Performance Benchmarks of Metal Nitride and Oxide Derived Catalysts in Electrochemical Nitrogen Reduction
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metal Nitride/Oxide Catalysts Background and Objectives
The electrochemical nitrogen reduction reaction (NRR) represents a groundbreaking approach to ammonia synthesis under ambient conditions, offering a sustainable alternative to the energy-intensive Haber-Bosch process. This technology has evolved significantly over the past decade, with metal nitride and oxide derived catalysts emerging as promising materials for efficient nitrogen fixation. The historical trajectory of these catalysts began with early theoretical predictions of their catalytic activity in the early 2000s, followed by experimental validation around 2010-2015.
Metal nitrides, particularly those of transition metals such as molybdenum, vanadium, and titanium, have demonstrated unique electronic properties that facilitate N₂ adsorption and activation. Their development has progressed from simple binary nitrides to complex ternary and quaternary systems with enhanced performance characteristics. Similarly, metal oxides have evolved from basic structures to sophisticated architectures with engineered oxygen vacancies and defect sites that serve as active centers for NRR.
Recent technological advancements have focused on nanostructuring these materials to maximize active site exposure and optimize electron transfer pathways. The integration of computational modeling with experimental approaches has accelerated catalyst design, enabling rational tuning of electronic properties and surface chemistry. This synergistic approach has led to significant improvements in Faradaic efficiency and ammonia yield rates.
The primary objective of current research is to develop catalysts that can achieve industrially relevant performance metrics: Faradaic efficiencies exceeding 60% and ammonia production rates above 10⁻⁹ mol cm⁻² s⁻¹ under ambient conditions. Secondary goals include enhancing catalyst stability for continuous operation beyond 100 hours and reducing precious metal content to improve economic viability.
Global research efforts are increasingly focused on understanding fundamental reaction mechanisms at the atomic level, particularly the rate-determining steps of N₂ activation and protonation. This mechanistic insight is crucial for designing next-generation catalysts with optimized binding energies for reaction intermediates.
The technology trajectory suggests convergence toward hybrid structures that combine the beneficial properties of both nitrides and oxides, potentially incorporating additional components such as carbon supports or secondary metals to create synergistic effects. These advanced materials aim to overcome the inherent scaling relations that limit conventional catalysts and push performance boundaries toward theoretical maximums.
As this field continues to mature, standardized testing protocols and benchmarking methodologies are being established to enable meaningful comparisons across different catalyst systems and research groups, accelerating progress toward commercially viable electrochemical ammonia synthesis technologies.
Metal nitrides, particularly those of transition metals such as molybdenum, vanadium, and titanium, have demonstrated unique electronic properties that facilitate N₂ adsorption and activation. Their development has progressed from simple binary nitrides to complex ternary and quaternary systems with enhanced performance characteristics. Similarly, metal oxides have evolved from basic structures to sophisticated architectures with engineered oxygen vacancies and defect sites that serve as active centers for NRR.
Recent technological advancements have focused on nanostructuring these materials to maximize active site exposure and optimize electron transfer pathways. The integration of computational modeling with experimental approaches has accelerated catalyst design, enabling rational tuning of electronic properties and surface chemistry. This synergistic approach has led to significant improvements in Faradaic efficiency and ammonia yield rates.
The primary objective of current research is to develop catalysts that can achieve industrially relevant performance metrics: Faradaic efficiencies exceeding 60% and ammonia production rates above 10⁻⁹ mol cm⁻² s⁻¹ under ambient conditions. Secondary goals include enhancing catalyst stability for continuous operation beyond 100 hours and reducing precious metal content to improve economic viability.
Global research efforts are increasingly focused on understanding fundamental reaction mechanisms at the atomic level, particularly the rate-determining steps of N₂ activation and protonation. This mechanistic insight is crucial for designing next-generation catalysts with optimized binding energies for reaction intermediates.
The technology trajectory suggests convergence toward hybrid structures that combine the beneficial properties of both nitrides and oxides, potentially incorporating additional components such as carbon supports or secondary metals to create synergistic effects. These advanced materials aim to overcome the inherent scaling relations that limit conventional catalysts and push performance boundaries toward theoretical maximums.
