Sodium Anode Protection And Dendrite Mitigation In Na–S Cells
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-S Battery Technology Background and Objectives
Sodium-sulfur (Na-S) battery technology represents one of the most promising next-generation energy storage solutions, offering theoretical energy densities up to 760 Wh/kg—significantly higher than current lithium-ion technologies. The development of Na-S batteries traces back to the 1960s when Ford Motor Company pioneered high-temperature (300-350°C) Na-S cells. These early systems demonstrated the fundamental electrochemical viability but faced substantial challenges in practical implementation due to safety concerns and material degradation at elevated temperatures.
The evolution of Na-S technology has since bifurcated into two distinct pathways: high-temperature systems that have found niche applications in grid-scale energy storage, and room-temperature Na-S batteries that represent the frontier of current research efforts. This technological trajectory has been driven by the increasing global demand for sustainable, high-capacity energy storage solutions that rely on earth-abundant materials.
Sodium's natural abundance (2.6% of Earth's crust compared to lithium's 0.002%) presents a compelling economic case for Na-S technology. The sulfur cathode material offers additional advantages of low cost ($0.02/kg versus $15/kg for cobalt), environmental benignity, and a high theoretical capacity of 1675 mAh/g. These factors position Na-S batteries as potentially transformative for large-scale energy storage applications.
The primary technical objective in Na-S battery development centers on addressing the critical challenges of sodium anode protection and dendrite mitigation. Sodium metal anodes suffer from high reactivity with electrolytes and uncontrolled dendrite growth during cycling, leading to capacity fade, internal short circuits, and safety hazards. Developing effective protection strategies for sodium anodes while maintaining high ionic conductivity represents the cornerstone challenge for viable room-temperature Na-S cells.
Secondary objectives include optimizing the sulfur cathode to mitigate the "shuttle effect" of polysulfide intermediates, enhancing electrolyte stability, and improving overall cycle life. The field aims to achieve practical energy densities exceeding 300 Wh/kg with cycle life beyond 1000 cycles—metrics that would position Na-S technology as a viable alternative to lithium-ion batteries for numerous applications.
Recent technological breakthroughs in solid-state electrolytes, artificial interphase engineering, and nanostructured electrode materials have accelerated progress toward these objectives. The convergence of computational materials science, advanced characterization techniques, and innovative synthetic approaches has created a fertile environment for addressing the fundamental challenges of sodium anode protection and dendrite suppression in next-generation Na-S batteries.
The evolution of Na-S technology has since bifurcated into two distinct pathways: high-temperature systems that have found niche applications in grid-scale energy storage, and room-temperature Na-S batteries that represent the frontier of current research efforts. This technological trajectory has been driven by the increasing global demand for sustainable, high-capacity energy storage solutions that rely on earth-abundant materials.
Sodium's natural abundance (2.6% of Earth's crust compared to lithium's 0.002%) presents a compelling economic case for Na-S technology. The sulfur cathode material offers additional advantages of low cost ($0.02/kg versus $15/kg for cobalt), environmental benignity, and a high theoretical capacity of 1675 mAh/g. These factors position Na-S batteries as potentially transformative for large-scale energy storage applications.
The primary technical objective in Na-S battery development centers on addressing the critical challenges of sodium anode protection and dendrite mitigation. Sodium metal anodes suffer from high reactivity with electrolytes and uncontrolled dendrite growth during cycling, leading to capacity fade, internal short circuits, and safety hazards. Developing effective protection strategies for sodium anodes while maintaining high ionic conductivity represents the cornerstone challenge for viable room-temperature Na-S cells.
Secondary objectives include optimizing the sulfur cathode to mitigate the "shuttle effect" of polysulfide intermediates, enhancing electrolyte stability, and improving overall cycle life. The field aims to achieve practical energy densities exceeding 300 Wh/kg with cycle life beyond 1000 cycles—metrics that would position Na-S technology as a viable alternative to lithium-ion batteries for numerous applications.
Recent technological breakthroughs in solid-state electrolytes, artificial interphase engineering, and nanostructured electrode materials have accelerated progress toward these objectives. The convergence of computational materials science, advanced characterization techniques, and innovative synthetic approaches has created a fertile environment for addressing the fundamental challenges of sodium anode protection and dendrite suppression in next-generation Na-S batteries.
Market Analysis for Na-S Energy Storage Solutions
The global energy storage market is witnessing a significant shift towards sustainable and cost-effective solutions, with Na-S (Sodium-Sulfur) batteries emerging as a promising alternative to traditional lithium-ion technologies. Current market valuations place the Na-S battery sector at approximately $300 million, with projections indicating growth to reach $600 million by 2025, representing a compound annual growth rate of 15%.
The primary market drivers for Na-S energy storage solutions include increasing grid-scale storage demands, rising renewable energy integration requirements, and growing concerns about lithium resource limitations. Utility companies constitute the largest customer segment, accounting for nearly 60% of current deployments, followed by industrial applications at 25% and emerging microgrid implementations at 15%.
Geographically, Japan leads the market with approximately 40% share due to NGK Insulators' early technological dominance. However, China and the United States are rapidly expanding their market presence, with growth rates of 22% and 18% respectively over the past three years. European adoption remains modest but is accelerating, particularly in Germany and the United Kingdom where renewable energy integration is prioritized.
Cost analysis reveals that while Na-S systems currently have higher upfront capital expenditure compared to some alternatives, their total cost of ownership over 15+ year lifespans presents compelling value. The average installation cost ranges between $500-700 per kWh, with operational expenses approximately 30% lower than comparable lithium-ion systems due to abundant sodium resources and simpler thermal management requirements.
Market segmentation shows distinct application clusters: grid-scale storage represents 65% of deployments, industrial power quality applications account for 20%, and remote/off-grid installations comprise 15%. The fastest growth is occurring in the renewable energy integration segment, where Na-S technology's long discharge duration (4-8 hours) provides advantages over shorter-duration alternatives.
Customer demand analysis indicates increasing interest in technologies addressing dendrite formation issues, with 78% of potential adopters citing safety and longevity as primary concerns. Market surveys show that solutions demonstrating effective sodium anode protection could potentially accelerate adoption by 35% among hesitant utility customers.
Competitive landscape assessment reveals approximately 25 companies actively developing Na-S technologies, with five holding 80% of current market share. Strategic partnerships between technology developers and utility companies have increased by 40% in the past two years, indicating growing commercial confidence in the technology's viability.
The primary market drivers for Na-S energy storage solutions include increasing grid-scale storage demands, rising renewable energy integration requirements, and growing concerns about lithium resource limitations. Utility companies constitute the largest customer segment, accounting for nearly 60% of current deployments, followed by industrial applications at 25% and emerging microgrid implementations at 15%.
