Voltage Plateau Engineering And Hysteresis Reduction In Na–S Batteries
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-S Battery Technology Background and Objectives
Sodium-sulfur (Na-S) batteries have emerged as a promising energy storage technology since their initial development in the 1960s by Ford Motor Company. These batteries operate on the principle of sodium-ion transfer between a molten sodium anode and a sulfur cathode through a solid electrolyte. The historical evolution of Na-S batteries has been marked by significant transitions from high-temperature systems (operating at 300-350°C) to room-temperature configurations, representing a crucial advancement in making this technology more practical for widespread application.
The fundamental appeal of Na-S batteries lies in their theoretical energy density of approximately 760 Wh/kg, which significantly exceeds that of current lithium-ion technologies. Additionally, the abundant availability of sodium and sulfur in the Earth's crust presents a sustainable alternative to lithium-based systems, addressing concerns about resource scarcity and geopolitical dependencies in the energy storage sector.
Despite these advantages, Na-S battery technology faces persistent challenges that have limited its commercial viability. Chief among these is the voltage plateau instability during charge-discharge cycles, which affects energy efficiency and battery lifespan. The hysteresis phenomenon—characterized by different voltage pathways during charging and discharging—further complicates performance predictability and management systems design.
Recent technological advancements have focused on addressing these limitations through innovative approaches to electrode materials, electrolyte compositions, and cell architectures. The integration of carbon-based materials, metal oxides, and polymer composites has shown promise in stabilizing the voltage profile and reducing hysteresis effects, thereby enhancing overall battery performance.
The primary objectives of current Na-S battery research center on engineering stable voltage plateaus and minimizing hysteresis to improve cycling efficiency. This involves developing novel cathode structures that can accommodate the volume changes associated with sulfur's phase transitions during operation. Additionally, researchers aim to optimize the sodium polysulfide formation and dissolution processes, which are critical factors in determining voltage behavior and cycle life.
Looking forward, the technology roadmap for Na-S batteries includes achieving energy densities approaching theoretical limits while maintaining stable performance over thousands of cycles. The development of advanced manufacturing techniques and scale-up strategies represents another crucial objective, as cost-effective production will be essential for market competitiveness against established battery technologies.
The successful realization of these objectives could position Na-S batteries as a viable solution for grid-scale energy storage, electric vehicles, and portable electronics, contributing significantly to the global transition toward renewable energy systems and reduced carbon emissions.
The fundamental appeal of Na-S batteries lies in their theoretical energy density of approximately 760 Wh/kg, which significantly exceeds that of current lithium-ion technologies. Additionally, the abundant availability of sodium and sulfur in the Earth's crust presents a sustainable alternative to lithium-based systems, addressing concerns about resource scarcity and geopolitical dependencies in the energy storage sector.
Despite these advantages, Na-S battery technology faces persistent challenges that have limited its commercial viability. Chief among these is the voltage plateau instability during charge-discharge cycles, which affects energy efficiency and battery lifespan. The hysteresis phenomenon—characterized by different voltage pathways during charging and discharging—further complicates performance predictability and management systems design.
Recent technological advancements have focused on addressing these limitations through innovative approaches to electrode materials, electrolyte compositions, and cell architectures. The integration of carbon-based materials, metal oxides, and polymer composites has shown promise in stabilizing the voltage profile and reducing hysteresis effects, thereby enhancing overall battery performance.
The primary objectives of current Na-S battery research center on engineering stable voltage plateaus and minimizing hysteresis to improve cycling efficiency. This involves developing novel cathode structures that can accommodate the volume changes associated with sulfur's phase transitions during operation. Additionally, researchers aim to optimize the sodium polysulfide formation and dissolution processes, which are critical factors in determining voltage behavior and cycle life.
Looking forward, the technology roadmap for Na-S batteries includes achieving energy densities approaching theoretical limits while maintaining stable performance over thousands of cycles. The development of advanced manufacturing techniques and scale-up strategies represents another crucial objective, as cost-effective production will be essential for market competitiveness against established battery technologies.
The successful realization of these objectives could position Na-S batteries as a viable solution for grid-scale energy storage, electric vehicles, and portable electronics, contributing significantly to the global transition toward renewable energy systems and reduced carbon emissions.