As this field continues to mature, standardized testing protocols and benchmarking methodologies are being established to enable meaningful comparisons across different catalyst systems and research groups, accelerating progress toward commercially viable electrochemical ammonia synthesis technologies.
Market Analysis for Electrochemical Nitrogen Reduction
The electrochemical nitrogen reduction reaction (NRR) market is experiencing significant growth driven by increasing demand for sustainable ammonia production methods. Traditional ammonia synthesis via the Haber-Bosch process consumes approximately 1-2% of global energy production and generates substantial CO2 emissions. This creates a compelling market opportunity for electrochemical alternatives that can operate under ambient conditions with renewable electricity.
The global ammonia market, valued at approximately $70 billion in 2022, is projected to reach $95 billion by 2030, growing at a CAGR of 4.3%. Electrochemical nitrogen reduction technologies are positioned to capture a portion of this market, particularly in regions with abundant renewable energy resources and stringent carbon regulations.
Agricultural applications represent the largest market segment, accounting for over 80% of ammonia consumption globally. The growing population and increasing food demand drive the need for fertilizers, creating sustained market pull for nitrogen fixation technologies. Additionally, emerging applications in hydrogen storage, fuel cells, and clean energy systems are expanding the potential market for electrochemical nitrogen reduction products.
Regional market analysis reveals significant opportunities in Asia-Pacific, particularly China and India, where agricultural intensification and industrial growth drive ammonia demand. North America and Europe present markets focused on green technology adoption, supported by favorable regulatory frameworks and sustainability initiatives.
Metal nitride and oxide derived catalysts represent a crucial segment within the electrochemical nitrogen reduction technology landscape. These materials offer advantages in selectivity, stability, and cost compared to precious metal catalysts. The market for advanced catalyst materials is expected to grow at a CAGR of 6.5% through 2030, outpacing the overall ammonia market growth.
Competitive analysis indicates increasing investment in this sector, with major chemical companies, agricultural technology firms, and clean energy startups actively developing commercial applications. Venture capital funding for electrochemical nitrogen reduction startups has exceeded $300 million since 2020, signaling strong market interest.
Key market drivers include carbon reduction policies, volatile natural gas prices affecting traditional ammonia production costs, and increasing demand for distributed manufacturing systems. Barriers to market adoption include scaling challenges, competition with established infrastructure, and the need for performance improvements in catalyst efficiency and selectivity.
The market for metal nitride and oxide derived catalysts specifically shows promising growth potential, with applications extending beyond ammonia to specialty chemicals and pharmaceutical intermediates. This diversification of end markets enhances the commercial viability of these catalyst technologies and expands their total addressable market.
The global ammonia market, valued at approximately $70 billion in 2022, is projected to reach $95 billion by 2030, growing at a CAGR of 4.3%. Electrochemical nitrogen reduction technologies are positioned to capture a portion of this market, particularly in regions with abundant renewable energy resources and stringent carbon regulations.
Agricultural applications represent the largest market segment, accounting for over 80% of ammonia consumption globally. The growing population and increasing food demand drive the need for fertilizers, creating sustained market pull for nitrogen fixation technologies. Additionally, emerging applications in hydrogen storage, fuel cells, and clean energy systems are expanding the potential market for electrochemical nitrogen reduction products.
Regional market analysis reveals significant opportunities in Asia-Pacific, particularly China and India, where agricultural intensification and industrial growth drive ammonia demand. North America and Europe present markets focused on green technology adoption, supported by favorable regulatory frameworks and sustainability initiatives.
Metal nitride and oxide derived catalysts represent a crucial segment within the electrochemical nitrogen reduction technology landscape. These materials offer advantages in selectivity, stability, and cost compared to precious metal catalysts. The market for advanced catalyst materials is expected to grow at a CAGR of 6.5% through 2030, outpacing the overall ammonia market growth.
