In Situ Spectroscopy Methods for Identifying Intermediates in Electrochemical Nitrogen Reduction
AUG 26, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical NRR Spectroscopy Background and Objectives
Electrochemical nitrogen reduction reaction (NRR) represents a revolutionary approach to ammonia synthesis that could potentially replace the energy-intensive Haber-Bosch process, which currently consumes approximately 1-2% of global energy production. The development of this technology has evolved significantly since the early 2000s, with major breakthroughs occurring in the last decade as researchers have focused on creating efficient electrocatalysts capable of converting atmospheric nitrogen to ammonia under ambient conditions.
The technical evolution in this field has progressed from early proof-of-concept studies to increasingly sophisticated catalyst designs and reaction systems. Initial research primarily utilized simple metal electrodes, while recent advances have incorporated nanomaterials, single-atom catalysts, and complex hybrid structures to enhance reaction efficiency and selectivity.
A critical challenge in advancing electrochemical NRR technology has been the identification and characterization of reaction intermediates. These transient species exist briefly during the multi-step electron transfer process that converts N₂ to NH₃, making them exceptionally difficult to detect and analyze using conventional methods.
In situ spectroscopy has emerged as a vital tool for addressing this challenge, allowing researchers to observe reaction mechanisms in real-time under operating conditions. The development of these spectroscopic techniques has paralleled advancements in NRR catalyst design, with increasingly sophisticated methods being deployed to capture the elusive intermediate species.
The primary objective of in situ spectroscopy application in electrochemical NRR is to elucidate the complete reaction pathway, including the formation and transformation of key intermediates such as *N₂H, *NNH₂, and *NH₂. Understanding these mechanisms is fundamental to rational catalyst design and optimization of reaction conditions.
Secondary objectives include correlating spectroscopic data with electrochemical performance metrics, distinguishing between competing reaction pathways (associative vs. dissociative mechanisms), and identifying rate-determining steps that limit overall efficiency.
The technical trajectory indicates a convergence of advanced spectroscopic methods with computational modeling to create a comprehensive understanding of NRR mechanisms. This synergistic approach aims to bridge the gap between theoretical predictions and experimental observations, ultimately enabling the design of catalysts with significantly improved Faradaic efficiency and production rates.
Recent trends suggest increasing focus on operando techniques that combine multiple spectroscopic methods simultaneously with electrochemical measurements, providing multi-dimensional insights into the complex surface chemistry of nitrogen reduction.
The technical evolution in this field has progressed from early proof-of-concept studies to increasingly sophisticated catalyst designs and reaction systems. Initial research primarily utilized simple metal electrodes, while recent advances have incorporated nanomaterials, single-atom catalysts, and complex hybrid structures to enhance reaction efficiency and selectivity.
A critical challenge in advancing electrochemical NRR technology has been the identification and characterization of reaction intermediates. These transient species exist briefly during the multi-step electron transfer process that converts N₂ to NH₃, making them exceptionally difficult to detect and analyze using conventional methods.
In situ spectroscopy has emerged as a vital tool for addressing this challenge, allowing researchers to observe reaction mechanisms in real-time under operating conditions. The development of these spectroscopic techniques has paralleled advancements in NRR catalyst design, with increasingly sophisticated methods being deployed to capture the elusive intermediate species.
The primary objective of in situ spectroscopy application in electrochemical NRR is to elucidate the complete reaction pathway, including the formation and transformation of key intermediates such as *N₂H, *NNH₂, and *NH₂. Understanding these mechanisms is fundamental to rational catalyst design and optimization of reaction conditions.
Secondary objectives include correlating spectroscopic data with electrochemical performance metrics, distinguishing between competing reaction pathways (associative vs. dissociative mechanisms), and identifying rate-determining steps that limit overall efficiency.
