Binder And Cathode Architecture Optimization For RT Na–S Batteries
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
RT Na-S Battery Technology Background and Objectives
Sodium-sulfur (Na-S) batteries have emerged as a promising energy storage technology due to their high theoretical energy density (1274 Wh/kg), abundant raw material resources, and cost-effectiveness compared to lithium-ion batteries. Traditionally, Na-S batteries operated at high temperatures (300-350°C), limiting their practical applications. The development of room temperature (RT) Na-S batteries represents a significant technological advancement, enabling broader deployment across various sectors including renewable energy integration, grid storage, and potentially electric vehicles.
The evolution of RT Na-S battery technology can be traced back to the early 2000s when researchers began exploring ways to overcome the challenges associated with high-temperature operation. Initial efforts focused on developing suitable electrolytes that could facilitate sodium ion transport at ambient temperatures. By 2010, several research groups had demonstrated proof-of-concept RT Na-S cells, albeit with limited cycle life and capacity retention.
Current technological trends in RT Na-S batteries are centered around addressing the fundamental challenges that hinder their commercial viability. These include the shuttle effect of polysulfides, volume expansion during cycling, slow reaction kinetics, and poor electronic conductivity of sulfur. The binder and cathode architecture have been identified as critical components that significantly influence battery performance, stability, and lifespan.
The primary technical objectives for RT Na-S battery development include achieving high specific capacity (approaching the theoretical value), extending cycle life to thousands of cycles, improving rate capability for fast charging applications, and ensuring safety under various operating conditions. Specifically for binder and cathode architecture optimization, the goals are to develop structures that can effectively contain polysulfides, accommodate volume changes, enhance electronic/ionic conductivity, and maintain structural integrity during long-term cycling.
Recent advancements in nanomaterials, conductive polymers, and composite structures have opened new avenues for cathode design. The integration of functional binders that can chemically interact with polysulfides represents a promising direction. Additionally, three-dimensional architectures that provide sufficient space for sulfur expansion while maintaining electronic pathways are being extensively investigated.
The optimization of binder and cathode architecture is expected to address approximately 40-50% of the current performance limitations in RT Na-S batteries. Successful development in this area could potentially enable energy densities exceeding 400 Wh/kg at the cell level, with cycle life approaching 1000 cycles at 80% capacity retention – metrics that would position RT Na-S batteries as competitive alternatives to current commercial battery technologies.
The evolution of RT Na-S battery technology can be traced back to the early 2000s when researchers began exploring ways to overcome the challenges associated with high-temperature operation. Initial efforts focused on developing suitable electrolytes that could facilitate sodium ion transport at ambient temperatures. By 2010, several research groups had demonstrated proof-of-concept RT Na-S cells, albeit with limited cycle life and capacity retention.
Current technological trends in RT Na-S batteries are centered around addressing the fundamental challenges that hinder their commercial viability. These include the shuttle effect of polysulfides, volume expansion during cycling, slow reaction kinetics, and poor electronic conductivity of sulfur. The binder and cathode architecture have been identified as critical components that significantly influence battery performance, stability, and lifespan.
The primary technical objectives for RT Na-S battery development include achieving high specific capacity (approaching the theoretical value), extending cycle life to thousands of cycles, improving rate capability for fast charging applications, and ensuring safety under various operating conditions. Specifically for binder and cathode architecture optimization, the goals are to develop structures that can effectively contain polysulfides, accommodate volume changes, enhance electronic/ionic conductivity, and maintain structural integrity during long-term cycling.
Recent advancements in nanomaterials, conductive polymers, and composite structures have opened new avenues for cathode design. The integration of functional binders that can chemically interact with polysulfides represents a promising direction. Additionally, three-dimensional architectures that provide sufficient space for sulfur expansion while maintaining electronic pathways are being extensively investigated.