Competitive analysis indicates increasing investment in this sector, with major chemical companies, agricultural technology firms, and clean energy startups actively developing commercial applications. Venture capital funding for electrochemical nitrogen reduction startups has exceeded $300 million since 2020, signaling strong market interest.
Key market drivers include carbon reduction policies, volatile natural gas prices affecting traditional ammonia production costs, and increasing demand for distributed manufacturing systems. Barriers to market adoption include scaling challenges, competition with established infrastructure, and the need for performance improvements in catalyst efficiency and selectivity.
The market for metal nitride and oxide derived catalysts specifically shows promising growth potential, with applications extending beyond ammonia to specialty chemicals and pharmaceutical intermediates. This diversification of end markets enhances the commercial viability of these catalyst technologies and expands their total addressable market.
Current Status and Challenges in Catalyst Development
The field of electrochemical nitrogen reduction reaction (NRR) has witnessed significant advancements in recent years, with metal nitride and oxide derived catalysts emerging as promising candidates for ammonia synthesis under ambient conditions. Currently, the development of these catalysts faces several critical challenges that hinder their practical application at industrial scales.
Globally, research efforts have primarily focused on improving the catalytic activity and selectivity of metal nitride and oxide derived catalysts. Despite these efforts, the Faradaic efficiency of most reported catalysts remains below 15%, with ammonia yield rates typically under 10 μg h⁻¹ mg⁻¹cat. These performance metrics fall significantly short of the benchmarks required for commercial viability, which experts estimate to be at least 60% Faradaic efficiency and yield rates exceeding 100 μg h⁻¹ mg⁻¹cat.
A major technical obstacle lies in the competing hydrogen evolution reaction (HER), which dominates the electron transfer process during NRR. This parasitic reaction significantly reduces nitrogen reduction efficiency, particularly in aqueous electrolytes. Researchers have attempted to address this through catalyst surface engineering and electrolyte optimization, but a definitive solution remains elusive.
Another substantial challenge is the accurate detection and quantification of ammonia at low concentrations. Current analytical methods, including spectrophotometric techniques (Nessler's reagent, indophenol blue method) and nuclear magnetic resonance spectroscopy, often suffer from interference issues and limited sensitivity. This has led to reproducibility concerns and debates regarding reported performance metrics in the scientific community.
The stability of catalysts presents another significant hurdle. Many metal nitride and oxide catalysts undergo structural changes or degradation during extended operation, resulting in performance deterioration. Long-term stability tests exceeding 100 hours are rarely reported in literature, leaving questions about the practical durability of these materials unaddressed.
From a geographical perspective, research in this field is concentrated primarily in East Asia (particularly China), North America, and Europe. Chinese institutions have published approximately 45% of all research papers on metal nitride and oxide catalysts for NRR in the past five years, followed by the United States (18%) and European countries (22% collectively).
The scalability of catalyst synthesis methods represents another critical challenge. Many high-performing catalysts rely on complex preparation techniques that are difficult to scale up, including atomic layer deposition, plasma treatment, and precise defect engineering. Bridging the gap between laboratory-scale synthesis and industrial production remains a significant barrier to commercialization.
Globally, research efforts have primarily focused on improving the catalytic activity and selectivity of metal nitride and oxide derived catalysts. Despite these efforts, the Faradaic efficiency of most reported catalysts remains below 15%, with ammonia yield rates typically under 10 μg h⁻¹ mg⁻¹cat. These performance metrics fall significantly short of the benchmarks required for commercial viability, which experts estimate to be at least 60% Faradaic efficiency and yield rates exceeding 100 μg h⁻¹ mg⁻¹cat.
A major technical obstacle lies in the competing hydrogen evolution reaction (HER), which dominates the electron transfer process during NRR. This parasitic reaction significantly reduces nitrogen reduction efficiency, particularly in aqueous electrolytes. Researchers have attempted to address this through catalyst surface engineering and electrolyte optimization, but a definitive solution remains elusive.
Another substantial challenge is the accurate detection and quantification of ammonia at low concentrations. Current analytical methods, including spectrophotometric techniques (Nessler's reagent, indophenol blue method) and nuclear magnetic resonance spectroscopy, often suffer from interference issues and limited sensitivity. This has led to reproducibility concerns and debates regarding reported performance metrics in the scientific community.