The technical trajectory indicates a convergence of advanced spectroscopic methods with computational modeling to create a comprehensive understanding of NRR mechanisms. This synergistic approach aims to bridge the gap between theoretical predictions and experimental observations, ultimately enabling the design of catalysts with significantly improved Faradaic efficiency and production rates.
Recent trends suggest increasing focus on operando techniques that combine multiple spectroscopic methods simultaneously with electrochemical measurements, providing multi-dimensional insights into the complex surface chemistry of nitrogen reduction.
Market Analysis for Sustainable Ammonia Production Technologies
The global ammonia market is experiencing significant transformation driven by sustainability imperatives, with the electrochemical nitrogen reduction reaction (NRR) emerging as a promising alternative to the conventional Haber-Bosch process. The current ammonia market, valued at approximately $72 billion in 2022, is projected to grow at a CAGR of 5.3% through 2030, primarily fueled by agricultural demands which account for over 80% of consumption.
Traditional ammonia production via the Haber-Bosch process consumes 1-2% of global energy and generates substantial CO2 emissions—approximately 1.8 tons of CO2 per ton of ammonia produced. This environmental burden has created a substantial market opportunity for sustainable alternatives, particularly electrochemical nitrogen reduction technologies that can operate at ambient conditions using renewable electricity.
Market analysis reveals growing investment in sustainable ammonia production, with venture capital funding for electrochemical nitrogen reduction startups increasing by 45% between 2019 and 2022. Major chemical companies including BASF, Yara, and CF Industries have announced strategic initiatives to incorporate green ammonia production into their portfolios, signaling strong commercial interest.
The agricultural sector represents the largest potential market for sustainably produced ammonia, driven by increasing demand for low-carbon fertilizers. Premium pricing models suggest farmers may accept a 15-20% price premium for carbon-neutral fertilizers, particularly in regions with stringent emissions regulations or carbon pricing mechanisms.
Beyond agriculture, emerging applications in energy storage and transportation fuel are expanding the potential market. Ammonia's high energy density (22.5 MJ/kg) makes it attractive as a hydrogen carrier, with projections suggesting the ammonia-as-fuel market could reach $10 billion by 2030 if technical and infrastructure challenges are addressed.
Regional market analysis indicates Europe leads in sustainable ammonia development due to aggressive decarbonization policies, while Asia-Pacific represents the largest growth market due to agricultural intensification and industrial expansion. North America shows increasing interest driven by renewable energy availability and agricultural sector demands.
The economic viability of electrochemical nitrogen reduction technologies remains challenging, with current production costs estimated at 3-4 times that of conventional methods. However, techno-economic analyses suggest cost parity could be achieved by 2030 with advances in catalyst efficiency, renewable electricity costs reduction, and scaled manufacturing.
Market adoption will likely follow a phased approach, beginning with premium niche applications where sustainability commands value, followed by broader adoption as economies of scale and technological improvements reduce costs. The development of in situ spectroscopy methods for identifying reaction intermediates represents a critical enabling technology that could accelerate this market evolution by improving process efficiency and selectivity.
Traditional ammonia production via the Haber-Bosch process consumes 1-2% of global energy and generates substantial CO2 emissions—approximately 1.8 tons of CO2 per ton of ammonia produced. This environmental burden has created a substantial market opportunity for sustainable alternatives, particularly electrochemical nitrogen reduction technologies that can operate at ambient conditions using renewable electricity.
Market analysis reveals growing investment in sustainable ammonia production, with venture capital funding for electrochemical nitrogen reduction startups increasing by 45% between 2019 and 2022. Major chemical companies including BASF, Yara, and CF Industries have announced strategic initiatives to incorporate green ammonia production into their portfolios, signaling strong commercial interest.
The agricultural sector represents the largest potential market for sustainably produced ammonia, driven by increasing demand for low-carbon fertilizers. Premium pricing models suggest farmers may accept a 15-20% price premium for carbon-neutral fertilizers, particularly in regions with stringent emissions regulations or carbon pricing mechanisms.