The optimization of binder and cathode architecture is expected to address approximately 40-50% of the current performance limitations in RT Na-S batteries. Successful development in this area could potentially enable energy densities exceeding 400 Wh/kg at the cell level, with cycle life approaching 1000 cycles at 80% capacity retention – metrics that would position RT Na-S batteries as competitive alternatives to current commercial battery technologies.
Market Analysis for Room Temperature Sodium-Sulfur Batteries
The global market for room temperature sodium-sulfur (RT Na-S) batteries is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current projections indicate the market could reach $2.7 billion by 2030, with a compound annual growth rate of approximately 18% from 2023 to 2030. This growth trajectory is primarily fueled by the expanding renewable energy sector and the urgent need for grid-scale energy storage systems.
The cost advantage of RT Na-S batteries represents a major market driver. Sodium is approximately 1000 times more abundant than lithium in the Earth's crust, making it substantially less expensive. Raw material costs for sodium-based batteries can be 30-40% lower than their lithium counterparts, offering a compelling economic incentive for adoption in price-sensitive applications.
Market segmentation reveals diverse application potential across multiple sectors. Grid energy storage currently dominates, accounting for roughly 45% of the market share, followed by electric vehicles (25%), consumer electronics (15%), and industrial applications (15%). The grid storage segment is particularly promising due to the inherent advantages of RT Na-S technology in stationary applications where energy density constraints are less critical than cost considerations.
Regional analysis shows Asia-Pacific leading the market with approximately 40% share, driven by aggressive renewable energy integration policies in China, Japan, and South Korea. North America follows at 30%, with Europe at 25%, both regions showing accelerated growth due to governmental clean energy initiatives and carbon reduction targets.
Customer demand patterns indicate growing interest from utility companies seeking cost-effective solutions for grid stabilization and peak shaving. Survey data suggests that 65% of utility operators consider sodium-based batteries as a viable alternative to lithium-ion for large-scale installations, primarily due to lower lifetime costs and reduced fire safety concerns.
Market barriers include technical challenges related to the binder and cathode architecture optimization. The shuttle effect and volume expansion issues during cycling remain significant obstacles to widespread commercial adoption. Additionally, the established lithium-ion ecosystem presents market entry challenges for newer sodium-based technologies.
Future market growth will likely be catalyzed by technological breakthroughs in binder materials and cathode architectures that enhance cycle life and energy density. Partnerships between research institutions and industrial manufacturers are accelerating, with over 30 major collaborative projects currently focused on RT Na-S battery optimization worldwide.
The cost advantage of RT Na-S batteries represents a major market driver. Sodium is approximately 1000 times more abundant than lithium in the Earth's crust, making it substantially less expensive. Raw material costs for sodium-based batteries can be 30-40% lower than their lithium counterparts, offering a compelling economic incentive for adoption in price-sensitive applications.
Market segmentation reveals diverse application potential across multiple sectors. Grid energy storage currently dominates, accounting for roughly 45% of the market share, followed by electric vehicles (25%), consumer electronics (15%), and industrial applications (15%). The grid storage segment is particularly promising due to the inherent advantages of RT Na-S technology in stationary applications where energy density constraints are less critical than cost considerations.
Regional analysis shows Asia-Pacific leading the market with approximately 40% share, driven by aggressive renewable energy integration policies in China, Japan, and South Korea. North America follows at 30%, with Europe at 25%, both regions showing accelerated growth due to governmental clean energy initiatives and carbon reduction targets.
Customer demand patterns indicate growing interest from utility companies seeking cost-effective solutions for grid stabilization and peak shaving. Survey data suggests that 65% of utility operators consider sodium-based batteries as a viable alternative to lithium-ion for large-scale installations, primarily due to lower lifetime costs and reduced fire safety concerns.
Market barriers include technical challenges related to the binder and cathode architecture optimization. The shuttle effect and volume expansion issues during cycling remain significant obstacles to widespread commercial adoption. Additionally, the established lithium-ion ecosystem presents market entry challenges for newer sodium-based technologies.