The stability of catalysts presents another significant hurdle. Many metal nitride and oxide catalysts undergo structural changes or degradation during extended operation, resulting in performance deterioration. Long-term stability tests exceeding 100 hours are rarely reported in literature, leaving questions about the practical durability of these materials unaddressed.
From a geographical perspective, research in this field is concentrated primarily in East Asia (particularly China), North America, and Europe. Chinese institutions have published approximately 45% of all research papers on metal nitride and oxide catalysts for NRR in the past five years, followed by the United States (18%) and European countries (22% collectively).
The scalability of catalyst synthesis methods represents another critical challenge. Many high-performing catalysts rely on complex preparation techniques that are difficult to scale up, including atomic layer deposition, plasma treatment, and precise defect engineering. Bridging the gap between laboratory-scale synthesis and industrial production remains a significant barrier to commercialization.
Benchmark Methodologies for Catalyst Performance Evaluation
01 Transition Metal Nitride Catalysts for Electrochemical Applications
Transition metal nitrides have emerged as effective catalysts for various electrochemical applications due to their unique electronic properties and high stability. These materials demonstrate excellent performance in hydrogen evolution reactions, oxygen reduction reactions, and other electrocatalytic processes. The incorporation of nitrogen into transition metal structures creates favorable electronic configurations that enhance catalytic activity while maintaining durability under harsh operating conditions.- Transition Metal Nitride Catalysts for Electrochemical Applications: Transition metal nitrides have emerged as promising catalysts for various electrochemical applications due to their high conductivity and catalytic activity. These materials demonstrate excellent performance in hydrogen evolution reactions, oxygen reduction reactions, and other electrochemical processes. The unique electronic structure of metal nitrides contributes to their enhanced catalytic properties, making them suitable alternatives to precious metal catalysts in energy conversion and storage systems.
- Metal Oxide Derived Catalysts for Environmental Applications: Metal oxide derived catalysts show remarkable performance in environmental applications such as pollutant degradation and emission control. These catalysts can be synthesized through various methods to achieve specific surface properties and reactivity. The performance benchmarks for these catalysts include conversion efficiency, selectivity, and stability under operating conditions. Metal oxide catalysts can be modified with dopants to enhance their catalytic activity and extend their operational lifetime in environmental remediation processes.
- Semiconductor Processing Using Metal Nitride and Oxide Materials: Metal nitrides and oxides serve as critical materials in semiconductor processing, offering excellent performance as diffusion barriers, gate dielectrics, and electrode materials. These materials provide superior electrical properties and thermal stability required for advanced semiconductor devices. Performance benchmarks include resistivity, dielectric constant, breakdown voltage, and compatibility with existing fabrication processes. The controlled deposition and patterning of these materials enable the development of high-performance electronic components.
- Synthesis Methods for High-Performance Metal Nitride and Oxide Catalysts: Various synthesis methods have been developed to produce high-performance metal nitride and oxide catalysts with controlled morphology, composition, and surface properties. These methods include sol-gel processing, hydrothermal synthesis, chemical vapor deposition, and plasma-assisted techniques. The synthesis parameters significantly influence the catalytic performance of the resulting materials. Advanced characterization techniques are employed to establish structure-property relationships and optimize the synthesis conditions for specific applications.
- Composite and Supported Metal Nitride/Oxide Catalysts: Composite and supported metal nitride/oxide catalysts demonstrate enhanced performance through synergistic effects between different components. These catalysts typically consist of active metal nitride or oxide phases dispersed on high-surface-area supports or combined with other functional materials. The support material can improve the dispersion, stability, and accessibility of the active sites. Performance benchmarks for these composite catalysts include activity per unit mass, selectivity toward desired products, and resistance to deactivation under reaction conditions.
