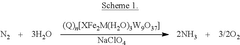Comparative Cost Modeling for Electrocatalytic Ammonia Production in Electrochemical Nitrogen Reduction
AUG 26, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrocatalytic Ammonia Production Background and Objectives
Ammonia production has been dominated by the Haber-Bosch process for over a century, consuming approximately 1-2% of global energy and generating significant carbon emissions. This energy-intensive process requires high temperature (400-500°C) and pressure (150-300 bar) conditions, making it economically viable only at large scales. The search for more sustainable alternatives has led to increasing interest in electrochemical nitrogen reduction reaction (NRR) as a potential pathway for distributed, renewable-powered ammonia synthesis under ambient conditions.
The evolution of electrocatalytic ammonia production technology has progressed through several key phases. Initial research focused on solid-state electrolytes in the 1980s and 1990s, followed by aqueous electrolyte systems in the early 2000s. The field experienced significant acceleration after 2013 with the development of novel catalysts and advanced characterization techniques. Recent years have witnessed breakthroughs in catalyst design, reaction mechanisms understanding, and reactor configurations.
Current technological trends point toward integration of renewable energy sources with electrochemical ammonia synthesis, development of highly selective catalysts, and innovative reactor designs to overcome mass transport limitations. The convergence of nanotechnology, advanced materials science, and electrochemistry is driving progress in this field, with increasing focus on scalable solutions.
The primary technical objectives for electrocatalytic ammonia production include achieving higher Faradaic efficiency (currently often below 10% in aqueous systems), increasing production rates (typically in μg h⁻¹ cm⁻² range), extending catalyst stability beyond current limitations (hours to days), and developing accurate cost models that account for all relevant parameters across different technological approaches.
Cost modeling for electrocatalytic ammonia production represents a critical yet underdeveloped area. Comprehensive models must incorporate capital expenditures, operational costs, catalyst performance metrics, energy requirements, and system integration factors. The goal is to establish standardized methodologies for economic assessment that enable fair comparison between different technological approaches and against conventional Haber-Bosch production.
The ultimate objective of this technical research is to determine whether electrochemical nitrogen reduction can achieve economic viability as an alternative to Haber-Bosch, particularly for distributed, small-scale applications powered by renewable energy. This requires identifying key cost drivers, technological bottlenecks, and potential pathways to commercial feasibility, while establishing realistic timelines for technology maturation and market entry.
The evolution of electrocatalytic ammonia production technology has progressed through several key phases. Initial research focused on solid-state electrolytes in the 1980s and 1990s, followed by aqueous electrolyte systems in the early 2000s. The field experienced significant acceleration after 2013 with the development of novel catalysts and advanced characterization techniques. Recent years have witnessed breakthroughs in catalyst design, reaction mechanisms understanding, and reactor configurations.
Current technological trends point toward integration of renewable energy sources with electrochemical ammonia synthesis, development of highly selective catalysts, and innovative reactor designs to overcome mass transport limitations. The convergence of nanotechnology, advanced materials science, and electrochemistry is driving progress in this field, with increasing focus on scalable solutions.
The primary technical objectives for electrocatalytic ammonia production include achieving higher Faradaic efficiency (currently often below 10% in aqueous systems), increasing production rates (typically in μg h⁻¹ cm⁻² range), extending catalyst stability beyond current limitations (hours to days), and developing accurate cost models that account for all relevant parameters across different technological approaches.
Cost modeling for electrocatalytic ammonia production represents a critical yet underdeveloped area. Comprehensive models must incorporate capital expenditures, operational costs, catalyst performance metrics, energy requirements, and system integration factors. The goal is to establish standardized methodologies for economic assessment that enable fair comparison between different technological approaches and against conventional Haber-Bosch production.