Future market growth will likely be catalyzed by technological breakthroughs in binder materials and cathode architectures that enhance cycle life and energy density. Partnerships between research institutions and industrial manufacturers are accelerating, with over 30 major collaborative projects currently focused on RT Na-S battery optimization worldwide.
Current Challenges in Binder and Cathode Development
Despite significant advancements in room temperature sodium-sulfur (RT Na-S) battery technology, several critical challenges persist in binder and cathode architecture development that impede commercial viability. The conventional polyvinylidene fluoride (PVDF) binder, widely used in lithium-ion batteries, demonstrates inadequate performance in Na-S systems due to its limited ability to accommodate the substantial volume changes during cycling. This limitation leads to electrode pulverization, active material detachment, and rapid capacity fading.
Water-soluble binders such as carboxymethyl cellulose (CMC) and polyacrylic acid (PAA) have shown improved performance but still struggle with long-term stability in the highly reactive sulfur environment. The binder's inability to maintain structural integrity throughout hundreds of cycles represents a significant barrier to practical application of RT Na-S batteries.
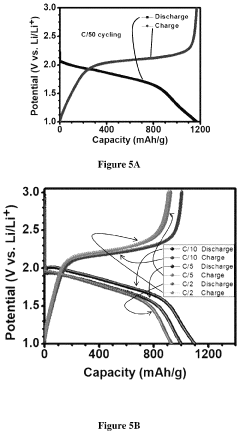
Cathode architecture optimization faces equally challenging obstacles. The insulating nature of sulfur (conductivity ~10^-30 S/cm) necessitates conductive additives, typically carbon materials, which dilute the active material content and reduce energy density. Current carbon hosts, including carbon nanotubes, graphene, and porous carbon, provide insufficient sulfur immobilization, resulting in polysulfide shuttling and capacity loss.
The cathode's pore structure presents another critical challenge. Micropores effectively trap sulfur but hinder ion transport, while mesopores facilitate ion movement but allow polysulfide leakage. This trade-off between confinement and transport efficiency remains unresolved in current cathode designs.
Sulfur loading represents a persistent dilemma in cathode development. Commercial viability requires high sulfur loading (>5 mg/cm²), but increased loading exacerbates electronic/ionic transport limitations and accelerates capacity decay. Current cathode architectures struggle to maintain performance at loadings necessary for practical energy density targets.
Interface engineering between the cathode and electrolyte presents additional complications. The formation of unstable solid-electrolyte interphase (SEI) layers on sodium metal anodes contributes to continuous electrolyte consumption and cell failure. Cathode-electrolyte interfaces similarly suffer from degradation mechanisms that current binder systems cannot adequately address.
Manufacturing scalability poses a significant challenge, as many laboratory-scale advanced cathode architectures rely on complex, multi-step synthesis procedures incompatible with industrial production. The development of simplified, scalable fabrication methods that maintain performance metrics remains an unresolved challenge in the field.
Water-soluble binders such as carboxymethyl cellulose (CMC) and polyacrylic acid (PAA) have shown improved performance but still struggle with long-term stability in the highly reactive sulfur environment. The binder's inability to maintain structural integrity throughout hundreds of cycles represents a significant barrier to practical application of RT Na-S batteries.
Cathode architecture optimization faces equally challenging obstacles. The insulating nature of sulfur (conductivity ~10^-30 S/cm) necessitates conductive additives, typically carbon materials, which dilute the active material content and reduce energy density. Current carbon hosts, including carbon nanotubes, graphene, and porous carbon, provide insufficient sulfur immobilization, resulting in polysulfide shuttling and capacity loss.
The cathode's pore structure presents another critical challenge. Micropores effectively trap sulfur but hinder ion transport, while mesopores facilitate ion movement but allow polysulfide leakage. This trade-off between confinement and transport efficiency remains unresolved in current cathode designs.