Beyond agriculture, emerging applications in energy storage and transportation fuel are expanding the potential market. Ammonia's high energy density (22.5 MJ/kg) makes it attractive as a hydrogen carrier, with projections suggesting the ammonia-as-fuel market could reach $10 billion by 2030 if technical and infrastructure challenges are addressed.
Regional market analysis indicates Europe leads in sustainable ammonia development due to aggressive decarbonization policies, while Asia-Pacific represents the largest growth market due to agricultural intensification and industrial expansion. North America shows increasing interest driven by renewable energy availability and agricultural sector demands.
The economic viability of electrochemical nitrogen reduction technologies remains challenging, with current production costs estimated at 3-4 times that of conventional methods. However, techno-economic analyses suggest cost parity could be achieved by 2030 with advances in catalyst efficiency, renewable electricity costs reduction, and scaled manufacturing.
Market adoption will likely follow a phased approach, beginning with premium niche applications where sustainability commands value, followed by broader adoption as economies of scale and technological improvements reduce costs. The development of in situ spectroscopy methods for identifying reaction intermediates represents a critical enabling technology that could accelerate this market evolution by improving process efficiency and selectivity.
Current In Situ Spectroscopy Techniques and Challenges
In situ spectroscopy techniques have emerged as powerful tools for real-time monitoring of electrochemical nitrogen reduction reaction (NRR) processes. Currently, several spectroscopic methods are employed to identify reaction intermediates, with Raman spectroscopy being one of the most widely utilized. Surface-enhanced Raman spectroscopy (SERS) offers significant advantages due to its ability to detect species at the electrode-electrolyte interface with enhanced sensitivity. This technique has successfully identified key nitrogen-containing intermediates such as N2H, NH, and NH2 during NRR operations.
Infrared (IR) spectroscopy, particularly in its attenuated total reflection (ATR) configuration, provides complementary information about vibrational modes of adsorbed species. ATR-FTIR has been instrumental in distinguishing between different nitrogen-containing intermediates based on their characteristic absorption bands. Recent advances in operando IR techniques have improved temporal resolution, allowing researchers to capture transient species with lifetimes in the millisecond range.
X-ray absorption spectroscopy (XAS) techniques, including X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), offer element-specific information about electronic structure and local coordination environments. These techniques have been valuable for tracking changes in catalyst oxidation states during NRR and correlating them with intermediate formation.
Despite these advances, significant challenges persist in the application of in situ spectroscopy for NRR intermediate identification. The primary challenge is the extremely low concentration of intermediates, often at sub-monolayer coverage, which pushes detection limits of most spectroscopic techniques. This is exacerbated by the short lifetime of many nitrogen reduction intermediates, requiring exceptional temporal resolution.
Signal interference presents another major obstacle. The aqueous environment necessary for electrochemical reactions creates strong background signals that can obscure the spectral features of interest. Water's strong IR absorption bands, for instance, overlap with many N-H stretching vibrations critical for identifying NRR intermediates.
Technical limitations also impede progress. Many spectroscopic cells compromise either electrochemical performance or spectroscopic sensitivity. The need for specialized transparent electrodes or thin-layer configurations often alters reaction conditions compared to practical NRR systems.
Data interpretation remains challenging due to the complexity of spectra obtained under reaction conditions. Distinguishing between similar nitrogen-containing species (e.g., NH2 vs. NH2OH) requires sophisticated computational methods and reference spectra that are not always available. Additionally, the dynamic nature of the electrode-electrolyte interface during potential cycling creates a constantly changing spectroscopic environment.
Infrared (IR) spectroscopy, particularly in its attenuated total reflection (ATR) configuration, provides complementary information about vibrational modes of adsorbed species. ATR-FTIR has been instrumental in distinguishing between different nitrogen-containing intermediates based on their characteristic absorption bands. Recent advances in operando IR techniques have improved temporal resolution, allowing researchers to capture transient species with lifetimes in the millisecond range.
