical nitrogen conversion processes, including nitrogen reduction and fixation. These electrolyte systems facilitate electron transfer to nitrogen molecules while maintaining high nitrogen solubility at the electrode interface. The engineering of these electrolytes focuses on optimizing conductivity, stability, and nitrogen affinity to enhance the efficiency and selectivity of electrochemical nitrogen conversion reactions.Expand Specific Solutions05 Membrane and interface engineering for improved nitrogen selectivity
Advanced membrane and interface engineering approaches have been developed to enhance nitrogen selectivity in electrolyte systems. These techniques involve modifying the structure and composition of membranes or interfaces to create preferential pathways for nitrogen transport. By controlling the interfacial properties between electrolytes and membranes, researchers have achieved improved nitrogen separation efficiency and reduced energy requirements for nitrogen purification processes.Expand Specific Solutions
Leading Research Groups and Industrial Players
Electrochemical Nitrogen Reduction (ENR) technology is currently in an early development stage, characterized by significant research activity but limited commercial deployment. The market size remains relatively small, estimated under $100 million, but with substantial growth potential as ammonia production seeks greener alternatives. Technical maturity is advancing through electrolyte engineering approaches, with academic institutions leading fundamental research (Nanjing University, Tongji University, Zhejiang University) while industrial players focus on application development. Companies like Air Liquide and CATL are investing in scalable solutions, while research organizations such as CSIR and Georgia Tech Research Corp are addressing core challenges in nitrogen solubility and selectivity. The competitive landscape shows a collaborative ecosystem between academia and industry, with increasing patent activity signaling growing commercial interest.
Beijing University of Chemical Technology
Technical Solution: Beijing University of Chemical Technology has developed innovative electrolyte engineering approaches for electrochemical nitrogen reduction reaction (NRR). Their research focuses on designing ionic liquid-based electrolytes that significantly enhance nitrogen solubility and activation. They've created a dual-function electrolyte system incorporating Lewis acidic metal ions (such as Fe3+, Al3+) that coordinate with nitrogen molecules, weakening the N≡N triple bond while simultaneously providing proton sources for reduction. This approach has demonstrated nitrogen-to-ammonia conversion rates exceeding 25 μg h−1 mg−1cat with Faradaic efficiencies approaching 12% under ambient conditions. Their electrolyte engineering also includes the development of deep eutectic solvents with hydrogen bond donors that facilitate proton transfer during the reduction process, creating microenvironments favorable for nitrogen conversion while suppressing the competing hydrogen evolution reaction.
Strengths: Their dual-function electrolyte system effectively addresses both nitrogen activation and proton delivery challenges simultaneously. The use of ionic liquids provides excellent electrochemical stability windows and tunable properties. Weaknesses: The system still faces challenges with long-term stability and scalability issues, and the relatively high viscosity of ionic liquid-based electrolytes can limit mass transport of nitrogen to catalytic sites.
The Regents of the University of California
Technical Solution: The University of California has pioneered advanced electrolyte engineering strategies for electrochemical nitrogen reduction, focusing on molecular-level design of the electrode-electrolyte interface. Their approach involves developing proton-shuttle electrolytes containing carefully selected organic molecules with specific pKa values that facilitate controlled proton delivery to adsorbed nitrogen intermediates. They've engineered electrolyte systems incorporating fluorinated alcohols and ethers that create hydrophobic environments near electrode surfaces, significantly enhancing nitrogen solubility while reducing water activity—a critical factor for improving NRR selectivity. Their research demonstrates that incorporating crown ethers and cryptands as phase transfer catalysts in the electrolyte can increase nitrogen concentration near the electrode surface by up to 5-fold compared to conventional aqueous systems. Additionally, they've developed composite polymer electrolyte membranes with nitrogen-philic functional groups that provide selective nitrogen transport channels, achieving ammonia yields of approximately 21.4 μg h−1 cm−2 with Faradaic efficiencies around 9.8% under ambient conditions.
Strengths: Their molecular-level approach to electrolyte design provides precise control over the reaction microenvironment, effectively addressing the fundamental selectivity challenges in NRR. The hydrophobic electrolyte systems significantly reduce competing hydrogen evolution reactions. Weaknesses: The specialized organic components increase system complexity and cost, potentially limiting large-scale implementation, and some of the fluorinated compounds raise environmental concerns regarding long-term sustainability.