Sulfur loading represents a persistent dilemma in cathode development. Commercial viability requires high sulfur loading (>5 mg/cm²), but increased loading exacerbates electronic/ionic transport limitations and accelerates capacity decay. Current cathode architectures struggle to maintain performance at loadings necessary for practical energy density targets.
Interface engineering between the cathode and electrolyte presents additional complications. The formation of unstable solid-electrolyte interphase (SEI) layers on sodium metal anodes contributes to continuous electrolyte consumption and cell failure. Cathode-electrolyte interfaces similarly suffer from degradation mechanisms that current binder systems cannot adequately address.
Manufacturing scalability poses a significant challenge, as many laboratory-scale advanced cathode architectures rely on complex, multi-step synthesis procedures incompatible with industrial production. The development of simplified, scalable fabrication methods that maintain performance metrics remains an unresolved challenge in the field.
Current Binder and Cathode Design Solutions
01 Polymer binders for RT Na-S batteries
Various polymer binders are used in room temperature sodium-sulfur batteries to improve the mechanical stability and electrochemical performance of the cathode. These binders help to maintain the structural integrity of the electrode during charge-discharge cycles and prevent the dissolution of polysulfides. Common polymer binders include PVDF (polyvinylidene fluoride), CMC (carboxymethyl cellulose), and water-soluble polymers that offer better adhesion between active materials and current collectors.- Polymer binders for room temperature Na-S batteries: Various polymer binders are used in room temperature sodium-sulfur batteries to improve the electrochemical performance and stability of the cathode. These binders help maintain the structural integrity of the electrode during charge-discharge cycles and enhance the contact between active materials. Common polymer binders include PVDF (polyvinylidene fluoride), CMC (carboxymethyl cellulose), and water-soluble polymers that offer better adhesion properties and environmental benefits. The selection of appropriate binders significantly affects the cycle life and capacity retention of RT Na-S batteries.
- Cathode architecture design for enhanced sulfur utilization: The architecture of the cathode plays a crucial role in the performance of room temperature Na-S batteries. Advanced cathode designs incorporate porous structures, hierarchical architectures, and optimized sulfur distribution to enhance active material utilization and mitigate the shuttle effect. Three-dimensional frameworks, carbon-based hosts, and layered structures are employed to accommodate volume changes during cycling and provide conductive pathways for electron transport. These architectural innovations help overcome the insulating nature of sulfur and its discharge products, leading to improved energy density and cycling stability.
- Carbon-based materials for sulfur immobilization: Carbon-based materials are extensively used in RT Na-S battery cathodes to immobilize sulfur, enhance conductivity, and prevent polysulfide dissolution. Various carbon structures including mesoporous carbon, carbon nanotubes, graphene, and carbon fibers serve as hosts for sulfur, creating physical confinement and chemical interactions that limit polysulfide shuttling. These carbon materials provide large surface areas and pore volumes for sulfur loading while maintaining electrical connectivity throughout the electrode. The integration of carbon-based materials significantly improves the electrochemical performance and cycle life of room temperature sodium-sulfur batteries.
- Functional additives for cathode performance enhancement: Various functional additives are incorporated into RT Na-S battery cathodes to enhance electrochemical performance. These include metal oxides, metal sulfides, and polar materials that can chemically interact with polysulfides and prevent their dissolution into the electrolyte. Conductive additives like carbon black improve the electronic conductivity of the cathode. Catalytic materials facilitate the conversion reactions of sulfur species. The strategic combination of these additives with appropriate binders creates synergistic effects that address multiple challenges in sodium-sulfur batteries, including poor conductivity, volume expansion, and polysulfide shuttling.