X-ray absorption spectroscopy (XAS) techniques, including X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), offer element-specific information about electronic structure and local coordination environments. These techniques have been valuable for tracking changes in catalyst oxidation states during NRR and correlating them with intermediate formation.
Despite these advances, significant challenges persist in the application of in situ spectroscopy for NRR intermediate identification. The primary challenge is the extremely low concentration of intermediates, often at sub-monolayer coverage, which pushes detection limits of most spectroscopic techniques. This is exacerbated by the short lifetime of many nitrogen reduction intermediates, requiring exceptional temporal resolution.
Signal interference presents another major obstacle. The aqueous environment necessary for electrochemical reactions creates strong background signals that can obscure the spectral features of interest. Water's strong IR absorption bands, for instance, overlap with many N-H stretching vibrations critical for identifying NRR intermediates.
Technical limitations also impede progress. Many spectroscopic cells compromise either electrochemical performance or spectroscopic sensitivity. The need for specialized transparent electrodes or thin-layer configurations often alters reaction conditions compared to practical NRR systems.
Data interpretation remains challenging due to the complexity of spectra obtained under reaction conditions. Distinguishing between similar nitrogen-containing species (e.g., NH2 vs. NH2OH) requires sophisticated computational methods and reference spectra that are not always available. Additionally, the dynamic nature of the electrode-electrolyte interface during potential cycling creates a constantly changing spectroscopic environment.
State-of-the-Art In Situ Spectroscopy Solutions for NRR
01 Raman spectroscopy for in situ reaction monitoring
Raman spectroscopy is utilized for real-time monitoring of chemical reactions and identification of intermediates. This non-destructive technique provides molecular fingerprinting capabilities that allow for the detection and characterization of reaction intermediates without sample preparation. The method enables researchers to track reaction progress, identify transient species, and optimize reaction conditions by observing spectral changes associated with the formation and consumption of intermediates.- Raman spectroscopy for in situ reaction monitoring: Raman spectroscopy techniques are employed for real-time monitoring of chemical reactions and identification of intermediates. This non-destructive method allows for the detection and characterization of reaction intermediates without interrupting the reaction process. The technique provides molecular-level information about the structural changes occurring during reactions, enabling researchers to track reaction pathways and identify key intermediates that may be short-lived or difficult to isolate using conventional methods.
- Infrared spectroscopy methods for intermediate analysis: Infrared spectroscopy techniques, including FTIR (Fourier Transform Infrared) spectroscopy, are utilized for the identification and characterization of reaction intermediates in situ. These methods detect the vibrational modes of molecules, providing valuable information about functional groups and molecular structures of intermediates. The ability to perform measurements in real-time allows researchers to monitor reaction progress and identify transient species that may play crucial roles in reaction mechanisms.
- NMR spectroscopy for intermediate structure elucidation: Nuclear Magnetic Resonance (NMR) spectroscopy is applied for in situ analysis of reaction intermediates, providing detailed structural information at the atomic level. This technique allows researchers to determine the molecular structure of intermediates by analyzing the magnetic properties of atomic nuclei. In situ NMR methods enable continuous monitoring of reactions, helping to identify and characterize intermediates that may be crucial for understanding reaction mechanisms and optimizing chemical processes.
- Mass spectrometry for real-time intermediate detection: Mass spectrometry techniques are employed for the real-time detection and identification of reaction intermediates. These methods provide information about the molecular weight and fragmentation patterns of intermediates, allowing for their identification even in complex reaction mixtures. In situ mass spectrometry enables researchers to monitor the formation and consumption of intermediates during reactions, providing insights into reaction pathways and mechanisms that can be used to optimize chemical processes.