The ultimate objective of this technical research is to determine whether electrochemical nitrogen reduction can achieve economic viability as an alternative to Haber-Bosch, particularly for distributed, small-scale applications powered by renewable energy. This requires identifying key cost drivers, technological bottlenecks, and potential pathways to commercial feasibility, while establishing realistic timelines for technology maturation and market entry.
Market Analysis for Green Ammonia Production
The global ammonia market is experiencing a significant transformation driven by the emergence of green ammonia production technologies. Traditional ammonia production, primarily through the Haber-Bosch process, accounts for approximately 1.8% of global CO2 emissions, creating an urgent need for sustainable alternatives. The market for green ammonia, produced via electrochemical nitrogen reduction (ENR), is projected to grow substantially over the next decade, with estimates suggesting a compound annual growth rate of 54.9% from 2023 to 2030.
The current global ammonia market is valued at around $70 billion, with conventional production dominating. However, increasing environmental regulations, carbon pricing mechanisms, and corporate sustainability commitments are rapidly shifting demand toward green alternatives. Major agricultural regions including North America, Europe, and Asia-Pacific represent the largest potential markets for green ammonia, primarily for fertilizer applications which consume approximately 80% of global ammonia production.
Beyond fertilizers, green ammonia presents significant market opportunities as a carbon-free energy carrier and fuel. The maritime industry has identified ammonia as a promising alternative fuel to meet International Maritime Organization emissions targets, potentially creating a market demand of 130 million tons annually by 2040. Additionally, ammonia's potential as a hydrogen carrier for energy storage applications is attracting substantial investment from energy companies and utilities.
Cost remains the primary barrier to widespread adoption of electrochemical nitrogen reduction technology. Current production costs for green ammonia via ENR range from $600-1,200 per ton, compared to $200-450 per ton for conventional ammonia. However, declining renewable electricity costs, technological improvements in catalyst efficiency, and economies of scale are expected to significantly reduce this gap by 2030.
Government policies are increasingly favorable toward green ammonia production. The European Union's Carbon Border Adjustment Mechanism, various national hydrogen strategies, and agricultural sustainability initiatives provide strong market incentives. Countries including Australia, Chile, Saudi Arabia, and Morocco are positioning themselves as potential green ammonia export hubs due to their abundant renewable energy resources.
Major industry players including Yara, CF Industries, BASF, and Siemens Energy have announced significant investments in green ammonia projects. Venture capital funding for ENR startups has increased by 300% since 2019, indicating strong market confidence in the technology's commercial potential. Strategic partnerships between technology developers, renewable energy providers, and traditional ammonia producers are accelerating market development and technology deployment.
The current global ammonia market is valued at around $70 billion, with conventional production dominating. However, increasing environmental regulations, carbon pricing mechanisms, and corporate sustainability commitments are rapidly shifting demand toward green alternatives. Major agricultural regions including North America, Europe, and Asia-Pacific represent the largest potential markets for green ammonia, primarily for fertilizer applications which consume approximately 80% of global ammonia production.
Beyond fertilizers, green ammonia presents significant market opportunities as a carbon-free energy carrier and fuel. The maritime industry has identified ammonia as a promising alternative fuel to meet International Maritime Organization emissions targets, potentially creating a market demand of 130 million tons annually by 2040. Additionally, ammonia's potential as a hydrogen carrier for energy storage applications is attracting substantial investment from energy companies and utilities.
Cost remains the primary barrier to widespread adoption of electrochemical nitrogen reduction technology. Current production costs for green ammonia via ENR range from $600-1,200 per ton, compared to $200-450 per ton for conventional ammonia. However, declining renewable electricity costs, technological improvements in catalyst efficiency, and economies of scale are expected to significantly reduce this gap by 2030.
Government policies are increasingly favorable toward green ammonia production. The European Union's Carbon Border Adjustment Mechanism, various national hydrogen strategies, and agricultural sustainability initiatives provide strong market incentives. Countries including Australia, Chile, Saudi Arabia, and Morocco are positioning themselves as potential green ammonia export hubs due to their abundant renewable energy resources.