- Novel electrolyte systems for RT Na-S batteries: Advanced electrolyte systems are crucial for the operation of room temperature sodium-sulfur batteries. These include solid-state electrolytes, gel polymer electrolytes, and liquid electrolytes with functional additives that suppress the polysulfide shuttle effect. Electrolyte composition significantly influences the formation of the solid electrolyte interphase (SEI) on electrodes and affects the sodium ion transport properties. The development of electrolytes with high ionic conductivity, wide electrochemical stability windows, and compatibility with both sodium metal anodes and sulfur cathodes is essential for achieving high-performance RT Na-S batteries with improved safety characteristics.
02 Cathode architecture optimization for RT Na-S batteries
The architecture of the cathode plays a crucial role in the performance of room temperature sodium-sulfur batteries. Various designs incorporate porous structures, hierarchical arrangements, and 3D frameworks to accommodate sulfur expansion during cycling and facilitate ion transport. Advanced cathode architectures also include carbon-based matrices that provide conductive pathways and help contain sulfur within the electrode structure, preventing capacity fade and improving cycle life.Expand Specific Solutions03 Carbon-based materials for sulfur immobilization
Carbon-based materials are widely used in RT Na-S battery cathodes to immobilize sulfur and trap polysulfides. These materials include carbon nanotubes, graphene, mesoporous carbon, and carbon fibers that provide high surface area and conductive networks. The carbon hosts help to address the shuttle effect by physically confining sulfur species and improving the utilization of active material, thereby enhancing capacity retention and cycling stability.Expand Specific Solutions04 Electrolyte modifications for RT Na-S batteries
Electrolyte composition significantly affects the performance of room temperature sodium-sulfur batteries. Modifications include the addition of electrolyte additives, use of ionic liquids, and development of solid or gel electrolytes to suppress the shuttle effect of polysulfides. These modifications aim to improve the ionic conductivity, interface stability, and compatibility with electrode materials, leading to enhanced battery performance and safety.Expand Specific Solutions05 Metal oxide/sulfide additives in cathode composites
Metal oxides and sulfides are incorporated into cathode composites to enhance the electrochemical performance of RT Na-S batteries. These additives serve as catalysts for the conversion reactions of sodium polysulfides and help to chemically bind polysulfides through polar interactions. The presence of these materials in the cathode architecture improves the reaction kinetics, reduces capacity fading, and extends the cycle life of the batteries.Expand Specific Solutions
Leading Companies and Research Institutions in Na-S Technology
Room temperature sodium-sulfur (RT Na-S) batteries are emerging as a promising energy storage technology, currently positioned in the early commercialization phase of industry development. The global market for these batteries is experiencing rapid growth, projected to reach significant scale as demand for cost-effective energy storage solutions increases. Technologically, RT Na-S batteries are advancing through critical optimization stages, with leading players demonstrating varied levels of maturity. Universities like Waterloo, Johns Hopkins, and Cornell are driving fundamental research, while commercial entities including LG Energy Solution, CATL (Ningde Amperex), and Honeycomb Battery are advancing practical applications. The competitive landscape shows a balanced ecosystem of academic institutions providing innovation foundations and industrial players focusing on scalable manufacturing processes and performance improvements, particularly in binder formulations and cathode architectures that address key challenges of capacity retention and cycle life.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced binder systems specifically for room temperature sodium-sulfur batteries that address the polysulfide shuttle effect. Their proprietary polymer-based binders incorporate functional groups that chemically interact with sodium polysulfides, effectively trapping them within the cathode structure. The company has implemented a dual-layer cathode architecture where an initial carbon-rich protective layer prevents direct contact between the sulfur cathode and the electrolyte, while the main cathode layer features a hierarchical porous structure with optimized sulfur loading (typically 70-75% by weight). This architecture facilitates both ion transport and accommodates volume changes during cycling. Their latest generation employs conductive polymer coatings on sulfur particles before integration into the cathode matrix, creating electronic pathways throughout the electrode structure while maintaining strong adhesion between active materials and current collectors even after hundreds of cycles.