- Combined spectroscopic approaches for comprehensive intermediate analysis: Multiple spectroscopic techniques are integrated to provide comprehensive analysis of reaction intermediates. By combining different methods such as Raman, infrared, NMR, and mass spectrometry, researchers can obtain complementary information about intermediates, leading to more accurate identification and characterization. These combined approaches enable the detection of a wider range of intermediates and provide more detailed information about their structures and properties, facilitating a deeper understanding of reaction mechanisms.
02 Infrared spectroscopy techniques for intermediate analysis
Infrared (IR) spectroscopy methods, including FTIR and ATR-IR, are employed for in situ identification of reaction intermediates. These techniques detect the vibrational modes of molecules, providing structural information about intermediates formed during chemical processes. The ability to perform measurements in real-time allows for monitoring reaction kinetics and mechanism elucidation by tracking the appearance and disappearance of characteristic absorption bands associated with intermediate species.Expand Specific Solutions03 NMR spectroscopy for in situ intermediate characterization
Nuclear Magnetic Resonance (NMR) spectroscopy enables the structural elucidation of reaction intermediates in situ. This technique provides detailed information about molecular structure, connectivity, and dynamics of intermediates during chemical transformations. Flow-through NMR systems and specialized probes allow for continuous monitoring of reactions, helping researchers understand reaction mechanisms by identifying key intermediate species and their transformation pathways.Expand Specific Solutions04 Mass spectrometry coupled with in situ sampling
Mass spectrometry techniques combined with specialized sampling interfaces enable real-time identification of reaction intermediates. These methods provide molecular weight and fragmentation pattern information that helps in characterizing transient species. Techniques such as electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) coupled with mass analyzers allow for sensitive detection of intermediates, even those present in low concentrations or with short lifetimes.Expand Specific Solutions05 Multimodal spectroscopic approaches for comprehensive intermediate analysis
Combining multiple spectroscopic techniques provides complementary information for more comprehensive identification and characterization of reaction intermediates. These integrated approaches utilize the strengths of different methods such as UV-Vis, Raman, IR, and NMR spectroscopy to overcome the limitations of individual techniques. Advanced data processing algorithms and chemometric methods help correlate spectral data from different sources, enabling more accurate identification of complex intermediate structures and reaction pathways.Expand Specific Solutions
Leading Research Groups and Industrial Players in NRR Spectroscopy
The field of In Situ Spectroscopy Methods for Electrochemical Nitrogen Reduction is in an early growth phase, characterized by intensive academic research with emerging industrial interest. The global market for these technologies is expanding, driven by sustainable ammonia production demands, though still relatively small compared to established spectroscopy markets. Leading academic institutions including Xiamen University, Dalian Institute of Chemical Physics, and University of California are advancing fundamental research, while companies like Shell, China Petroleum & Chemical Corp., and Agilent Technologies are beginning to develop commercial applications. The technology remains in early-to-mid maturity, with significant challenges in sensitivity and real-time detection of reaction intermediates still requiring breakthrough innovations before widespread industrial adoption.
Xiamen University
Technical Solution: Xiamen University has developed a comprehensive in situ spectroelectrochemical platform specifically for studying electrochemical nitrogen reduction reaction (NRR) intermediates. Their technical approach integrates attenuated total reflection surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS) with electrochemical measurements in custom-designed thin-layer flow cells. This setup allows for real-time monitoring of nitrogen-containing species at the electrode-electrolyte interface under reaction conditions. The university has pioneered the application of shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS) for NRR, enabling detection of reaction intermediates on practical catalyst surfaces without interference from the enhancing material. Their methodology incorporates potential-dependent spectroscopic mapping to correlate intermediate formation with electrochemical performance, providing crucial insights into reaction mechanisms and rate-determining steps.
Strengths: Innovative integration of multiple spectroscopic techniques with electrochemical measurements; expertise in surface-enhanced spectroscopy methods; strong capabilities in custom cell design for in situ measurements. Weaknesses: Highly specialized equipment and expertise requirements; potential challenges in distinguishing true intermediates from spectator species; limited throughput for catalyst screening applications.