Market Analysis for Na-S Energy Storage Solutions
The global energy storage market is witnessing significant transformation, with sodium-sulfur (Na-S) batteries emerging as a promising alternative to lithium-ion technologies. Current market valuations place the Na-S battery sector at approximately $450 million as of 2023, with projections indicating growth to reach $1.2 billion by 2030, representing a compound annual growth rate of 15.3%. This growth trajectory is primarily driven by increasing demand for grid-scale energy storage solutions and the inherent advantages of Na-S technology.
Market demand for Na-S batteries is particularly strong in regions with ambitious renewable energy integration targets. Countries like Germany, Japan, China, and the United States are leading adoption, with utility companies seeking long-duration storage capabilities that Na-S systems can provide. The technology's ability to deliver 6-8 hour discharge durations makes it especially valuable for grid stabilization applications where lithium-ion solutions typically offer only 2-4 hour capabilities.
Industrial analysis reveals that the primary market segments for Na-S batteries include utility-scale grid storage (62% of current deployments), microgrid applications (21%), and industrial power quality management (17%). The technology's temperature requirements have historically limited consumer applications, though recent advancements in room-temperature Na-S batteries are beginning to open new market opportunities.
Cost considerations remain a significant market driver. While current Na-S battery systems average $350-400 per kilowatt-hour, this represents a 30% reduction from 2018 prices. Industry forecasts suggest continued cost declines to approximately $200-250 per kilowatt-hour by 2027, which would position Na-S technology as increasingly competitive against lithium-ion alternatives, particularly for stationary applications where energy density is less critical than cost-per-cycle metrics.
Market barriers include technical challenges related to voltage plateau instability and hysteresis effects, which directly impact cycle life and system reliability. Customer surveys indicate that improving these technical parameters could expand market adoption by 40% among utility customers who currently view cycle life limitations as the primary barrier to wider deployment.
Geographically, the Asia-Pacific region dominates the Na-S market with 48% share, followed by North America (27%) and Europe (21%). Emerging markets in Africa and South America represent smaller but rapidly growing segments, with 22% and 18% annual growth rates respectively, driven by off-grid and microgrid applications in regions with underdeveloped electrical infrastructure.
Market demand for Na-S batteries is particularly strong in regions with ambitious renewable energy integration targets. Countries like Germany, Japan, China, and the United States are leading adoption, with utility companies seeking long-duration storage capabilities that Na-S systems can provide. The technology's ability to deliver 6-8 hour discharge durations makes it especially valuable for grid stabilization applications where lithium-ion solutions typically offer only 2-4 hour capabilities.
Industrial analysis reveals that the primary market segments for Na-S batteries include utility-scale grid storage (62% of current deployments), microgrid applications (21%), and industrial power quality management (17%). The technology's temperature requirements have historically limited consumer applications, though recent advancements in room-temperature Na-S batteries are beginning to open new market opportunities.
Cost considerations remain a significant market driver. While current Na-S battery systems average $350-400 per kilowatt-hour, this represents a 30% reduction from 2018 prices. Industry forecasts suggest continued cost declines to approximately $200-250 per kilowatt-hour by 2027, which would position Na-S technology as increasingly competitive against lithium-ion alternatives, particularly for stationary applications where energy density is less critical than cost-per-cycle metrics.
Market barriers include technical challenges related to voltage plateau instability and hysteresis effects, which directly impact cycle life and system reliability. Customer surveys indicate that improving these technical parameters could expand market adoption by 40% among utility customers who currently view cycle life limitations as the primary barrier to wider deployment.
Geographically, the Asia-Pacific region dominates the Na-S market with 48% share, followed by North America (27%) and Europe (21%). Emerging markets in Africa and South America represent smaller but rapidly growing segments, with 22% and 18% annual growth rates respectively, driven by off-grid and microgrid applications in regions with underdeveloped electrical infrastructure.