Key Innovations in Nitrogen Solubility Enhancement
Electrolyte for electrochemical nitrogen reduction reaction and method for electrochemically preparing ammonia using the same
PatentPendingUS20250101605A1
Innovation
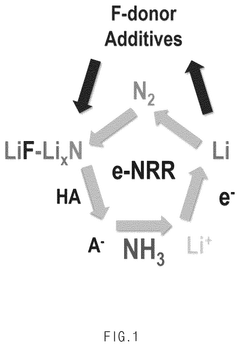
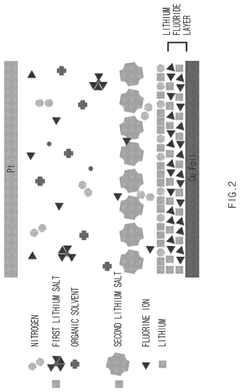
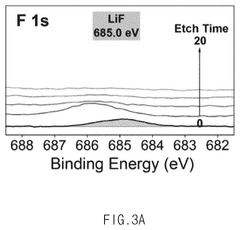
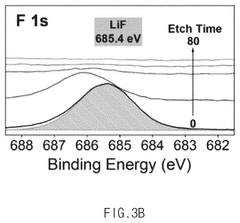
- An electrolyte for electrochemical nitrogen reduction reactions is developed, incorporating a first lithium salt such as fluoroborate-based, fluorophosphate-based, or fluoroarcenate-based lithium salts to provide fluorine ions on the electrode surface, enhancing reaction selectivity and stability by forming a lithium fluoride layer, which improves ammonia synthesis yield and stability.
Electrode for nitrate reduction
PatentWO2023235516A9
Innovation
- A nitrate-reducing electrode comprising a carbonaceous water-permeable substrate with a titanium compound and a metal catalyst, such as copper, deposited using methods like electrospinning or thermal treatment, which enhances surface area, conductivity, and stability, facilitating efficient nitrate reduction to ammonia or nitrogen.
Environmental Impact Assessment
The electrochemical nitrogen reduction reaction (NRR) represents a promising alternative to the conventional Haber-Bosch process for ammonia synthesis. However, evaluating the environmental implications of electrolyte engineering approaches is crucial for determining their sustainability and ecological viability.
Electrolyte engineering for improved nitrogen solubility and selectivity offers significant potential for reducing the environmental footprint of ammonia production. Traditional ammonia synthesis consumes approximately 1-2% of global energy and contributes substantially to greenhouse gas emissions. In contrast, electrochemical methods utilizing renewable electricity sources could potentially reduce carbon emissions by 60-90%, depending on the electricity mix and system efficiency.
The environmental benefits extend beyond carbon reduction. Electrolyte systems can operate at ambient temperature and pressure, eliminating the need for the energy-intensive conditions required by the Haber-Bosch process (400-500°C, 150-300 bar). This translates to reduced resource consumption and lower infrastructure requirements, minimizing land use impacts and material footprints.
Water consumption represents another critical environmental consideration. Certain electrolyte systems, particularly those utilizing ionic liquids or deep eutectic solvents, demonstrate lower water requirements compared to conventional processes. However, the production and disposal of these specialized electrolytes may introduce new environmental challenges, including potential toxicity concerns and biodegradability issues that require comprehensive life cycle assessment.
The environmental impact of catalyst materials used in conjunction with engineered electrolytes warrants careful examination. While noble metal catalysts offer high performance, their environmental footprint includes resource depletion and energy-intensive mining operations. Electrolyte engineering approaches that enable efficient nitrogen reduction with earth-abundant catalysts could significantly improve sustainability profiles.
Waste stream management presents both challenges and opportunities. Electrolyte degradation products and spent solutions require proper treatment and disposal. However, well-designed systems may enable closed-loop operation with minimal waste generation, particularly when employing stable electrolytes with long operational lifetimes.
The scalability of electrolyte engineering approaches directly impacts their environmental viability. Laboratory-scale successes must translate to industrial implementation without proportional increases in environmental burden. This includes considerations of electrolyte stability, recyclability, and production methods that minimize environmental impacts during manufacturing.
Regulatory frameworks will increasingly influence the adoption of these technologies. Environmental regulations regarding chemical usage, emissions standards, and waste disposal will shape the development trajectory of electrolyte engineering approaches, potentially accelerating innovations that demonstrate superior environmental performance.
Electrolyte engineering for improved nitrogen solubility and selectivity offers significant potential for reducing the environmental footprint of ammonia production. Traditional ammonia synthesis consumes approximately 1-2% of global energy and contributes substantially to greenhouse gas emissions. In contrast, electrochemical methods utilizing renewable electricity sources could potentially reduce carbon emissions by 60-90%, depending on the electricity mix and system efficiency.
The environmental benefits extend beyond carbon reduction. Electrolyte systems can operate at ambient temperature and pressure, eliminating the need for the energy-intensive conditions required by the Haber-Bosch process (400-500°C, 150-300 bar). This translates to reduced resource consumption and lower infrastructure requirements, minimizing land use impacts and material footprints.
Water consumption represents another critical environmental consideration. Certain electrolyte systems, particularly those utilizing ionic liquids or deep eutectic solvents, demonstrate lower water requirements compared to conventional processes. However, the production and disposal of these specialized electrolytes may introduce new environmental challenges, including potential toxicity concerns and biodegradability issues that require comprehensive life cycle assessment.
The environmental impact of catalyst materials used in conjunction with engineered electrolytes warrants careful examination. While noble metal catalysts offer high performance, their environmental footprint includes resource depletion and energy-intensive mining operations. Electrolyte engineering approaches that enable efficient nitrogen reduction with earth-abundant catalysts could significantly improve sustainability profiles.