Dalian Institute of Chemical Physics Chinese Academy of Sciences
Technical Solution: Dalian Institute has developed advanced in situ spectroscopic techniques specifically for electrochemical nitrogen reduction reaction (NRR), combining operando Raman spectroscopy with differential electrochemical mass spectrometry (DEMS) to identify reaction intermediates in real-time. Their approach utilizes surface-enhanced Raman spectroscopy (SERS) with carefully designed electrodes featuring plasmonic nanostructures that enhance signal detection of adsorbed nitrogen species. The institute has pioneered the use of isotope labeling (15N2) combined with in situ infrared spectroscopy to distinguish between different nitrogen-containing intermediates and track reaction pathways. Their technical solution incorporates multimodal spectroscopy, integrating X-ray absorption spectroscopy (XAS) with vibrational spectroscopy to simultaneously monitor catalyst electronic structure changes and surface-adsorbed species during nitrogen reduction.
Strengths: Exceptional expertise in combining multiple spectroscopic techniques; strong capabilities in isotope labeling methodologies; established infrastructure for advanced characterization. Weaknesses: Highly specialized equipment requirements limit widespread adoption; complex data interpretation necessitates significant expertise; potential challenges in translating laboratory findings to industrial applications.
Critical Spectroscopic Innovations for Nitrogen Reduction Intermediates
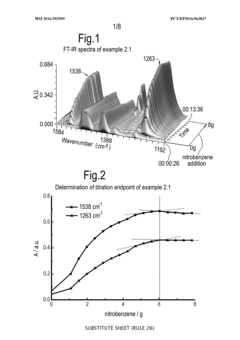
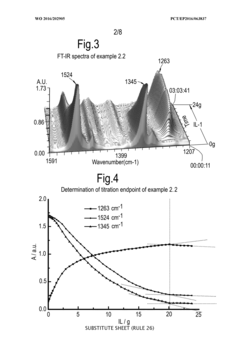
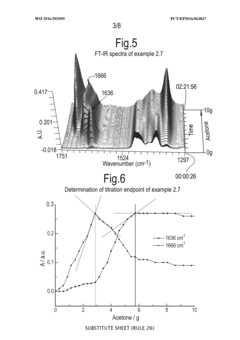
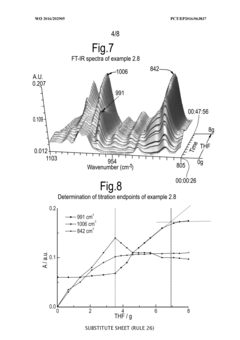
Process for monitoring the catalytic activity of an ionic liquid
PatentWO2016202905A1
Innovation
- A process involving in-situ infrared-complexometric titration using organic compounds with nitrogen, oxygen, or sulfur groups to quantify the catalytic activity of acidic ionic liquids, specifically chloroaluminate ionic liquids, by recording absorption peaks and calculating the catalytic activity based on the amount of reagents added during titration.
Environmental Impact and Sustainability Assessment
The electrochemical nitrogen reduction reaction (NRR) represents a promising approach for sustainable ammonia production, potentially replacing the energy-intensive Haber-Bosch process. When evaluating in situ spectroscopy methods for identifying intermediates in this process, environmental impact and sustainability considerations are paramount to ensuring the technology's alignment with green chemistry principles.
The development of in situ spectroscopic techniques for NRR offers significant environmental benefits through process optimization. By enabling real-time identification of reaction intermediates, these methods allow researchers to design more efficient catalysts and reaction conditions that minimize energy consumption. This directly addresses one of the most pressing environmental concerns with conventional ammonia production, which currently consumes approximately 1-2% of global energy and generates substantial CO2 emissions.
Water consumption represents another critical environmental factor in electrochemical nitrogen reduction. In situ spectroscopy helps optimize reaction pathways that favor nitrogen reduction over competing hydrogen evolution reactions, potentially improving water utilization efficiency. This is particularly important as water scarcity becomes an increasingly urgent global challenge, with agricultural fertilizer production being a significant consumer of water resources.