Major industry players including Yara, CF Industries, BASF, and Siemens Energy have announced significant investments in green ammonia projects. Venture capital funding for ENR startups has increased by 300% since 2019, indicating strong market confidence in the technology's commercial potential. Strategic partnerships between technology developers, renewable energy providers, and traditional ammonia producers are accelerating market development and technology deployment.
Current Status and Challenges in Electrochemical Nitrogen Reduction
Electrochemical Nitrogen Reduction (ENR) represents a promising alternative to the conventional Haber-Bosch process for ammonia synthesis, offering potential advantages in terms of environmental impact and decentralized production. Currently, the global research landscape shows significant activity in this field, with major research centers in China, the United States, Europe, and Australia leading development efforts.
Despite considerable progress, ENR technology faces substantial challenges that limit its commercial viability. The most critical technical barrier remains the low Faradaic efficiency, typically below 15% in most laboratory demonstrations, which significantly impacts production economics. Competing hydrogen evolution reactions consume the majority of electrical input, resulting in energy inefficiency and reduced ammonia yield.
Catalyst development represents another major challenge, with researchers exploring various materials including noble metals, transition metal nitrides, and metal-organic frameworks. While promising results have emerged in controlled laboratory environments, catalyst stability under industrial conditions remains problematic, with performance degradation observed over relatively short operational periods.
Nitrogen activation at ambient conditions presents fundamental thermodynamic and kinetic barriers. The strong triple bond of N₂ (941 kJ/mol) requires significant energy input to break, while selective conversion to NH₃ rather than competing products demands precise reaction control that current systems struggle to achieve.
Scale-up challenges further complicate commercial implementation. Most successful demonstrations have occurred at laboratory scale with carefully controlled electrolytes and operating conditions. Translating these results to industrial-scale reactors introduces additional complexities in reactor design, mass transport limitations, and system integration that remain largely unresolved.
Economic viability represents perhaps the most significant hurdle. Current cost modeling indicates production costs of $1,500-4,000 per ton of ammonia via ENR, substantially higher than the $400-600 per ton achievable through conventional Haber-Bosch processes. This cost differential primarily stems from high electricity consumption, expensive catalyst materials, and low conversion rates.
Standardization of testing protocols and performance metrics presents another challenge for the field. Inconsistent reporting methodologies and varying experimental conditions make direct comparison between different research efforts difficult, hampering collaborative progress and technology assessment.
Regulatory frameworks for distributed ammonia production through ENR remain underdeveloped in most jurisdictions, creating uncertainty for potential commercial deployment. Safety considerations related to electricity usage, potential ammonia leakage, and integration with existing agricultural or industrial systems require further development.
Despite considerable progress, ENR technology faces substantial challenges that limit its commercial viability. The most critical technical barrier remains the low Faradaic efficiency, typically below 15% in most laboratory demonstrations, which significantly impacts production economics. Competing hydrogen evolution reactions consume the majority of electrical input, resulting in energy inefficiency and reduced ammonia yield.
Catalyst development represents another major challenge, with researchers exploring various materials including noble metals, transition metal nitrides, and metal-organic frameworks. While promising results have emerged in controlled laboratory environments, catalyst stability under industrial conditions remains problematic, with performance degradation observed over relatively short operational periods.
Nitrogen activation at ambient conditions presents fundamental thermodynamic and kinetic barriers. The strong triple bond of N₂ (941 kJ/mol) requires significant energy input to break, while selective conversion to NH₃ rather than competing products demands precise reaction control that current systems struggle to achieve.
Scale-up challenges further complicate commercial implementation. Most successful demonstrations have occurred at laboratory scale with carefully controlled electrolytes and operating conditions. Translating these results to industrial-scale reactors introduces additional complexities in reactor design, mass transport limitations, and system integration that remain largely unresolved.