Strengths: Superior polysulfide retention compared to conventional PVDF binders, resulting in significantly reduced capacity fading. Their dual-layer approach effectively balances sulfur utilization with cycle stability. Weaknesses: The complex manufacturing process increases production costs, and the specialized binders may be sensitive to precise processing conditions, potentially affecting quality control in mass production.
Honeycomb Battery Co.
Technical Solution: Honeycomb Battery has developed a distinctive approach to RT Na-S batteries centered around their patented "cellular cathode architecture." This design mimics natural honeycomb structures to create a three-dimensional framework that maximizes sulfur loading while providing efficient pathways for both electron and ion transport. Their cathode utilizes a specialized carbon scaffold with controlled macro/mesoporous structure that physically confines sulfur and its discharge products. For the binder system, Honeycomb employs a bio-derived polymer blend that combines excellent adhesion properties with functional groups specifically designed to interact with sodium polysulfides. Their manufacturing process involves a proprietary "sequential deposition" technique where layers of carbon, sulfur, and functional additives are built up in a controlled manner to create a highly integrated structure. This approach allows for sulfur loadings of up to 75% by weight while maintaining structural integrity throughout cycling. Additionally, Honeycomb has implemented an innovative interface layer between the cathode and separator composed of a sodium-ion conductive polymer that selectively filters ions, allowing Na+ transport while blocking polysulfide migration.
Strengths: Their biomimetic cellular architecture provides exceptional mechanical stability during cycling while maintaining high active material utilization. The bio-derived binder system offers environmental advantages and superior polysulfide retention compared to conventional binders. Weaknesses: The complex sequential deposition manufacturing process may limit production scalability and increase costs. The specialized carbon scaffolds require precise synthesis conditions, potentially creating supply chain challenges for mass production.
Key Patents and Research on Cathode Architecture
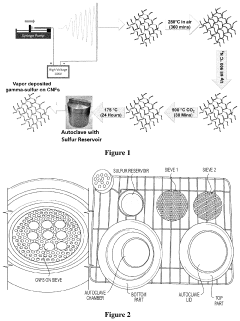
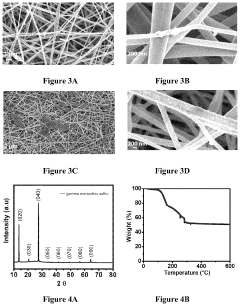
Synthesis of gamma monoclinic sulfur and sulfur batteries containing monoclinic sulfur
PatentActiveUS11831016B2
Innovation
- Depositing monoclinic gamma phase sulfur on a substrate using vapor deposition, allowing for the use of carbonate-based electrolytes without polysulfide formation, enabling a stable and reversible redox mechanism in sulfur batteries.
Room-temperature sodium-sulfur battery and preparation method thereof
PatentPendingCN118099560A
Innovation
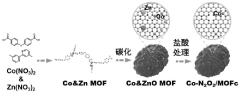
- Metal-organic framework materials (MOF) are used as precursors to synthesize single-atom catalyst Co-N2O2/MOFc composites as sulfur storage materials, which can be used as cathodes of room-temperature sodium-sulfur batteries to improve polysulfide conversion efficiency and prevent shuttle and loss.
Material Sustainability and Supply Chain Considerations
The sustainability of materials used in RT Na-S batteries represents a critical consideration for their large-scale deployment. Sulfur offers significant advantages as a cathode material due to its natural abundance, low cost, and environmental benignity. As the fifth most abundant element on Earth, sulfur is readily available as a byproduct of petroleum refining processes, making it a sustainable choice compared to cobalt and nickel used in conventional lithium-ion batteries. This abundance translates to lower material costs and reduced supply chain vulnerabilities.