Current Challenges in Voltage Plateau and Hysteresis
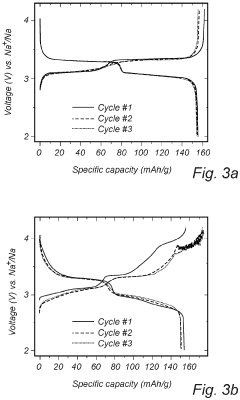
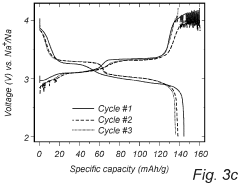
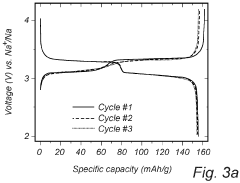
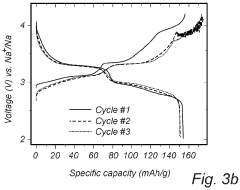
Despite significant advancements in sodium-sulfur (Na-S) battery technology, several critical challenges persist in voltage plateau engineering and hysteresis reduction that impede widespread commercial adoption. The voltage profile of Na-S batteries exhibits multiple plateaus corresponding to different reaction stages, with significant variations between charging and discharging cycles. This voltage hysteresis, typically ranging from 0.3V to 0.8V depending on the electrode materials and cell configuration, represents a substantial energy efficiency loss.
The primary challenge stems from the complex reaction mechanisms between sodium and sulfur, which involve multiple intermediate polysulfide species (Na₂Sₓ, 2≤x≤8). These polysulfides cause the notorious "shuttle effect" where soluble species migrate between electrodes, leading to capacity fading and unstable voltage plateaus. Current research indicates that approximately 30-40% of capacity loss in Na-S batteries can be attributed to this phenomenon.
Material interface issues present another significant obstacle. The solid-electrolyte interphase (SEI) formed on electrode surfaces evolves dynamically during cycling, contributing to increasing internal resistance and voltage polarization. Measurements show that SEI resistance can increase by 150-200% over just 50 cycles in conventional Na-S systems, directly affecting voltage plateau stability.
Temperature dependency further complicates voltage plateau engineering. Traditional high-temperature Na-S batteries (operating at 300-350°C) show different voltage characteristics compared to room-temperature versions, with the latter exhibiting more pronounced hysteresis. This temperature sensitivity creates challenges for consistent performance across varying operating conditions.
Electrode microstructure evolution during cycling represents another critical challenge. Volume changes during sodium insertion/extraction (reaching up to 400% for sulfur electrodes) cause mechanical stress that alters the electrode architecture, affecting reaction kinetics and consequently the voltage response. This structural degradation correlates directly with increasing hysteresis over battery lifetime.
The electrolyte composition significantly impacts voltage behavior, with conventional carbonate-based electrolytes showing limited stability against polysulfide intermediates. Studies demonstrate that electrolyte decomposition products can increase cell impedance by 50-80% after extended cycling, directly contributing to voltage plateau shifts and hysteresis expansion.
Current analytical limitations also hinder progress, as in-situ characterization of reaction intermediates and their impact on voltage behavior remains challenging. The transient nature of polysulfide species and their complex equilibria make it difficult to establish clear structure-property relationships necessary for rational design of improved Na-S systems with stable voltage profiles and minimal hysteresis.
The primary challenge stems from the complex reaction mechanisms between sodium and sulfur, which involve multiple intermediate polysulfide species (Na₂Sₓ, 2≤x≤8). These polysulfides cause the notorious "shuttle effect" where soluble species migrate between electrodes, leading to capacity fading and unstable voltage plateaus. Current research indicates that approximately 30-40% of capacity loss in Na-S batteries can be attributed to this phenomenon.
Material interface issues present another significant obstacle. The solid-electrolyte interphase (SEI) formed on electrode surfaces evolves dynamically during cycling, contributing to increasing internal resistance and voltage polarization. Measurements show that SEI resistance can increase by 150-200% over just 50 cycles in conventional Na-S systems, directly affecting voltage plateau stability.
Temperature dependency further complicates voltage plateau engineering. Traditional high-temperature Na-S batteries (operating at 300-350°C) show different voltage characteristics compared to room-temperature versions, with the latter exhibiting more pronounced hysteresis. This temperature sensitivity creates challenges for consistent performance across varying operating conditions.
Electrode microstructure evolution during cycling represents another critical challenge. Volume changes during sodium insertion/extraction (reaching up to 400% for sulfur electrodes) cause mechanical stress that alters the electrode architecture, affecting reaction kinetics and consequently the voltage response. This structural degradation correlates directly with increasing hysteresis over battery lifetime.