Waste stream management presents both challenges and opportunities. Electrolyte degradation products and spent solutions require proper treatment and disposal. However, well-designed systems may enable closed-loop operation with minimal waste generation, particularly when employing stable electrolytes with long operational lifetimes.
The scalability of electrolyte engineering approaches directly impacts their environmental viability. Laboratory-scale successes must translate to industrial implementation without proportional increases in environmental burden. This includes considerations of electrolyte stability, recyclability, and production methods that minimize environmental impacts during manufacturing.
Regulatory frameworks will increasingly influence the adoption of these technologies. Environmental regulations regarding chemical usage, emissions standards, and waste disposal will shape the development trajectory of electrolyte engineering approaches, potentially accelerating innovations that demonstrate superior environmental performance.
Techno-economic Analysis
The techno-economic analysis of electrolyte engineering approaches for electrochemical nitrogen reduction reveals significant economic implications across the ammonia production value chain. Current industrial ammonia production via the Haber-Bosch process costs approximately $400-600 per ton, consuming 1-2% of global energy and generating substantial CO2 emissions. In contrast, electrochemical nitrogen reduction reaction (NRR) systems offer potential decentralized production with lower carbon footprints when powered by renewable energy.
Capital expenditure (CAPEX) for NRR systems varies significantly based on electrolyte engineering approaches. Aqueous electrolytes represent the lowest initial investment at $500-1,000 per kW of capacity, while ionic liquid and organic electrolyte systems require $1,500-3,000 per kW due to higher material costs and specialized handling requirements. However, these higher-cost electrolytes often deliver improved nitrogen solubility and Faradaic efficiency, potentially justifying their increased initial investment.
Operational expenditure (OPEX) analysis indicates that electrolyte stability and longevity significantly impact economic viability. Conventional aqueous electrolytes require replacement every 3-6 months, while advanced engineered electrolytes with stabilizing additives can extend operational life to 12-18 months. This extension reduces maintenance costs by approximately 40-60% over a five-year operational period.
Energy consumption represents another critical economic factor. Traditional aqueous NRR systems operate at 50-70 kWh per kg NH3 produced. Engineered electrolytes incorporating nitrogen-philic ionic components have demonstrated reduced energy requirements of 35-45 kWh per kg NH3, representing potential energy cost savings of 30-40%.
Sensitivity analysis reveals that nitrogen solubility improvements directly correlate with production economics. Each 10% increase in nitrogen solubility typically yields a 7-12% decrease in production costs through improved reaction kinetics and reduced energy requirements. Similarly, selectivity improvements that reduce competing hydrogen evolution reactions can improve ammonia yield by 15-25% without additional energy input.
Market adoption scenarios suggest that engineered electrolytes could achieve economic parity with conventional Haber-Bosch production by 2030 if current technological trajectories continue. This would require achieving Faradaic efficiencies above 35% and nitrogen-to-ammonia conversion rates exceeding 10^-9 mol cm^-2 s^-1, both of which appear feasible through continued electrolyte engineering advances focusing on nitrogen solubility and selectivity optimization.
Capital expenditure (CAPEX) for NRR systems varies significantly based on electrolyte engineering approaches. Aqueous electrolytes represent the lowest initial investment at $500-1,000 per kW of capacity, while ionic liquid and organic electrolyte systems require $1,500-3,000 per kW due to higher material costs and specialized handling requirements. However, these higher-cost electrolytes often deliver improved nitrogen solubility and Faradaic efficiency, potentially justifying their increased initial investment.
Operational expenditure (OPEX) analysis indicates that electrolyte stability and longevity significantly impact economic viability. Conventional aqueous electrolytes require replacement every 3-6 months, while advanced engineered electrolytes with stabilizing additives can extend operational life to 12-18 months. This extension reduces maintenance costs by approximately 40-60% over a five-year operational period.
Energy consumption represents another critical economic factor. Traditional aqueous NRR systems operate at 50-70 kWh per kg NH3 produced. Engineered electrolytes incorporating nitrogen-philic ionic components have demonstrated reduced energy requirements of 35-45 kWh per kg NH3, representing potential energy cost savings of 30-40%.
Sensitivity analysis reveals that nitrogen solubility improvements directly correlate with production economics. Each 10% increase in nitrogen solubility typically yields a 7-12% decrease in production costs through improved reaction kinetics and reduced energy requirements. Similarly, selectivity improvements that reduce competing hydrogen evolution reactions can improve ammonia yield by 15-25% without additional energy input.
Market adoption scenarios suggest that engineered electrolytes could achieve economic parity with conventional Haber-Bosch production by 2030 if current technological trajectories continue. This would require achieving Faradaic efficiencies above 35% and nitrogen-to-ammonia conversion rates exceeding 10^-9 mol cm^-2 s^-1, both of which appear feasible through continued electrolyte engineering advances focusing on nitrogen solubility and selectivity optimization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!