However, the binder materials commonly used in Na-S battery cathodes present sustainability challenges. Polyvinylidene fluoride (PVDF), a prevalent binder, requires toxic solvents like N-methyl-2-pyrrolidone (NMP) during electrode manufacturing, raising environmental and health concerns. The development of water-based binders such as carboxymethyl cellulose (CMC) and styrene-butadiene rubber (SBR) offers more environmentally friendly alternatives that maintain or even enhance electrochemical performance while reducing processing costs and environmental impact.
The carbon materials used in cathode architectures also warrant sustainability assessment. While carbon additives are essential for enhancing conductivity, their production often involves energy-intensive processes. Research into sustainable carbon sources, including biomass-derived carbons from agricultural waste, presents promising alternatives that could reduce the environmental footprint of Na-S batteries while potentially improving performance through tailored porous structures.
Supply chain considerations for RT Na-S batteries appear favorable compared to lithium-ion technologies. Sodium resources are geographically distributed more evenly than lithium, with abundant reserves in seawater and rock salt deposits worldwide. This distribution reduces geopolitical supply risks and potential price volatilities. The simplified supply chain for sulfur, primarily sourced as a byproduct of desulfurization processes in petroleum refining, further enhances the economic and logistical advantages of Na-S technology.
Manufacturing scalability represents another important dimension of sustainability. Current cathode architecture optimization efforts must consider not only performance metrics but also compatibility with existing battery manufacturing infrastructure to facilitate industrial adoption. Techniques that require specialized equipment or complex processing steps may face barriers to commercialization despite superior performance characteristics.
End-of-life management and recyclability of Na-S batteries must also be integrated into material selection and cathode design decisions. Binders that facilitate easier separation of active materials during recycling processes would enhance the circular economy potential of these batteries, reducing waste and further improving their environmental credentials.
However, the binder materials commonly used in Na-S battery cathodes present sustainability challenges. Polyvinylidene fluoride (PVDF), a prevalent binder, requires toxic solvents like N-methyl-2-pyrrolidone (NMP) during electrode manufacturing, raising environmental and health concerns. The development of water-based binders such as carboxymethyl cellulose (CMC) and styrene-butadiene rubber (SBR) offers more environmentally friendly alternatives that maintain or even enhance electrochemical performance while reducing processing costs and environmental impact.
The carbon materials used in cathode architectures also warrant sustainability assessment. While carbon additives are essential for enhancing conductivity, their production often involves energy-intensive processes. Research into sustainable carbon sources, including biomass-derived carbons from agricultural waste, presents promising alternatives that could reduce the environmental footprint of Na-S batteries while potentially improving performance through tailored porous structures.
Supply chain considerations for RT Na-S batteries appear favorable compared to lithium-ion technologies. Sodium resources are geographically distributed more evenly than lithium, with abundant reserves in seawater and rock salt deposits worldwide. This distribution reduces geopolitical supply risks and potential price volatilities. The simplified supply chain for sulfur, primarily sourced as a byproduct of desulfurization processes in petroleum refining, further enhances the economic and logistical advantages of Na-S technology.
Manufacturing scalability represents another important dimension of sustainability. Current cathode architecture optimization efforts must consider not only performance metrics but also compatibility with existing battery manufacturing infrastructure to facilitate industrial adoption. Techniques that require specialized equipment or complex processing steps may face barriers to commercialization despite superior performance characteristics.
End-of-life management and recyclability of Na-S batteries must also be integrated into material selection and cathode design decisions. Binders that facilitate easier separation of active materials during recycling processes would enhance the circular economy potential of these batteries, reducing waste and further improving their environmental credentials.
Safety and Performance Testing Protocols
The development of robust safety and performance testing protocols is essential for the advancement of room temperature sodium-sulfur (RT Na-S) batteries, particularly when optimizing binder and cathode architectures. These protocols must comprehensively evaluate both the safety aspects and performance metrics to ensure reliable and commercially viable battery systems.