02 Metal Oxide Derived Catalysts for Energy Conversion
Metal oxide derived catalysts show promising performance in energy conversion applications, particularly in fuel cells and electrolyzers. These catalysts are typically synthesized through controlled oxidation processes, resulting in high surface area materials with abundant active sites. The performance benchmarks for these catalysts include high catalytic activity, selectivity, and long-term stability. Various synthesis methods can be employed to tune the properties of metal oxide catalysts for specific applications.Expand Specific Solutions03 Nitride-Oxide Composite Catalysts with Enhanced Performance
Composite catalysts combining metal nitrides and oxides exhibit synergistic effects that enhance catalytic performance beyond what either component could achieve individually. These hybrid materials benefit from complementary properties: metal nitrides often provide excellent electrical conductivity and activity, while metal oxides contribute stability and additional active sites. The interface between nitride and oxide phases creates unique catalytic environments that can significantly improve reaction kinetics and selectivity for various chemical transformations.Expand Specific Solutions04 Semiconductor Processing Applications of Metal Nitride and Oxide Catalysts
Metal nitride and oxide materials serve as important catalysts in semiconductor processing applications. These materials facilitate critical reactions during device fabrication, including chemical vapor deposition, atomic layer deposition, and etching processes. Performance benchmarks for these catalysts include precise control over reaction rates, uniformity of deposition or etching, and compatibility with existing semiconductor manufacturing processes. The catalytic properties can be tuned by controlling the composition, crystal structure, and surface properties of the materials.Expand Specific Solutions05 Synthesis Methods and Performance Optimization for Nitride and Oxide Catalysts
Various synthesis methods have been developed to optimize the performance of metal nitride and oxide catalysts. These include sol-gel processes, hydrothermal synthesis, solid-state reactions, and plasma-assisted techniques. The performance of these catalysts can be benchmarked through parameters such as specific surface area, active site density, turnover frequency, and stability under reaction conditions. Post-synthesis treatments, such as annealing and surface modification, can further enhance catalytic activity by optimizing the electronic structure and surface properties.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrochemical nitrogen reduction (ENR) technology market is currently in an early development stage, characterized by intensive research activities but limited commercial deployment. The global market size remains relatively small but is expected to grow significantly as ENR technologies mature and become economically viable for industrial applications. From a technical maturity perspective, metal nitride and oxide derived catalysts show promising performance but still face challenges in efficiency and scalability. Leading players in this field include academic institutions like Kyushu University, Tianjin University, and KAIST, alongside industrial entities such as SK Innovation, BASF, and Toyota Motor Corp. Research collaborations between academic institutions (Central South University, Monash University) and industrial partners (Resonac Holdings, LG Electronics) are accelerating catalyst development, with significant breakthroughs in nitrogen conversion efficiency and selectivity being reported in recent publications.
BASF Corp.
Technical Solution: BASF Corporation has developed an industrial-scale approach to metal nitride and oxide catalyst benchmarking for electrochemical nitrogen reduction, focusing on scalability and economic viability. Their research team has engineered several catalyst systems including supported Fe3O4 nanoparticles, Ti3N4-based materials, and mixed metal oxide composites designed for integration into commercial electrolyzer systems. Their benchmarking framework emphasizes performance metrics relevant to industrial application, including catalyst durability under high current densities, tolerance to common electrolyte impurities, and production costs. Notable developments include spray-dried mixed metal oxide catalysts achieving Faradaic efficiencies of 7.5% with ammonia production rates of 0.3 g h−1 cm−2 in prototype systems, and nitride-based catalysts with exceptional resistance to poisoning by common contaminants. BASF's comparative analysis indicates that while nitride catalysts generally demonstrate higher intrinsic activity, certain oxide formulations offer better economic performance when considering full lifecycle costs including synthesis, operation, and longevity.
Strengths: Catalyst designs optimized for industrial-scale implementation rather than just laboratory performance; comprehensive economic analysis incorporating full production costs; robust testing under realistic operating conditions including presence of contaminants. Weaknesses: Generally lower peak performance metrics compared to academic research catalysts; higher emphasis on stability sometimes comes at the cost of maximum activity; more conservative design approaches limiting exploration of cutting-edge materials.