Geographically, Japan leads the market with approximately 40% share due to NGK Insulators' early technological dominance. However, China and the United States are rapidly expanding their market presence, with growth rates of 22% and 18% respectively over the past three years. European adoption remains modest but is accelerating, particularly in Germany and the United Kingdom where renewable energy integration is prioritized.
Cost analysis reveals that while Na-S systems currently have higher upfront capital expenditure compared to some alternatives, their total cost of ownership over 15+ year lifespans presents compelling value. The average installation cost ranges between $500-700 per kWh, with operational expenses approximately 30% lower than comparable lithium-ion systems due to abundant sodium resources and simpler thermal management requirements.
Market segmentation shows distinct application clusters: grid-scale storage represents 65% of deployments, industrial power quality applications account for 20%, and remote/off-grid installations comprise 15%. The fastest growth is occurring in the renewable energy integration segment, where Na-S technology's long discharge duration (4-8 hours) provides advantages over shorter-duration alternatives.
Customer demand analysis indicates increasing interest in technologies addressing dendrite formation issues, with 78% of potential adopters citing safety and longevity as primary concerns. Market surveys show that solutions demonstrating effective sodium anode protection could potentially accelerate adoption by 35% among hesitant utility customers.
Competitive landscape assessment reveals approximately 25 companies actively developing Na-S technologies, with five holding 80% of current market share. Strategic partnerships between technology developers and utility companies have increased by 40% in the past two years, indicating growing commercial confidence in the technology's viability.
Sodium Anode Protection Challenges and Limitations
Despite the promising potential of sodium-sulfur (Na-S) batteries as a cost-effective energy storage solution, sodium anode protection presents significant challenges that hinder widespread commercial adoption. The high reactivity of sodium metal with electrolytes leads to continuous side reactions, forming an unstable solid electrolyte interphase (SEI) that consumes active material and reduces coulombic efficiency. Unlike lithium-based systems, sodium's larger ionic radius (102 pm vs. 76 pm for lithium) results in slower diffusion kinetics and more challenging ion transport through protective layers.
A critical limitation in Na-S cells is the formation and growth of sodium dendrites during cycling. These tree-like structures grow from the anode surface during charging, potentially penetrating separators and causing catastrophic short circuits. The dendrite formation mechanism in sodium systems differs subtly from lithium due to sodium's distinct electrochemical properties, including lower surface energy and different crystal growth patterns, making mitigation strategies developed for lithium batteries not directly transferable.
The operating temperature presents another significant challenge. High-temperature Na-S batteries (operating at 300-350°C) face thermal management issues, safety concerns, and accelerated corrosion of cell components. Conversely, room-temperature Na-S cells struggle with slow reaction kinetics, poor sulfur utilization, and rapid capacity fading due to polysulfide shuttle effects, where soluble sodium polysulfides migrate between electrodes.
Existing protective strategies show limitations in long-term stability. Artificial SEI layers often crack during repeated volume changes of the sodium anode during cycling. Polymer-based protective layers, while flexible, typically exhibit insufficient ionic conductivity at room temperature. Ceramic protectors offer excellent mechanical strength but suffer from brittleness and poor interfacial contact with the sodium metal.
The polysulfide shuttle effect compounds protection challenges, as dissolved polysulfides react with the sodium anode, contaminating the protective layer and accelerating degradation. This creates a complex protection requirement where the anode must be shielded not only from the electrolyte but also from migrating polysulfide species.
Scale-up and manufacturing considerations present additional hurdles. Many laboratory-scale protection strategies involve complex processes incompatible with large-scale production. The high sensitivity of sodium metal to air and moisture complicates handling during manufacturing, requiring specialized equipment and controlled environments that increase production costs significantly.
A critical limitation in Na-S cells is the formation and growth of sodium dendrites during cycling. These tree-like structures grow from the anode surface during charging, potentially penetrating separators and causing catastrophic short circuits. The dendrite formation mechanism in sodium systems differs subtly from lithium due to sodium's distinct electrochemical properties, including lower surface energy and different crystal growth patterns, making mitigation strategies developed for lithium batteries not directly transferable.
The operating temperature presents another significant challenge. High-temperature Na-S batteries (operating at 300-350°C) face thermal management issues, safety concerns, and accelerated corrosion of cell components. Conversely, room-temperature Na-S cells struggle with slow reaction kinetics, poor sulfur utilization, and rapid capacity fading due to polysulfide shuttle effects, where soluble sodium polysulfides migrate between electrodes.
Existing protective strategies show limitations in long-term stability. Artificial SEI layers often crack during repeated volume changes of the sodium anode during cycling. Polymer-based protective layers, while flexible, typically exhibit insufficient ionic conductivity at room temperature. Ceramic protectors offer excellent mechanical strength but suffer from brittleness and poor interfacial contact with the sodium metal.
The polysulfide shuttle effect compounds protection challenges, as dissolved polysulfides react with the sodium anode, contaminating the protective layer and accelerating degradation. This creates a complex protection requirement where the anode must be shielded not only from the electrolyte but also from migrating polysulfide species.
Scale-up and manufacturing considerations present additional hurdles. Many laboratory-scale protection strategies involve complex processes incompatible with large-scale production. The high sensitivity of sodium metal to air and moisture complicates handling during manufacturing, requiring specialized equipment and controlled environments that increase production costs significantly.
Current Sodium Anode Protection Methodologies
01 Solid electrolyte interfaces for sodium anode protection
Solid electrolyte interfaces (SEI) can be engineered to protect sodium anodes in Na-S cells. These interfaces act as barriers between the sodium metal and the electrolyte, preventing unwanted reactions and dendrite formation. Various materials and coatings can be used to create stable SEI layers that allow sodium ion transport while inhibiting dendrite growth. These protective interfaces significantly improve the cycling stability and safety of Na-S batteries by maintaining the integrity of the sodium anode during repeated charge-discharge cycles.- Solid electrolyte interfaces for sodium anode protection: Solid electrolyte interfaces (SEI) can be engineered to protect sodium anodes in Na-S cells. These interfaces act as barriers between the sodium metal and the electrolyte, preventing unwanted reactions and dendrite formation. Various materials and coatings can be used to create stable SEI layers that allow sodium ion transport while inhibiting dendrite growth. These protective interfaces improve the cycling stability and safety of Na-S batteries by maintaining the integrity of the sodium anode during repeated charge-discharge cycles.