From a life cycle assessment perspective, in situ spectroscopic techniques generally have minimal direct environmental footprint compared to the processes they help optimize. The materials used in spectroscopic equipment typically include optical components, electronics, and specialized detection systems. While some rare elements may be required, their quantities are negligible compared to the environmental benefits gained through process improvements.
Regarding sustainability metrics, the implementation of advanced in situ monitoring enables precise quantification of Faradaic efficiency and selectivity. These parameters directly correlate with resource utilization efficiency and waste generation. Higher selectivity means fewer side products and waste streams requiring treatment or disposal, contributing to circular economy principles.
The scalability of spectroscopic methods also influences their sustainability profile. While laboratory-scale implementations may use expensive components with limited lifespans, industrial adaptations often emphasize durability and resource efficiency. Recent developments in fiber optics and miniaturized spectroscopic systems have reduced material requirements while extending operational lifetimes.
When considering broader environmental implications, improved NRR processes enabled by in situ spectroscopy could significantly reduce agriculture's carbon footprint. Ammonia-based fertilizers produced through renewable electricity-powered electrochemical processes could potentially achieve carbon neutrality, representing a transformative shift from current fossil fuel-dependent production methods.
In conclusion, in situ spectroscopy methods for identifying intermediates in electrochemical nitrogen reduction offer substantial environmental benefits through process optimization, reduced resource consumption, and potential decarbonization of ammonia production. These sustainability advantages must be weighed against the material and energy requirements of implementing the spectroscopic systems themselves, though the net environmental benefit appears strongly positive.
The development of in situ spectroscopic techniques for NRR offers significant environmental benefits through process optimization. By enabling real-time identification of reaction intermediates, these methods allow researchers to design more efficient catalysts and reaction conditions that minimize energy consumption. This directly addresses one of the most pressing environmental concerns with conventional ammonia production, which currently consumes approximately 1-2% of global energy and generates substantial CO2 emissions.
Water consumption represents another critical environmental factor in electrochemical nitrogen reduction. In situ spectroscopy helps optimize reaction pathways that favor nitrogen reduction over competing hydrogen evolution reactions, potentially improving water utilization efficiency. This is particularly important as water scarcity becomes an increasingly urgent global challenge, with agricultural fertilizer production being a significant consumer of water resources.
From a life cycle assessment perspective, in situ spectroscopic techniques generally have minimal direct environmental footprint compared to the processes they help optimize. The materials used in spectroscopic equipment typically include optical components, electronics, and specialized detection systems. While some rare elements may be required, their quantities are negligible compared to the environmental benefits gained through process improvements.
Regarding sustainability metrics, the implementation of advanced in situ monitoring enables precise quantification of Faradaic efficiency and selectivity. These parameters directly correlate with resource utilization efficiency and waste generation. Higher selectivity means fewer side products and waste streams requiring treatment or disposal, contributing to circular economy principles.
The scalability of spectroscopic methods also influences their sustainability profile. While laboratory-scale implementations may use expensive components with limited lifespans, industrial adaptations often emphasize durability and resource efficiency. Recent developments in fiber optics and miniaturized spectroscopic systems have reduced material requirements while extending operational lifetimes.
When considering broader environmental implications, improved NRR processes enabled by in situ spectroscopy could significantly reduce agriculture's carbon footprint. Ammonia-based fertilizers produced through renewable electricity-powered electrochemical processes could potentially achieve carbon neutrality, representing a transformative shift from current fossil fuel-dependent production methods.
In conclusion, in situ spectroscopy methods for identifying intermediates in electrochemical nitrogen reduction offer substantial environmental benefits through process optimization, reduced resource consumption, and potential decarbonization of ammonia production. These sustainability advantages must be weighed against the material and energy requirements of implementing the spectroscopic systems themselves, though the net environmental benefit appears strongly positive.