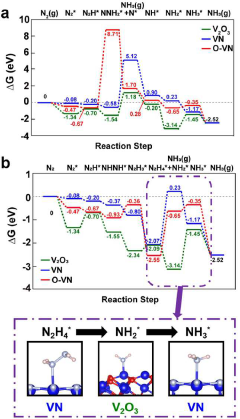
Tianjin University
Technical Solution: Tianjin University has developed a comprehensive benchmarking framework for metal nitride and oxide catalysts in electrochemical nitrogen reduction, focusing on performance standardization and mechanistic understanding. Their research team has created a series of nanostructured catalysts including VN nanosheets, MoO3-x with oxygen vacancies, and hybrid nitride-oxide structures that demonstrate synergistic effects. Their benchmarking protocol includes standardized testing conditions (electrolyte composition, potential ranges, and detection methods) that allow for direct comparison between different catalyst systems. Notable achievements include the development of Fe-doped TiO2 catalysts achieving Faradaic efficiencies of 9.8% and ammonia yields of 23.1 μg h−1 mg−1cat, as well as Mo2N catalysts with enhanced N2 adsorption properties through surface engineering. Their work has established correlations between electronic structure parameters and catalytic performance, providing design principles for next-generation catalysts.
Strengths: Comprehensive standardized testing protocols enabling reliable comparisons; innovative hybrid structures combining benefits of both nitride and oxide systems; detailed mechanistic insights guiding rational catalyst design. Weaknesses: Some catalysts show performance degradation in scaled-up testing conditions; complex synthesis procedures for hybrid structures limiting commercial viability; challenges in maintaining performance consistency across different batches.
Key Patents and Scientific Breakthroughs in Catalyst Design
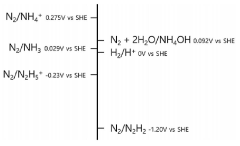
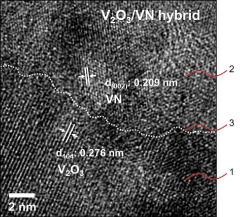
Vanadium oxide-nitride hybrid electrocatalyst for nitrogen reduction reaction and method for manufacturing same
PatentActiveKR1020220055308A
Innovation
- A vanadium oxide-nitride hybrid electrocatalyst is developed, featuring a first region of vanadium oxide nanoparticles, a second region of vanadium nitride nanoparticles, and an amorphous boundary region where both are mixed, facilitating parallelization of nitrogen reduction reaction intermediates to accelerate the overall reaction.
Oxidized surface layer on transition metal nitrides: active catalysts for the oxygen reduction reaction
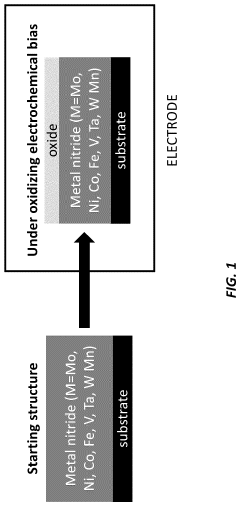
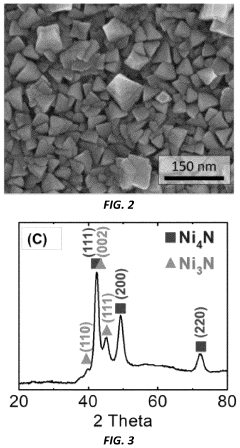
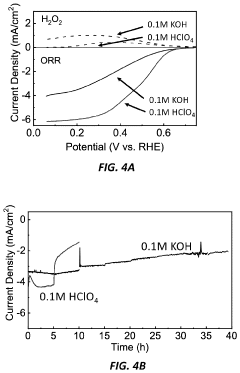
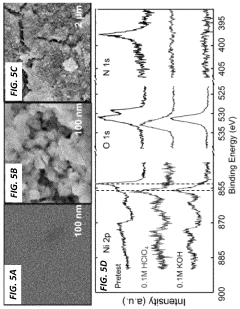
PatentActiveUS11929512B2
Innovation
- A method involving the deposition of a transition metal nitride layer on a substrate with an in-situ formed oxidized surface layer using an oxidizing electrochemical bias, creating a thin oxide layer that enhances ORR activity and stability, specifically using transition metals like Mo, Ni, Co, Fe, V, Ta, W, and Mn in amorphous or crystalline structures with nanostructured or thin film morphologies.