- Electrolyte additives for dendrite mitigation: Specific additives can be incorporated into the electrolyte to suppress sodium dendrite formation. These additives modify the electrochemical deposition behavior of sodium ions, promoting more uniform plating and reducing the tendency for dendrite growth. Some additives work by forming protective films on the sodium surface, while others alter the ion transport properties near the electrode interface. By carefully selecting and optimizing electrolyte additives, the cycle life and safety of Na-S cells can be significantly improved.
- Structured sodium anodes and interface engineering: Engineering the structure of sodium anodes and their interfaces can effectively mitigate dendrite formation. This approach includes creating porous or 3D structured sodium anodes that distribute current density more evenly, reducing localized dendrite nucleation. Interface engineering techniques involve surface modifications of the sodium anode to control the deposition behavior of sodium ions. These structured designs help maintain the mechanical integrity of the anode while accommodating volume changes during cycling.
- Protective membranes and separators: Specialized membranes and separators can be employed to physically block dendrite growth in Na-S cells. These components are designed with specific pore structures and surface properties that allow sodium ion transport while preventing dendrite penetration. Some advanced separators incorporate functional materials that react with or redirect dendrite growth. The use of these protective membranes enhances the safety and longevity of Na-S batteries by preventing short circuits caused by dendrite penetration.
- Novel cell designs and operating strategies: Innovative cell designs and operating strategies can be implemented to protect sodium anodes and prevent dendrite formation. These approaches include optimized cell configurations that control sodium plating/stripping behavior, pressure-modulated designs that suppress dendrite growth, and thermal management systems that maintain optimal operating conditions. Additionally, specific charging protocols and current density control strategies can be employed to minimize dendrite nucleation and growth, thereby extending the cycle life and improving the safety of Na-S batteries.
02 Electrolyte additives for dendrite mitigation
Specific additives can be incorporated into the electrolyte to suppress sodium dendrite formation. These additives modify the electrochemical deposition process of sodium ions, promoting more uniform plating and reducing the tendency for dendrite growth. Some additives form protective films on the sodium surface, while others alter the ion transport mechanisms near the electrode surface. By carefully selecting and optimizing electrolyte additives, the cycle life and safety of Na-S cells can be significantly improved through effective dendrite mitigation.Expand Specific Solutions03 Structured sodium anodes and interface engineering
Engineered sodium anode structures can be designed to control sodium deposition and prevent dendrite formation. These structures include porous frameworks, 3D architectures, and patterned surfaces that distribute current density more evenly across the anode. Interface engineering approaches modify the surface properties of the sodium anode to guide ion deposition in a more uniform manner. These structural modifications help maintain the physical integrity of the anode during cycling and significantly reduce the risk of dendrite-induced short circuits.Expand Specific Solutions04 Protective membranes and separators
Specialized membranes and separators can be employed to physically block dendrite penetration while maintaining efficient sodium ion transport. These components can be made from various materials including ceramics, polymers, or composite structures with tailored porosity and surface properties. Some advanced separators incorporate functional coatings that react with and neutralize any dendrites that begin to form. The mechanical strength and ion selectivity of these membranes play crucial roles in preventing short circuits caused by dendrite growth in Na-S cells.Expand Specific Solutions05 Temperature and pressure management systems
Controlling operating conditions such as temperature and pressure can significantly impact dendrite formation in Na-S cells. Elevated temperatures can improve sodium ion mobility and promote more uniform deposition, while carefully managed pressure can help maintain physical contact between cell components. Advanced thermal management systems can be integrated into Na-S batteries to maintain optimal operating conditions that minimize dendrite growth. These approaches often work synergistically with other protection strategies to enhance the overall stability and safety of sodium anodes.Expand Specific Solutions
Key Industry Players in Na-S Battery Development
The sodium-sulfur (Na-S) battery market is currently in an early growth phase, characterized by increasing research activity but limited commercial deployment. The global market size remains relatively small compared to lithium-ion technologies, though projections indicate significant expansion potential due to sodium's abundance and cost advantages. Technologically, Na-S cells face critical challenges in sodium anode protection and dendrite mitigation, with varying maturity levels across competitors. Major players like NGK Insulators have established commercial presence in grid-scale applications, while research institutions (USTC, Fudan University) and battery manufacturers (CATL, LG Energy Solution, Northvolt) are advancing fundamental solutions. Companies including Honeycomb Battery and SES Holdings are developing proprietary technologies to address dendrite formation, while automotive firms (Honda, Bosch) explore integration opportunities for transportation applications.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a comprehensive sodium anode protection strategy for Na-S batteries focusing on electrolyte engineering and advanced separator technologies. Their approach utilizes dual-salt electrolyte formulations containing sodium bis(fluorosulfonyl)imide (NaFSI) and sodium bis(trifluoromethanesulfonyl)imide (NaTFSI) in ether-based solvents, which form stable and uniform solid electrolyte interphase (SEI) layers on sodium metal surfaces. This engineered SEI significantly reduces side reactions and promotes homogeneous sodium deposition. The company has implemented a proprietary separator coating technology using aluminum oxide and silicon dioxide nanoparticles that creates a ceramic-reinforced barrier against dendrite penetration while maintaining high ionic conductivity. Their research demonstrates that this combination of electrolyte optimization and separator engineering reduces interfacial impedance by approximately 40% compared to conventional systems and enables stable cycling performance exceeding 1000 cycles with capacity retention above 80%. LG Energy Solution has also developed sodium-ion conducting polymer additives that further enhance the mechanical properties of the protective layers, effectively suppressing dendrite growth even at higher current densities (>1 mA/cm²). This integrated protection strategy addresses both the chemical and mechanical aspects of sodium anode degradation.
Strengths: Comprehensive approach combining electrolyte engineering and separator technologies provides multi-layer protection against dendrite formation. Their commercial manufacturing capabilities enable potential scale-up of these technologies. Weaknesses: The complex electrolyte formulations may increase production costs and present thermal stability challenges. The long-term stability of their engineered interfaces under extreme temperature conditions requires further validation.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: Contemporary Amperex Technology (CATL) has developed an innovative sodium anode protection system for Na-S batteries utilizing a multi-functional artificial interface approach. Their technology incorporates a sodium-ion conductive polymer matrix (modified polyethylene oxide) infused with ceramic nanoparticles (Na3Zr2Si2PO12) that creates a flexible yet mechanically robust barrier against dendrite penetration. This composite protective layer is applied directly to the sodium metal anode through a proprietary deposition process that ensures uniform coverage and strong adhesion. CATL's approach also features an advanced electrolyte formulation containing fluoroethylene carbonate (FEC) and sodium hexafluorophosphate (NaPF6) additives that contribute to the formation of a stable and ion-conductive solid electrolyte interphase. Their research demonstrates that this protection strategy reduces interfacial resistance by over 50% compared to conventional systems and enables stable sodium plating/stripping behavior with Coulombic efficiencies consistently above 99%. The company has successfully integrated this technology into pouch cell prototypes that demonstrate stable cycling performance exceeding 800 cycles with minimal capacity degradation. CATL's sodium anode protection system effectively addresses both the chemical degradation and mechanical failure modes that typically limit Na-S battery performance.