Economic viability represents perhaps the most significant hurdle. Current cost modeling indicates production costs of $1,500-4,000 per ton of ammonia via ENR, substantially higher than the $400-600 per ton achievable through conventional Haber-Bosch processes. This cost differential primarily stems from high electricity consumption, expensive catalyst materials, and low conversion rates.
Standardization of testing protocols and performance metrics presents another challenge for the field. Inconsistent reporting methodologies and varying experimental conditions make direct comparison between different research efforts difficult, hampering collaborative progress and technology assessment.
Regulatory frameworks for distributed ammonia production through ENR remain underdeveloped in most jurisdictions, creating uncertainty for potential commercial deployment. Safety considerations related to electricity usage, potential ammonia leakage, and integration with existing agricultural or industrial systems require further development.
Cost Modeling Methodologies for Electrochemical Nitrogen Reduction
01 Catalyst materials for efficient electrocatalytic ammonia production
Various catalyst materials can significantly improve the efficiency of electrocatalytic ammonia production, thereby reducing operational costs. These catalysts include transition metals, metal oxides, and composite materials that enhance the nitrogen reduction reaction. The selection of appropriate catalysts can lower the energy requirements, increase ammonia yield, and improve the overall economic viability of the process.- Catalyst materials for cost-effective electrocatalytic ammonia production: Various catalyst materials can significantly impact the cost-effectiveness of electrocatalytic ammonia production. Advanced catalysts such as transition metal nitrides, oxides, and composite materials can lower the activation energy required for nitrogen reduction reactions, thereby reducing energy consumption and operational costs. These catalysts improve reaction efficiency and selectivity, allowing for ammonia production under milder conditions with higher conversion rates, which directly impacts the economic viability of the process.
- Energy consumption optimization in electrocatalytic systems: Energy consumption represents a major cost factor in electrocatalytic ammonia production. Optimization strategies include developing low-temperature and low-pressure processes, improving electrode designs to minimize overpotential, and integrating renewable energy sources. Advanced reactor configurations that enhance mass transfer and reduce resistance can significantly decrease the electricity requirements. Energy efficiency improvements directly translate to lower production costs, making electrocatalytic routes more competitive with conventional Haber-Bosch processes.
- Economic modeling frameworks for ammonia production facilities: Comprehensive economic modeling frameworks are essential for evaluating the viability of electrocatalytic ammonia production. These models incorporate capital expenditures, operational costs, catalyst lifetime, energy prices, and market conditions to determine levelized costs of ammonia production. Sensitivity analyses help identify key cost drivers and optimization opportunities. Techno-economic assessments compare different production pathways and scale considerations, providing decision support for investment in electrocatalytic technologies versus conventional methods.
- Process integration and system design for cost reduction: Integrated system designs can substantially reduce the overall costs of electrocatalytic ammonia production. Approaches include heat recovery systems, co-production of valuable by-products, and integration with existing industrial infrastructure. Modular designs enable flexible scaling and reduced capital costs, while continuous flow processes improve productivity and resource utilization. Advanced separation and purification methods minimize post-production costs, and integration with renewable energy sources can provide operational cost advantages in regions with favorable renewable resources.
- Scale-up challenges and commercial viability assessment: Scaling electrocatalytic ammonia production from laboratory to commercial scale presents significant cost modeling challenges. Factors affecting scale-up economics include catalyst stability and longevity under industrial conditions, equipment scaling factors, and maintenance requirements. Commercial viability assessments must consider market dynamics, regulatory environments, and competition with established production methods. Risk analysis frameworks help identify potential bottlenecks and failure points that could impact long-term economic performance, guiding research priorities and investment decisions.