Safety testing for RT Na-S batteries requires specialized protocols due to the reactive nature of sodium metal and sulfur compounds. Thermal stability tests must be conducted at various states of charge to evaluate the potential for thermal runaway, with differential scanning calorimetry (DSC) and accelerating rate calorimetry (ARC) being critical methodologies. The interaction between binder materials and sodium polysulfides demands particular attention, as some polymer binders may degrade in the presence of these reactive species.
Mechanical integrity testing represents another crucial safety protocol, evaluating the structural stability of cathode architectures under various mechanical stresses. This includes compression tests, vibration resistance, and impact tolerance assessments. The cohesion between sulfur particles, conductive additives, and binder materials must withstand these mechanical challenges without compromising electrochemical performance or safety.
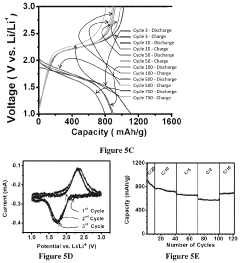
Electrochemical performance testing protocols for RT Na-S batteries with optimized binder and cathode architectures should include standardized cycling protocols at various C-rates to evaluate rate capability and capacity retention. Long-term cycling tests (>500 cycles) are essential to assess the stability of the cathode architecture and the effectiveness of the binder in maintaining structural integrity and preventing active material dissolution.
Impedance spectroscopy measurements provide valuable insights into the interfacial resistance changes during cycling, helping to identify degradation mechanisms related to binder decomposition or cathode structural collapse. These measurements should be conducted at different states of charge and after various cycle numbers to track the evolution of resistance components.
Self-discharge testing protocols are particularly important for RT Na-S batteries due to the shuttle effect of polysulfides. Standardized rest periods between cycling should be implemented to quantify capacity loss during idle states, with particular attention to how different binder chemistries and cathode architectures mitigate this phenomenon.
Environmental testing protocols must evaluate performance across a wide temperature range (-20°C to 60°C) to assess the practical applicability of the battery technology. The interaction between temperature fluctuations and binder properties can significantly impact both safety and performance metrics, necessitating comprehensive thermal cycling tests that simulate real-world usage conditions.
Safety testing for RT Na-S batteries requires specialized protocols due to the reactive nature of sodium metal and sulfur compounds. Thermal stability tests must be conducted at various states of charge to evaluate the potential for thermal runaway, with differential scanning calorimetry (DSC) and accelerating rate calorimetry (ARC) being critical methodologies. The interaction between binder materials and sodium polysulfides demands particular attention, as some polymer binders may degrade in the presence of these reactive species.
Mechanical integrity testing represents another crucial safety protocol, evaluating the structural stability of cathode architectures under various mechanical stresses. This includes compression tests, vibration resistance, and impact tolerance assessments. The cohesion between sulfur particles, conductive additives, and binder materials must withstand these mechanical challenges without compromising electrochemical performance or safety.
Electrochemical performance testing protocols for RT Na-S batteries with optimized binder and cathode architectures should include standardized cycling protocols at various C-rates to evaluate rate capability and capacity retention. Long-term cycling tests (>500 cycles) are essential to assess the stability of the cathode architecture and the effectiveness of the binder in maintaining structural integrity and preventing active material dissolution.
Impedance spectroscopy measurements provide valuable insights into the interfacial resistance changes during cycling, helping to identify degradation mechanisms related to binder decomposition or cathode structural collapse. These measurements should be conducted at different states of charge and after various cycle numbers to track the evolution of resistance components.
Self-discharge testing protocols are particularly important for RT Na-S batteries due to the shuttle effect of polysulfides. Standardized rest periods between cycling should be implemented to quantify capacity loss during idle states, with particular attention to how different binder chemistries and cathode architectures mitigate this phenomenon.
Environmental testing protocols must evaluate performance across a wide temperature range (-20°C to 60°C) to assess the practical applicability of the battery technology. The interaction between temperature fluctuations and binder properties can significantly impact both safety and performance metrics, necessitating comprehensive thermal cycling tests that simulate real-world usage conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!