Strengths: Integrated approach combining materials science and manufacturing expertise enables practical implementation of sodium protection technologies. Their artificial interface design effectively suppresses dendrite formation while maintaining high ionic conductivity. Weaknesses: The complex composite materials may present manufacturing scalability challenges. The long-term stability of polymer-based protective layers under various operating conditions requires further validation.
Critical Patents in Dendrite Suppression Technologies
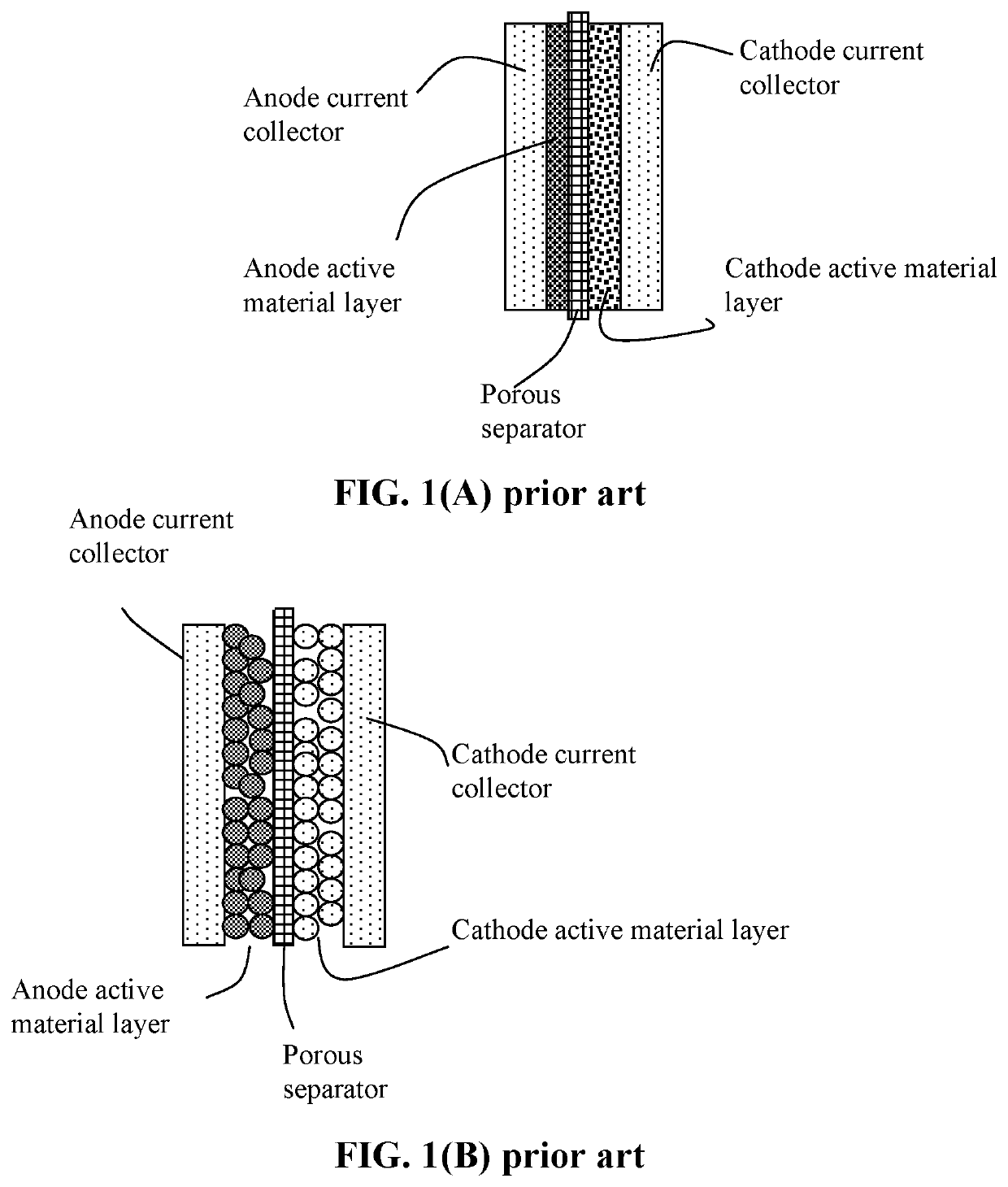
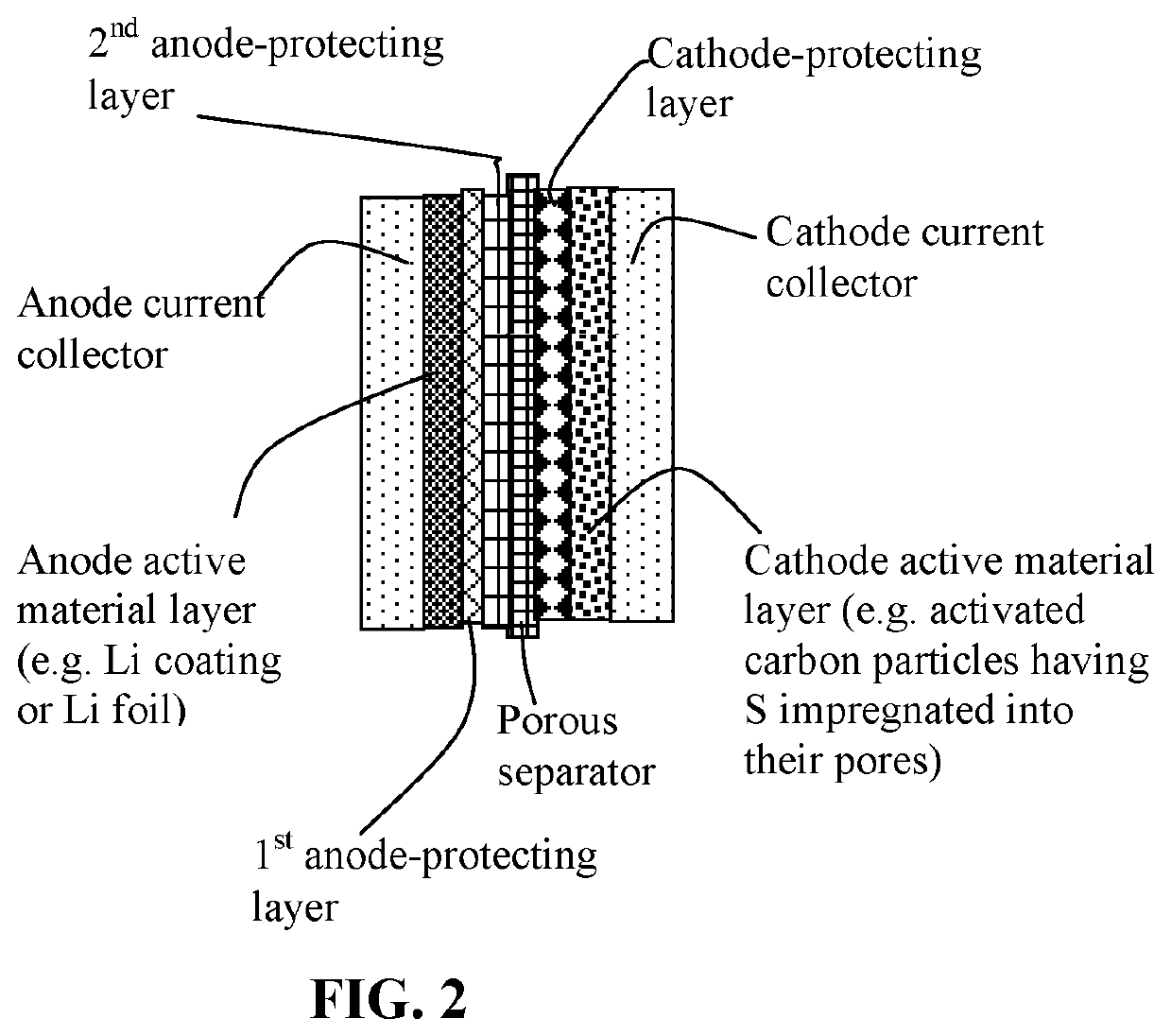
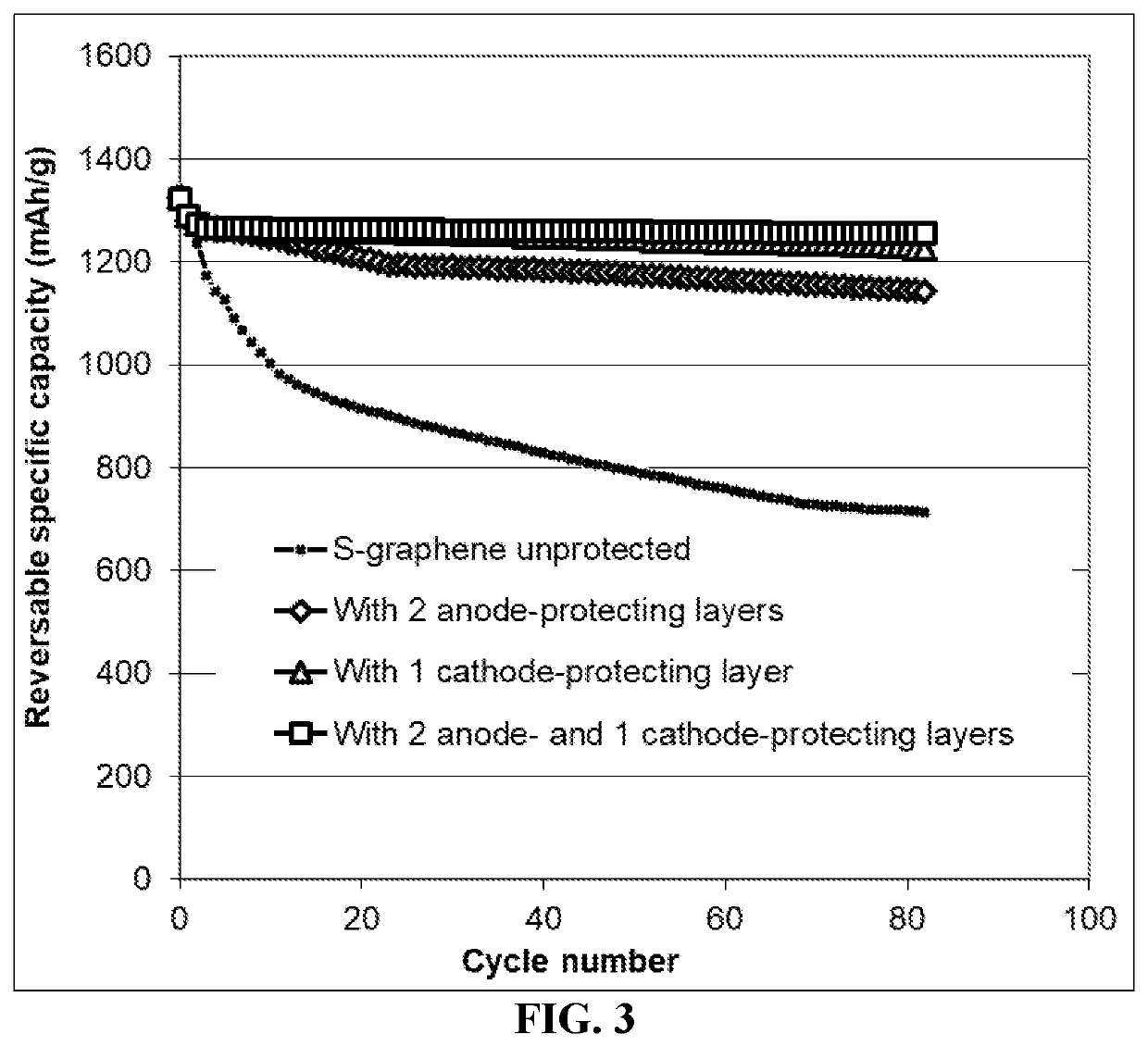
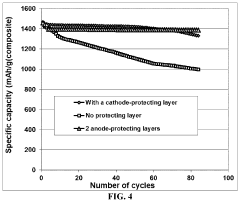
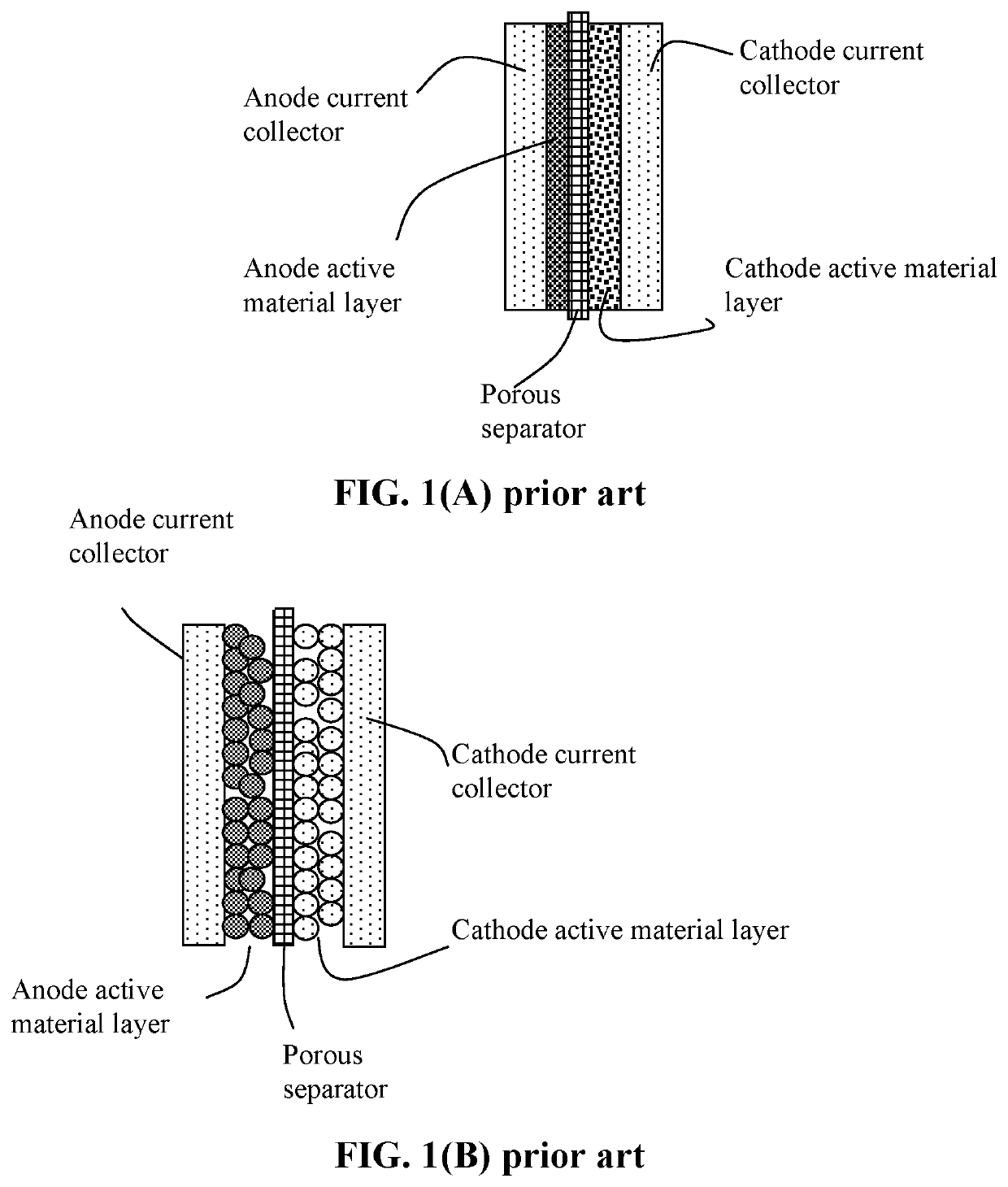
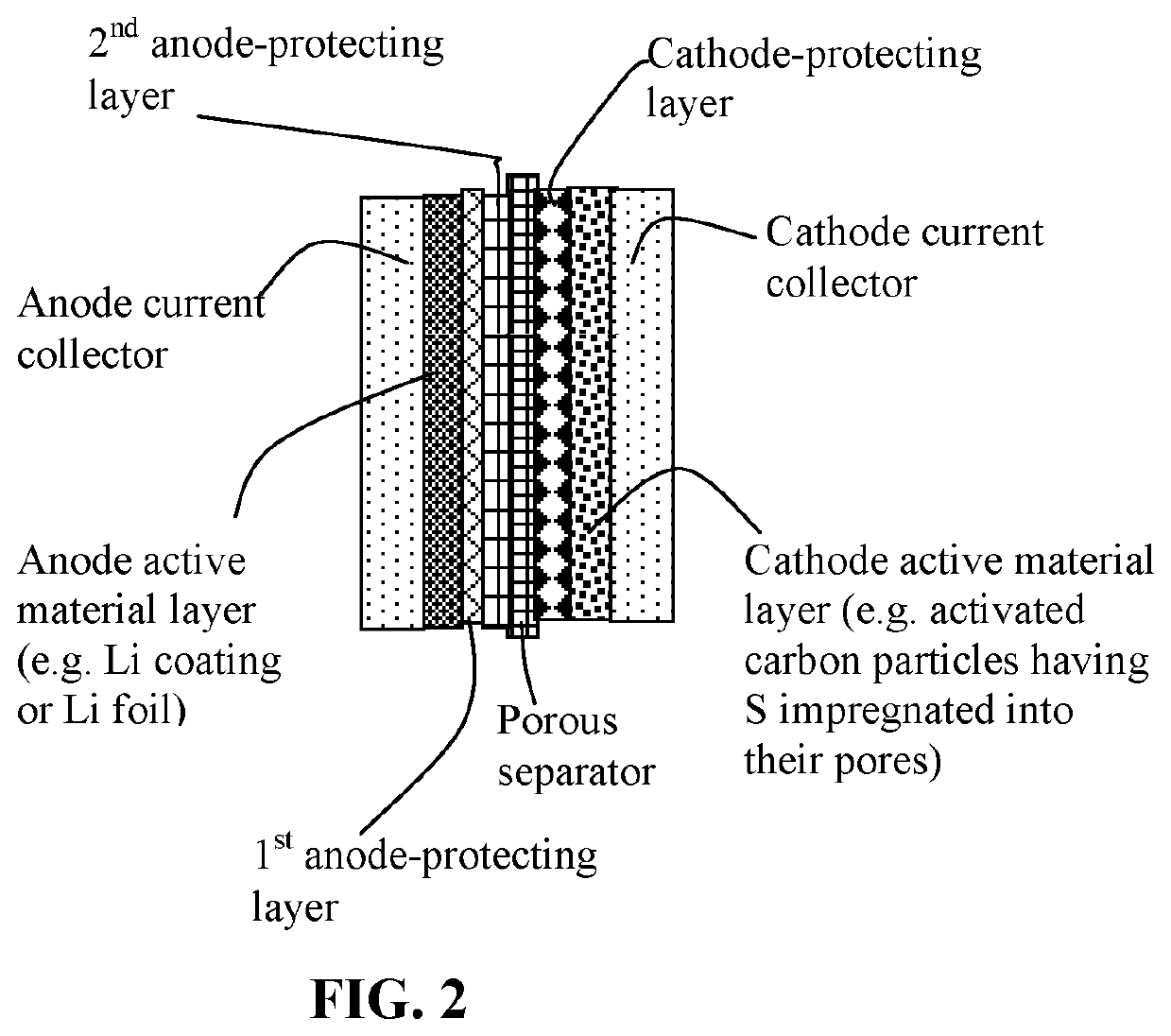
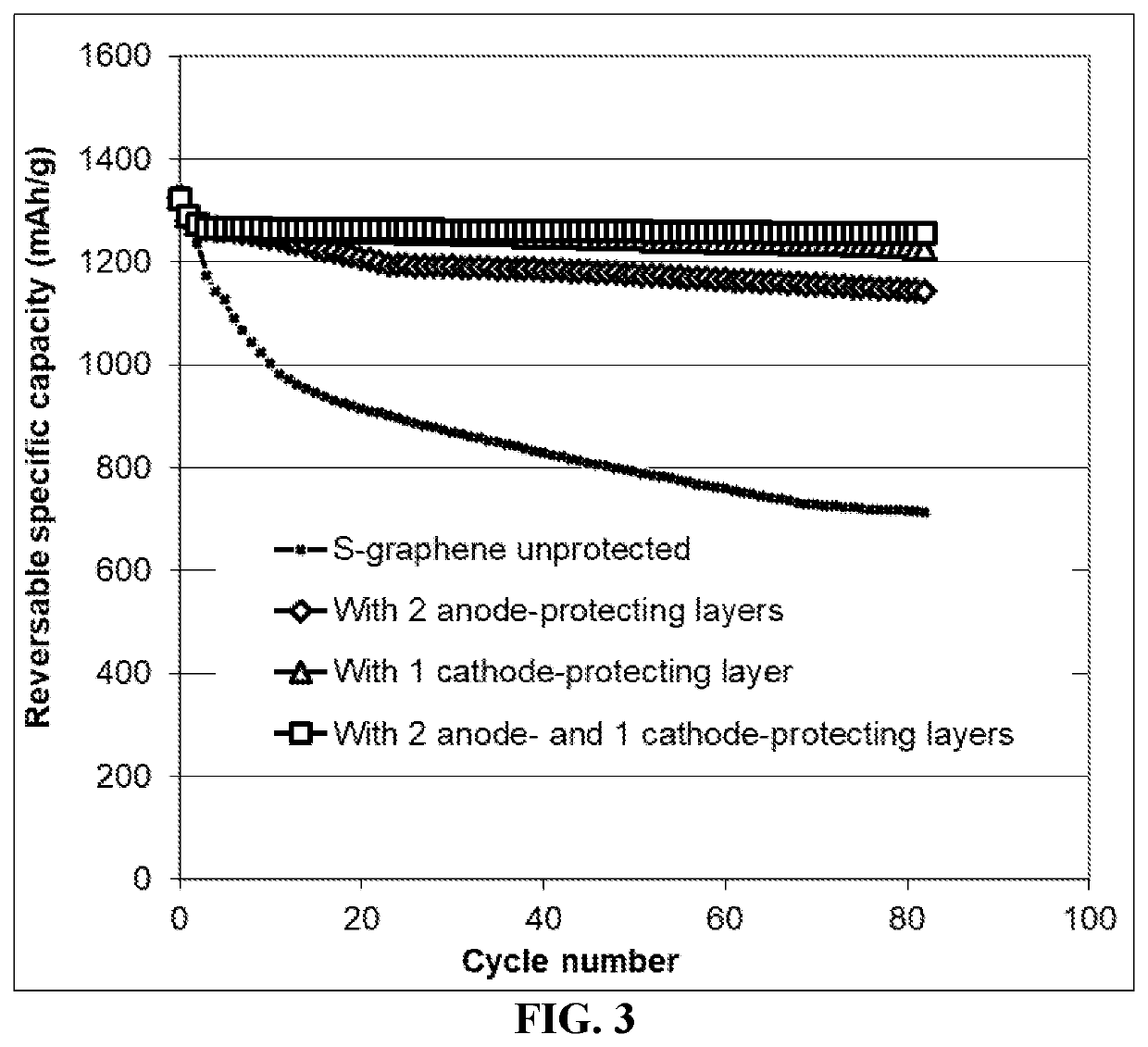