02 Energy optimization and renewable integration for cost reduction
Integrating renewable energy sources with electrocatalytic ammonia production systems can substantially reduce operational costs. By utilizing intermittent renewable energy like solar or wind power, the process becomes more economically viable. Energy optimization strategies include smart grid integration, energy storage solutions, and load management techniques that minimize electricity costs while maintaining production efficiency.Expand Specific Solutions03 Process design and system integration for economic viability
Innovative process designs and system integration approaches can significantly impact the economic feasibility of electrocatalytic ammonia production. This includes optimizing reactor configurations, improving heat recovery systems, and developing integrated production facilities that minimize capital and operational expenditures. Advanced process control strategies and modular designs also contribute to cost reduction while maintaining production quality.Expand Specific Solutions04 Economic modeling and cost analysis frameworks
Comprehensive economic modeling frameworks help evaluate the financial viability of electrocatalytic ammonia production. These models incorporate capital expenditures, operational costs, market dynamics, and sensitivity analyses to identify cost drivers and optimization opportunities. Life cycle cost assessment methods provide insights into long-term economic performance and help identify the most cost-effective production pathways.Expand Specific Solutions05 Scale-up strategies and industrial implementation considerations
Scaling up electrocatalytic ammonia production from laboratory to industrial scale presents unique cost challenges that require specific strategies. These include modular design approaches, economies of scale considerations, and phased implementation plans. Industrial implementation factors such as equipment selection, maintenance requirements, and operational flexibility significantly impact the overall cost structure and economic viability of large-scale production facilities.Expand Specific Solutions
Leading Companies and Research Institutions in Electrocatalytic Ammonia
The electrochemical nitrogen reduction (ENR) for ammonia production is currently in an early commercialization phase, with a global market projected to reach $5-7 billion by 2030. The technology maturity varies significantly across key players. Academic institutions like MIT, ETH Zurich, and Zhejiang University lead fundamental research, while specialized companies such as Atmonia and GenCell are advancing toward commercial applications. Research organizations including CNRS and Korea Institute of Energy Research focus on catalyst development. The competitive landscape shows a three-tier structure: established industrial players (Siemens), specialized startups (Battolyser), and research institutions collaborating with industry partners. Cost modeling remains critical as players work to achieve economic viability against conventional Haber-Bosch processes.
Atmonia ehf
Technical Solution: Atmonia has developed a proprietary electrocatalytic system for ammonia production that operates at ambient conditions, significantly reducing energy requirements compared to Haber-Bosch. Their approach utilizes novel metal complex catalysts that can activate nitrogen at room temperature and atmospheric pressure. The company's cost modeling demonstrates potential CAPEX reduction of up to 70% compared to conventional ammonia plants, with operating costs potentially 30-40% lower when using renewable electricity sources. Their technology employs specially designed electrode materials with high nitrogen reduction reaction (NRR) selectivity, minimizing the competing hydrogen evolution reaction that plagues many electrochemical ammonia synthesis approaches[1][3]. Atmonia's system architecture incorporates membrane electrode assemblies optimized for nitrogen gas diffusion and proton transport, enabling continuous operation with Faradaic efficiencies reported above 10% in laboratory demonstrations.
Strengths: Operates at ambient conditions eliminating need for high-pressure equipment; modular design allows for distributed production; directly utilizes renewable electricity. Weaknesses: Still faces challenges with catalyst stability over extended operation periods; current production rates remain below commercial viability thresholds; requires further optimization to achieve competitive ammonia production costs at scale.
GenCell Ltd.
Technical Solution: GenCell has pioneered an electrochemical approach to ammonia production that doubles as both a synthesis and energy storage solution. Their technology integrates with renewable energy sources to produce green ammonia through a proprietary alkaline electrolysis system. The company's cost modeling indicates potential production costs of $450-550 per ton of ammonia when operating at scale with renewable electricity at $0.04/kWh. GenCell's system employs specialized catalysts based on non-precious metals that demonstrate enhanced nitrogen reduction reaction (NRR) activity while minimizing the competing hydrogen evolution reaction. Their process architecture includes a three-chamber cell design that separates the nitrogen reduction and hydrogen evolution processes, improving overall efficiency and selectivity[2]. The company has developed comprehensive techno-economic models that account for capital costs, operational expenses, catalyst lifetimes, and electricity pricing scenarios across different geographical regions, demonstrating pathways to cost-competitive ammonia production compared to conventional methods.