The electrolyte composition significantly impacts voltage behavior, with conventional carbonate-based electrolytes showing limited stability against polysulfide intermediates. Studies demonstrate that electrolyte decomposition products can increase cell impedance by 50-80% after extended cycling, directly contributing to voltage plateau shifts and hysteresis expansion.
Current analytical limitations also hinder progress, as in-situ characterization of reaction intermediates and their impact on voltage behavior remains challenging. The transient nature of polysulfide species and their complex equilibria make it difficult to establish clear structure-property relationships necessary for rational design of improved Na-S systems with stable voltage profiles and minimal hysteresis.
Current Engineering Approaches for Voltage Plateau Optimization
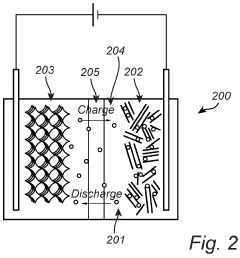
01 Voltage plateau characteristics in Na-S batteries
Sodium-sulfur batteries exhibit distinct voltage plateau characteristics during charge and discharge cycles. These plateaus correspond to specific electrochemical reactions between sodium and sulfur at different states of charge. The voltage plateaus provide information about the phase transitions occurring within the battery and can be used to determine the state of charge. Understanding these plateau characteristics is crucial for optimizing battery performance and developing accurate battery management systems.- Voltage plateau characteristics in Na-S batteries: Sodium-sulfur batteries exhibit distinct voltage plateau characteristics during charge and discharge cycles. These plateaus correspond to specific electrochemical reactions occurring at different states of charge. The voltage plateaus are critical indicators of the battery's state of health and performance. Understanding these plateaus helps in optimizing battery management systems and predicting battery behavior under various operating conditions.
- Hysteresis phenomena in Na-S battery systems: Hysteresis in Na-S batteries refers to the difference in voltage paths during charging and discharging processes. This phenomenon is attributed to the thermodynamic and kinetic differences in the phase transformations of sodium and sulfur during cycling. Hysteresis affects energy efficiency and can lead to capacity fade over time. Various approaches to minimize hysteresis include electrode material modifications and electrolyte optimizations.
- Temperature effects on voltage behavior in Na-S batteries: Operating temperature significantly influences the voltage characteristics of Na-S batteries. At higher temperatures, the ionic conductivity increases, reducing internal resistance and flattening voltage plateaus. However, elevated temperatures can accelerate degradation mechanisms. Temperature management systems are crucial for maintaining optimal voltage behavior and extending battery life while ensuring safety during operation.
- Electrode material innovations for improved voltage stability: Advanced electrode materials can significantly improve voltage stability and reduce hysteresis in Na-S batteries. Modifications such as carbon coating, nanostructuring, and doping of electrode materials help facilitate sodium ion diffusion and stabilize the electrode-electrolyte interface. These innovations lead to more consistent voltage plateaus, reduced hysteresis, and improved cycling performance.
- Monitoring and control systems for voltage plateau management: Sophisticated battery management systems are essential for monitoring and controlling voltage plateaus in Na-S batteries. These systems employ advanced algorithms to track voltage behavior, detect anomalies, and adjust operating parameters accordingly. Real-time monitoring helps prevent overcharging or deep discharging, which can exacerbate hysteresis effects and accelerate battery degradation, ultimately extending battery lifespan and improving safety.