Sustainability and Scalability Considerations
The sustainability and scalability of metal nitride and oxide derived catalysts represent critical factors in determining their viability for large-scale electrochemical nitrogen reduction applications. Current laboratory-scale demonstrations, while promising, face significant challenges when considered for industrial implementation. The environmental footprint of catalyst production processes requires thorough assessment, particularly regarding energy-intensive synthesis methods that often involve high temperatures and pressures.
Material sourcing presents another crucial consideration, as many high-performance catalysts incorporate precious or rare earth metals with limited global reserves. For instance, ruthenium-based catalysts demonstrate excellent activity but face supply constraints that could impede widespread adoption. Developing catalysts based on earth-abundant elements such as iron, molybdenum, and vanadium offers a more sustainable alternative, though often with performance trade-offs that must be carefully evaluated.
The energy efficiency of the overall electrochemical nitrogen reduction process significantly impacts its sustainability profile. Current benchmarks indicate that most metal nitride and oxide catalysts operate at energy efficiencies below 10%, substantially lower than the theoretical maximum and insufficient for economically viable industrial applications. Improving energy efficiency through catalyst design, reactor engineering, and process optimization represents a critical research direction.
Catalyst stability and longevity directly affect both sustainability and economic feasibility. Performance degradation over time necessitates catalyst replacement, increasing operational costs and material consumption. Recent studies demonstrate promising stability for certain metal nitride catalysts, particularly those with core-shell structures that protect active sites from poisoning and dissolution, potentially enabling continuous operation for thousands of hours.
Manufacturing scalability presents technical challenges that extend beyond laboratory synthesis methods. Current production techniques for high-performance catalysts often involve complex multi-step processes with precise control requirements that prove difficult to scale. Developing simplified synthesis routes compatible with existing industrial infrastructure would accelerate commercial implementation.
Water consumption and management represent additional considerations, particularly for applications in water-stressed regions. Catalyst systems requiring ultrapure water inputs face implementation barriers in areas with limited water resources. Developing catalysts tolerant of impurities and compatible with recycled water streams would enhance deployment flexibility and reduce environmental impact.
Material sourcing presents another crucial consideration, as many high-performance catalysts incorporate precious or rare earth metals with limited global reserves. For instance, ruthenium-based catalysts demonstrate excellent activity but face supply constraints that could impede widespread adoption. Developing catalysts based on earth-abundant elements such as iron, molybdenum, and vanadium offers a more sustainable alternative, though often with performance trade-offs that must be carefully evaluated.
The energy efficiency of the overall electrochemical nitrogen reduction process significantly impacts its sustainability profile. Current benchmarks indicate that most metal nitride and oxide catalysts operate at energy efficiencies below 10%, substantially lower than the theoretical maximum and insufficient for economically viable industrial applications. Improving energy efficiency through catalyst design, reactor engineering, and process optimization represents a critical research direction.
Catalyst stability and longevity directly affect both sustainability and economic feasibility. Performance degradation over time necessitates catalyst replacement, increasing operational costs and material consumption. Recent studies demonstrate promising stability for certain metal nitride catalysts, particularly those with core-shell structures that protect active sites from poisoning and dissolution, potentially enabling continuous operation for thousands of hours.
Manufacturing scalability presents technical challenges that extend beyond laboratory synthesis methods. Current production techniques for high-performance catalysts often involve complex multi-step processes with precise control requirements that prove difficult to scale. Developing simplified synthesis routes compatible with existing industrial infrastructure would accelerate commercial implementation.
Water consumption and management represent additional considerations, particularly for applications in water-stressed regions. Catalyst systems requiring ultrapure water inputs face implementation barriers in areas with limited water resources. Developing catalysts tolerant of impurities and compatible with recycled water streams would enhance deployment flexibility and reduce environmental impact.