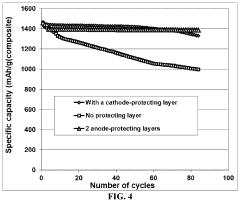
Lithium-sulfur battery containing two anode-protecting layers
PatentActiveUS20190386296A1
Innovation
- The implementation of an alkali metal-sulfur cell design featuring a sulfur-carbon hybrid cathode and a dual anode-protecting layer system, comprising a thin electron-conducting layer and an elastomer layer with high tensile elastic strain, to prevent dendrite formation and enhance sulfur utilization.
Method of protecting anode of a lithium-sulfur battery
PatentActiveUS20190386342A1
Innovation
- The implementation of an alkali metal-sulfur battery design featuring a sulfur-carbon hybrid cathode and a dual anode-protecting layer system, comprising a thin electron-conducting layer and an elastomer layer with high tensile elasticity, to prevent dendrite formation and enhance sulfur utilization.
Safety and Performance Standards for Na-S Batteries
The establishment of comprehensive safety and performance standards is critical for the widespread adoption of Na-S battery technology, particularly concerning sodium anode protection and dendrite mitigation. Current standards primarily focus on lithium-based systems, creating a regulatory gap for sodium-sulfur technologies. International organizations such as IEC, IEEE, and UL are gradually developing specific guidelines, but these remain in nascent stages compared to their lithium counterparts.
Safety standards for Na-S batteries must address the unique challenges posed by sodium metal anodes, including their high reactivity with moisture and oxygen, as well as the potential for thermal runaway at elevated temperatures. Particular attention must be given to dendrite formation, which can lead to internal short circuits and catastrophic failure. Testing protocols should include accelerated cycling under various temperature conditions to evaluate dendrite growth patterns and effectiveness of mitigation strategies.
Performance standards need to establish benchmarks for key metrics including energy density, power capability, cycle life, and temperature operating range. Current Na-S cells demonstrate theoretical energy densities of 760 Wh/kg, but practical implementations typically achieve only 150-200 Wh/kg due to protective measures required for anode stabilization. Standardized testing procedures should evaluate the trade-offs between protective layer thickness and electrochemical performance.