Scalability and Industrial Implementation Considerations
The scalability of in situ spectroscopy methods for electrochemical nitrogen reduction represents a critical consideration for transitioning from laboratory-scale research to industrial implementation. Current spectroscopic techniques such as Raman, FTIR, and X-ray absorption spectroscopy often require specialized equipment and controlled environments that present significant challenges for large-scale operations. The miniaturization and adaptation of these technologies for industrial settings remains a substantial engineering challenge that must be addressed before widespread adoption.
Cost considerations play a pivotal role in industrial implementation. High-resolution spectroscopic equipment typically involves substantial capital investment, with maintenance requirements that can impact operational expenses. A comprehensive cost-benefit analysis must evaluate whether the improved process control and efficiency gained through real-time intermediate detection justifies the financial outlay, particularly for commercial-scale nitrogen reduction operations.
Integration with existing industrial infrastructure presents another dimension of complexity. Most current electrochemical nitrogen reduction processes were not designed with in situ monitoring capabilities in mind. Retrofitting established systems requires careful engineering to ensure spectroscopic components do not interfere with process efficiency or safety protocols. The development of standardized interfaces between spectroscopic equipment and industrial electrochemical cells would significantly accelerate adoption.
Data management infrastructure represents an often overlooked aspect of scalability. In situ spectroscopy generates enormous volumes of spectral data that require sophisticated processing algorithms and storage solutions. Industrial implementation necessitates robust data pipelines capable of real-time analysis to provide actionable insights for process control, along with secure long-term storage for quality assurance and process optimization purposes.
Regulatory considerations must also be addressed when scaling spectroscopic monitoring technologies. Different jurisdictions may have varying requirements regarding process validation, equipment certification, and data integrity. Developing standardized protocols that satisfy global regulatory frameworks would facilitate broader industrial adoption while ensuring consistent quality and safety standards.
The workforce implications of implementing advanced spectroscopic technologies should not be underestimated. Industrial adoption requires personnel with specialized training in both electrochemistry and spectroscopic analysis. Developing comprehensive training programs and user-friendly interfaces would mitigate this challenge and accelerate technology transfer from research laboratories to production environments.
Cost considerations play a pivotal role in industrial implementation. High-resolution spectroscopic equipment typically involves substantial capital investment, with maintenance requirements that can impact operational expenses. A comprehensive cost-benefit analysis must evaluate whether the improved process control and efficiency gained through real-time intermediate detection justifies the financial outlay, particularly for commercial-scale nitrogen reduction operations.
Integration with existing industrial infrastructure presents another dimension of complexity. Most current electrochemical nitrogen reduction processes were not designed with in situ monitoring capabilities in mind. Retrofitting established systems requires careful engineering to ensure spectroscopic components do not interfere with process efficiency or safety protocols. The development of standardized interfaces between spectroscopic equipment and industrial electrochemical cells would significantly accelerate adoption.
Data management infrastructure represents an often overlooked aspect of scalability. In situ spectroscopy generates enormous volumes of spectral data that require sophisticated processing algorithms and storage solutions. Industrial implementation necessitates robust data pipelines capable of real-time analysis to provide actionable insights for process control, along with secure long-term storage for quality assurance and process optimization purposes.
Regulatory considerations must also be addressed when scaling spectroscopic monitoring technologies. Different jurisdictions may have varying requirements regarding process validation, equipment certification, and data integrity. Developing standardized protocols that satisfy global regulatory frameworks would facilitate broader industrial adoption while ensuring consistent quality and safety standards.
The workforce implications of implementing advanced spectroscopic technologies should not be underestimated. Industrial adoption requires personnel with specialized training in both electrochemistry and spectroscopic analysis. Developing comprehensive training programs and user-friendly interfaces would mitigate this challenge and accelerate technology transfer from research laboratories to production environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!