Strengths: Integrated approach that serves both chemical production and energy storage needs; alkaline system avoids expensive platinum group metal catalysts; modular design enables deployment across various scales. Weaknesses: Current energy efficiency remains below theoretical optimum; system requires further engineering to reduce overall capital costs; catalyst performance degradation over time necessitates periodic replacement, impacting long-term operational costs.
Key Patents and Literature on Electrocatalytic Ammonia Production
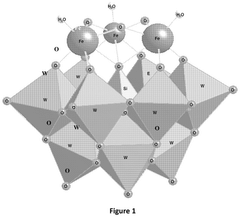
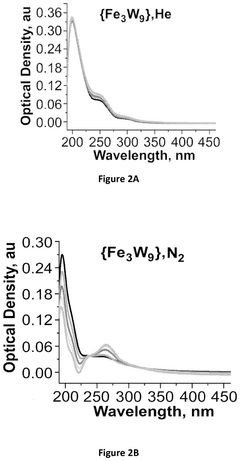
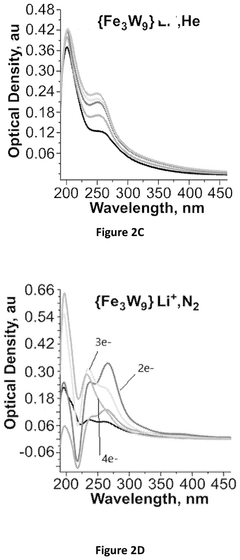
Electrochemical reduction of nitrogen to ammonia catalyzed by polyoxometalates
PatentPendingUS20250034724A1
Innovation
- The use of a polyoxometalate catalyst in combination with an alkali metal cation and a donor of protons and/or electrons in an electrochemical cell to reduce dinitrogen to ammonia at low negative cathodic potentials and ambient conditions.
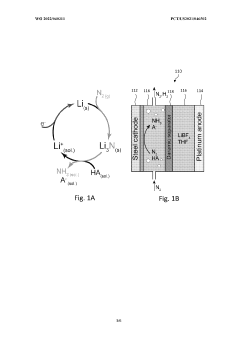
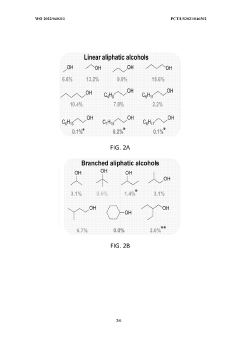
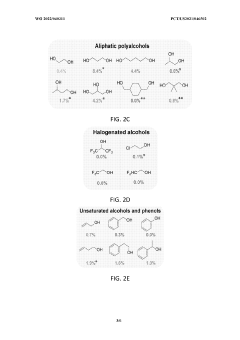
Lithium-mediated electrochemical ammonia synthesis
PatentWO2022040311A1
Innovation
- The use of a lithium-mediated electrochemical cell with specific hydrogen donors, such as alcohols or ionic liquids with high Kamlet-Taft alpha and beta parameters, to facilitate the conversion of nitrogen gas to ammonia at ambient temperature and pressure, utilizing lithium ions converted to metal at the cathode and reacting with nitrogen to form lithium nitride, which then reacts with protons to produce ammonia.
Energy Efficiency Analysis of Competing Ammonia Production Methods
The energy efficiency of ammonia production methods represents a critical metric for evaluating their economic and environmental viability. Traditional Haber-Bosch process, despite its industrial maturity, operates under energy-intensive conditions requiring temperatures of 400-500°C and pressures of 150-300 bar, resulting in an energy consumption of approximately 9-13 MWh per ton of ammonia produced. This significant energy requirement contributes to the process accounting for approximately 1-2% of global energy consumption.