Thermal management requirements represent another critical aspect of Na-S battery standards. Unlike lithium-ion systems that operate near room temperature, high-temperature Na-S batteries function optimally at 300-350°C, necessitating specialized thermal insulation and management systems. Emerging room-temperature Na-S technologies require different thermal considerations, particularly regarding heat generation during dendrite formation and breakdown.
Environmental and disposal standards must account for the materials used in dendrite mitigation strategies, including potential electrolyte additives, artificial SEI components, and separator modifications. While sodium itself presents fewer environmental concerns than lithium, certain protective coatings and electrolyte formulations may contain toxic or environmentally persistent compounds requiring specific handling protocols.
Harmonization of standards across different regions remains challenging but essential for global market development. The European Battery Directive, China's GB/T standards, and emerging US regulations through ANSI/CAN/UL must align on fundamental safety requirements while accommodating regional manufacturing variations. Industry consortia are increasingly collaborating with academic institutions to develop standardized testing methodologies specifically addressing sodium dendrite formation and mitigation effectiveness.
Safety standards for Na-S batteries must address the unique challenges posed by sodium metal anodes, including their high reactivity with moisture and oxygen, as well as the potential for thermal runaway at elevated temperatures. Particular attention must be given to dendrite formation, which can lead to internal short circuits and catastrophic failure. Testing protocols should include accelerated cycling under various temperature conditions to evaluate dendrite growth patterns and effectiveness of mitigation strategies.
Performance standards need to establish benchmarks for key metrics including energy density, power capability, cycle life, and temperature operating range. Current Na-S cells demonstrate theoretical energy densities of 760 Wh/kg, but practical implementations typically achieve only 150-200 Wh/kg due to protective measures required for anode stabilization. Standardized testing procedures should evaluate the trade-offs between protective layer thickness and electrochemical performance.
Thermal management requirements represent another critical aspect of Na-S battery standards. Unlike lithium-ion systems that operate near room temperature, high-temperature Na-S batteries function optimally at 300-350°C, necessitating specialized thermal insulation and management systems. Emerging room-temperature Na-S technologies require different thermal considerations, particularly regarding heat generation during dendrite formation and breakdown.
Environmental and disposal standards must account for the materials used in dendrite mitigation strategies, including potential electrolyte additives, artificial SEI components, and separator modifications. While sodium itself presents fewer environmental concerns than lithium, certain protective coatings and electrolyte formulations may contain toxic or environmentally persistent compounds requiring specific handling protocols.
Harmonization of standards across different regions remains challenging but essential for global market development. The European Battery Directive, China's GB/T standards, and emerging US regulations through ANSI/CAN/UL must align on fundamental safety requirements while accommodating regional manufacturing variations. Industry consortia are increasingly collaborating with academic institutions to develop standardized testing methodologies specifically addressing sodium dendrite formation and mitigation effectiveness.
Environmental Impact and Sustainability Considerations
The environmental impact and sustainability considerations of Na-S battery technology present a compelling case for its continued development as an alternative to lithium-based systems. Sodium resources are approximately 1000 times more abundant than lithium in the Earth's crust, with widespread geographical distribution that reduces geopolitical supply risks. This abundance translates to significantly lower resource extraction impacts compared to lithium mining operations, which often involve extensive water usage and potential habitat disruption.
The production processes for sodium anodes generally require less energy than their lithium counterparts, contributing to a lower carbon footprint during manufacturing. Additionally, the sulfur cathode material represents an excellent example of industrial symbiosis, as it can be sourced as a byproduct from petroleum refining processes, effectively repurposing what would otherwise be considered industrial waste.
Life cycle assessments of Na-S cells indicate potential advantages in terms of embodied energy and carbon emissions compared to other battery technologies. However, these benefits are contingent upon effective dendrite mitigation strategies that do not introduce environmentally problematic materials. Some current protection approaches involve fluorinated compounds or synthetic polymers that may present end-of-life disposal challenges.
The recyclability of Na-S batteries represents another important sustainability consideration. The materials used in sodium anode protection systems must be designed with circularity in mind. Current research indicates that many of the solid electrolyte interphase (SEI) forming additives and artificial protective layers can be recovered through established recycling processes, though optimization is still needed to improve recovery rates.
Safety considerations also intersect with environmental impact, as dendrite-induced cell failures can lead to thermal events that release harmful substances. Effective dendrite mitigation not only improves battery performance but also reduces the risk of environmental contamination from battery failures. The development of non-toxic, biodegradable protection strategies represents a frontier in environmentally responsible battery design.
Regulatory frameworks are increasingly emphasizing full life-cycle environmental performance for energy storage technologies. Na-S cells with effective dendrite mitigation and anode protection systems are well-positioned to meet these evolving standards, particularly if research continues to focus on green chemistry principles in electrolyte and interface design. The integration of sustainability metrics into early-stage research could accelerate the development of environmentally superior protection strategies.
The production processes for sodium anodes generally require less energy than their lithium counterparts, contributing to a lower carbon footprint during manufacturing. Additionally, the sulfur cathode material represents an excellent example of industrial symbiosis, as it can be sourced as a byproduct from petroleum refining processes, effectively repurposing what would otherwise be considered industrial waste.
Life cycle assessments of Na-S cells indicate potential advantages in terms of embodied energy and carbon emissions compared to other battery technologies. However, these benefits are contingent upon effective dendrite mitigation strategies that do not introduce environmentally problematic materials. Some current protection approaches involve fluorinated compounds or synthetic polymers that may present end-of-life disposal challenges.
The recyclability of Na-S batteries represents another important sustainability consideration. The materials used in sodium anode protection systems must be designed with circularity in mind. Current research indicates that many of the solid electrolyte interphase (SEI) forming additives and artificial protective layers can be recovered through established recycling processes, though optimization is still needed to improve recovery rates.
Safety considerations also intersect with environmental impact, as dendrite-induced cell failures can lead to thermal events that release harmful substances. Effective dendrite mitigation not only improves battery performance but also reduces the risk of environmental contamination from battery failures. The development of non-toxic, biodegradable protection strategies represents a frontier in environmentally responsible battery design.
Regulatory frameworks are increasingly emphasizing full life-cycle environmental performance for energy storage technologies. Na-S cells with effective dendrite mitigation and anode protection systems are well-positioned to meet these evolving standards, particularly if research continues to focus on green chemistry principles in electrolyte and interface design. The integration of sustainability metrics into early-stage research could accelerate the development of environmentally superior protection strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!