Regulatory Framework for Ammonia Production Technologies
The regulatory landscape governing ammonia production technologies is undergoing significant transformation as electrochemical nitrogen reduction (ENR) emerges as a potential alternative to the conventional Haber-Bosch process. Current regulations primarily address traditional ammonia production methods, which are energy-intensive and carbon-heavy, contributing approximately 1.8% of global CO2 emissions.
International frameworks, including the Paris Agreement and various national carbon reduction commitments, are increasingly influencing ammonia production regulations. These frameworks establish greenhouse gas emission limits that indirectly affect the viability of different catalyst technologies in ENR processes. Metal nitride and oxide derived catalysts must demonstrate performance that enables compliance with these evolving standards.
Safety regulations present another critical dimension, as ammonia is classified as a hazardous substance. Regulatory bodies such as the U.S. Occupational Safety and Health Administration (OSHA) and the European Chemicals Agency (ECHA) impose strict guidelines on ammonia handling, storage, and transportation. ENR technologies utilizing metal nitride and oxide catalysts must incorporate safety features that satisfy these requirements, particularly when designed for distributed or smaller-scale production.
Water quality regulations also impact ENR implementation, as the process involves aqueous electrolytes. Discharge permits and wastewater treatment requirements vary by jurisdiction but generally restrict the release of metal ions and nitrogen compounds. This necessitates careful catalyst design to minimize leaching and degradation during operation.
Emerging regulatory frameworks specifically addressing green ammonia production are beginning to appear in progressive jurisdictions. These frameworks often include incentive structures such as tax credits, subsidies, or preferential market access for low-carbon ammonia. The performance benchmarks of metal nitride and oxide catalysts directly affect producers' ability to qualify for these incentives.
Certification and standardization efforts are also developing, with organizations like the International Organization for Standardization (ISO) working on standards for green ammonia production. These standards will likely include specific performance metrics for catalysts, including Faradaic efficiency, ammonia yield rate, and catalyst stability parameters.
Regulatory compliance costs represent a significant factor in technology adoption decisions. ENR technologies using advanced catalysts must demonstrate not only technical performance but also economic viability within the regulatory cost structure, which includes emissions permits, safety compliance, and reporting requirements.
International frameworks, including the Paris Agreement and various national carbon reduction commitments, are increasingly influencing ammonia production regulations. These frameworks establish greenhouse gas emission limits that indirectly affect the viability of different catalyst technologies in ENR processes. Metal nitride and oxide derived catalysts must demonstrate performance that enables compliance with these evolving standards.
Safety regulations present another critical dimension, as ammonia is classified as a hazardous substance. Regulatory bodies such as the U.S. Occupational Safety and Health Administration (OSHA) and the European Chemicals Agency (ECHA) impose strict guidelines on ammonia handling, storage, and transportation. ENR technologies utilizing metal nitride and oxide catalysts must incorporate safety features that satisfy these requirements, particularly when designed for distributed or smaller-scale production.
Water quality regulations also impact ENR implementation, as the process involves aqueous electrolytes. Discharge permits and wastewater treatment requirements vary by jurisdiction but generally restrict the release of metal ions and nitrogen compounds. This necessitates careful catalyst design to minimize leaching and degradation during operation.
Emerging regulatory frameworks specifically addressing green ammonia production are beginning to appear in progressive jurisdictions. These frameworks often include incentive structures such as tax credits, subsidies, or preferential market access for low-carbon ammonia. The performance benchmarks of metal nitride and oxide catalysts directly affect producers' ability to qualify for these incentives.
Certification and standardization efforts are also developing, with organizations like the International Organization for Standardization (ISO) working on standards for green ammonia production. These standards will likely include specific performance metrics for catalysts, including Faradaic efficiency, ammonia yield rate, and catalyst stability parameters.
Regulatory compliance costs represent a significant factor in technology adoption decisions. ENR technologies using advanced catalysts must demonstrate not only technical performance but also economic viability within the regulatory cost structure, which includes emissions permits, safety compliance, and reporting requirements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!