Electrochemical nitrogen reduction (ENR), by contrast, offers theoretical advantages in energy efficiency by operating at ambient conditions. Current ENR systems demonstrate energy efficiencies ranging from 0.5% to 10%, depending on catalyst selection, cell design, and operating parameters. The most promising laboratory-scale ENR systems report energy consumption figures of approximately 20-30 MWh per ton of ammonia, still higher than conventional methods but showing consistent improvement trajectories.
When comparing these methods, it is essential to consider the source of energy. Haber-Bosch typically relies on fossil fuels, while ENR can be powered by renewable electricity. This distinction becomes significant when calculating full lifecycle efficiency. ENR systems powered by renewable sources may achieve carbon neutrality despite lower conversion efficiencies, providing a net environmental benefit over more efficient but carbon-intensive conventional processes.
The energy return on investment (EROI) analysis reveals that current ENR technologies require approximately 2-3 times more primary energy input than Haber-Bosch when using grid electricity. However, this gap narrows significantly when renewable electricity sources are considered, particularly in regions with high renewable penetration.
System-level efficiency comparisons must account for auxiliary processes. ENR eliminates the need for hydrogen production and high-pressure compression equipment required by Haber-Bosch, potentially offsetting some efficiency disadvantages. Additionally, the modular nature of ENR allows for distributed production, reducing transportation energy requirements in the ammonia supply chain.
Recent technological breakthroughs in catalyst design have demonstrated potential for efficiency improvements of 15-20% in ENR systems. Particularly promising are dual-function catalysts that simultaneously activate nitrogen and facilitate proton transfer, reducing the overpotential required for ammonia formation and consequently improving energy efficiency.
Electrochemical nitrogen reduction (ENR), by contrast, offers theoretical advantages in energy efficiency by operating at ambient conditions. Current ENR systems demonstrate energy efficiencies ranging from 0.5% to 10%, depending on catalyst selection, cell design, and operating parameters. The most promising laboratory-scale ENR systems report energy consumption figures of approximately 20-30 MWh per ton of ammonia, still higher than conventional methods but showing consistent improvement trajectories.
When comparing these methods, it is essential to consider the source of energy. Haber-Bosch typically relies on fossil fuels, while ENR can be powered by renewable electricity. This distinction becomes significant when calculating full lifecycle efficiency. ENR systems powered by renewable sources may achieve carbon neutrality despite lower conversion efficiencies, providing a net environmental benefit over more efficient but carbon-intensive conventional processes.
The energy return on investment (EROI) analysis reveals that current ENR technologies require approximately 2-3 times more primary energy input than Haber-Bosch when using grid electricity. However, this gap narrows significantly when renewable electricity sources are considered, particularly in regions with high renewable penetration.
System-level efficiency comparisons must account for auxiliary processes. ENR eliminates the need for hydrogen production and high-pressure compression equipment required by Haber-Bosch, potentially offsetting some efficiency disadvantages. Additionally, the modular nature of ENR allows for distributed production, reducing transportation energy requirements in the ammonia supply chain.
Recent technological breakthroughs in catalyst design have demonstrated potential for efficiency improvements of 15-20% in ENR systems. Particularly promising are dual-function catalysts that simultaneously activate nitrogen and facilitate proton transfer, reducing the overpotential required for ammonia formation and consequently improving energy efficiency.
Environmental Impact and Sustainability Assessment
The environmental impact of electrocatalytic ammonia production through electrochemical nitrogen reduction represents a critical dimension in evaluating this emerging technology's viability. Conventional ammonia production via the Haber-Bosch process accounts for approximately 1-2% of global energy consumption and generates substantial CO2 emissions—approximately 1.9 tons of CO2 per ton of ammonia produced. Electrochemical nitrogen reduction offers a potentially transformative alternative with significantly reduced carbon footprint, particularly when powered by renewable electricity sources.
Life cycle assessment (LCA) studies indicate that electrochemical ammonia synthesis could reduce greenhouse gas emissions by 60-90% compared to conventional methods when utilizing renewable energy. This dramatic reduction stems from eliminating natural gas as both feedstock and energy source, which constitutes the primary environmental burden in traditional ammonia production. However, these environmental benefits are highly dependent on the electricity source, with coal-powered electrochemical production potentially generating higher emissions than optimized Haber-Bosch facilities.
Water consumption presents another important sustainability consideration. While electrochemical processes require water as a hydrogen source, their overall water footprint can be lower than conventional methods when considering the entire production chain. Preliminary studies suggest a potential reduction of 30-50% in water usage, though this varies significantly based on catalyst systems and cell designs.
Resource efficiency must also be evaluated through the lens of critical materials used in electrocatalysts. Many high-performance catalysts incorporate precious metals or rare earth elements, raising concerns about resource depletion and supply chain vulnerability. Sustainable development of this technology necessitates research into abundant, earth-abundant catalyst alternatives that maintain performance while reducing dependence on scarce materials.
Land use impacts differ substantially between conventional and electrochemical approaches. Distributed electrochemical ammonia production could reduce transportation requirements and associated emissions by enabling localized production closer to agricultural end-users. This decentralization potential represents a significant sustainability advantage, particularly for remote agricultural regions currently dependent on long-distance ammonia transport.
Waste generation and management considerations include catalyst degradation products and membrane disposal. Current research indicates minimal toxic waste generation compared to conventional processes, though long-term studies on catalyst stability and degradation pathways remain limited. Developing circular economy approaches for catalyst recycling and regeneration will be essential for maximizing sustainability benefits.
Regulatory frameworks and sustainability certification systems will play crucial roles in guiding the environmental performance of emerging electrochemical ammonia technologies. Standardized metrics for comparing environmental impacts across different production methods will be necessary to inform policy decisions and market adoption strategies as this technology advances toward commercialization.
Life cycle assessment (LCA) studies indicate that electrochemical ammonia synthesis could reduce greenhouse gas emissions by 60-90% compared to conventional methods when utilizing renewable energy. This dramatic reduction stems from eliminating natural gas as both feedstock and energy source, which constitutes the primary environmental burden in traditional ammonia production. However, these environmental benefits are highly dependent on the electricity source, with coal-powered electrochemical production potentially generating higher emissions than optimized Haber-Bosch facilities.
Water consumption presents another important sustainability consideration. While electrochemical processes require water as a hydrogen source, their overall water footprint can be lower than conventional methods when considering the entire production chain. Preliminary studies suggest a potential reduction of 30-50% in water usage, though this varies significantly based on catalyst systems and cell designs.
Resource efficiency must also be evaluated through the lens of critical materials used in electrocatalysts. Many high-performance catalysts incorporate precious metals or rare earth elements, raising concerns about resource depletion and supply chain vulnerability. Sustainable development of this technology necessitates research into abundant, earth-abundant catalyst alternatives that maintain performance while reducing dependence on scarce materials.
Land use impacts differ substantially between conventional and electrochemical approaches. Distributed electrochemical ammonia production could reduce transportation requirements and associated emissions by enabling localized production closer to agricultural end-users. This decentralization potential represents a significant sustainability advantage, particularly for remote agricultural regions currently dependent on long-distance ammonia transport.
Waste generation and management considerations include catalyst degradation products and membrane disposal. Current research indicates minimal toxic waste generation compared to conventional processes, though long-term studies on catalyst stability and degradation pathways remain limited. Developing circular economy approaches for catalyst recycling and regeneration will be essential for maximizing sustainability benefits.
Regulatory frameworks and sustainability certification systems will play crucial roles in guiding the environmental performance of emerging electrochemical ammonia technologies. Standardized metrics for comparing environmental impacts across different production methods will be necessary to inform policy decisions and market adoption strategies as this technology advances toward commercialization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!