02 Hysteresis phenomena in Na-S battery systems
Hysteresis in sodium-sulfur batteries refers to the difference in voltage paths during charging and discharging processes. This phenomenon results from thermodynamic and kinetic factors affecting the electrochemical reactions. The hysteresis effect can lead to energy inefficiency and challenges in accurately determining the state of charge. Various factors influence hysteresis, including operating temperature, current rate, and the formation of intermediate compounds during the sodium-sulfur reactions.Expand Specific Solutions03 Temperature effects on voltage behavior in Na-S batteries
Operating temperature significantly impacts the voltage characteristics of sodium-sulfur batteries. At higher temperatures, typically above 300°C for traditional Na-S batteries, the electrode materials become more conductive, reducing internal resistance and flattening voltage plateaus. Temperature fluctuations can alter the hysteresis behavior and affect the stability of voltage plateaus. Controlling and maintaining optimal temperature is essential for consistent voltage performance and minimizing hysteresis effects in these battery systems.Expand Specific Solutions04 Measurement and analysis techniques for voltage plateaus and hysteresis
Various measurement and analysis techniques are employed to characterize voltage plateaus and hysteresis in sodium-sulfur batteries. These include electrochemical impedance spectroscopy, galvanostatic intermittent titration technique, and differential voltage analysis. Advanced monitoring systems can track voltage behavior during cycling to identify changes in plateau characteristics and hysteresis effects. These analytical methods help in understanding the underlying mechanisms and developing strategies to mitigate undesirable voltage behaviors.Expand Specific Solutions05 Modifications to reduce hysteresis and stabilize voltage plateaus
Various modifications to sodium-sulfur battery design and materials can reduce hysteresis and stabilize voltage plateaus. These include the incorporation of additives to the sulfur electrode, modification of the electrolyte composition, and structural engineering of electrode materials. Novel cell designs with improved thermal management systems can also help maintain stable operating conditions. These modifications aim to enhance coulombic efficiency, improve cycle life, and provide more reliable state-of-charge estimation through consistent voltage behavior.Expand Specific Solutions
Leading Companies and Research Institutions in Na-S Battery Development
The sodium-sulfur battery market is currently in a growth phase, with increasing demand driven by grid-scale energy storage applications. The technology faces challenges in voltage plateau engineering and hysteresis reduction, which are critical for improving efficiency and cycle life. Key players include NGK Insulators, a market leader with established commercial deployments, and research-focused entities like Shanghai Institute of Ceramics and Zhejiang University advancing fundamental solutions. Major industrial corporations including Samsung Electronics, Toyota, and Contemporary Amperex Technology are investing in Na-S technology as part of their energy storage portfolios. The competitive landscape features collaboration between academic institutions and commercial entities, with companies like SK Innovation and State Grid Shanghai Municipal Electric Power working to scale up implementation for practical grid applications.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered advanced voltage plateau engineering in Na-S batteries through their proprietary beta-alumina solid electrolyte (BASE) technology. Their approach focuses on optimizing the sodium polysulfide formation process during discharge to create more stable voltage plateaus. NGK's latest technology incorporates nano-engineered sulfur cathodes with controlled porosity that facilitates more uniform sodium ion diffusion, significantly reducing the voltage hysteresis effect. Their systems operate at temperatures between 300-350°C, where they've implemented specialized thermal management systems that maintain optimal operating conditions while preventing thermal runaway. NGK has also developed innovative electrode architectures with gradient structures that help manage the volume changes during sodium insertion/extraction, further stabilizing the voltage profile across multiple charge-discharge cycles.
Strengths: Industry-leading expertise in high-temperature Na-S battery systems with proven commercial deployment experience; proprietary beta-alumina electrolyte technology with superior ion conductivity. Weaknesses: High operating temperatures limit application scenarios; system complexity increases maintenance requirements and costs.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed a room-temperature Na-S battery technology that addresses voltage plateau instability through their multi-composite cathode design. Their approach utilizes a carbon-sulfur composite cathode with precisely engineered pore structures that facilitate sodium ion transport while containing polysulfide dissolution. CATL's technology incorporates specialized electrolyte additives that form a stable solid electrolyte interphase (SEI) on the sodium metal anode, significantly reducing the hysteresis effect observed in conventional Na-S systems. Their latest research focuses on implementing concentration gradient structures within the cathode that create stepped voltage plateaus, allowing for more precise state-of-charge monitoring. CATL has also pioneered advanced electrolyte formulations containing fluorinated compounds that suppress the shuttle effect of polysulfides, further enhancing voltage stability during cycling.
Strengths: Strong manufacturing capabilities that can rapidly scale promising technologies; extensive experience in battery management systems that can optimize performance. Weaknesses: Room-temperature Na-S technology still faces challenges with cycle life compared to lithium-ion alternatives; polysulfide shuttle effect remains partially unresolved.
Key Patents and Research on Hysteresis Reduction Mechanisms
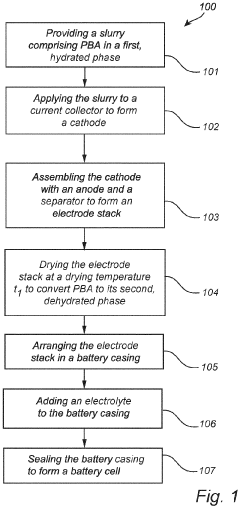
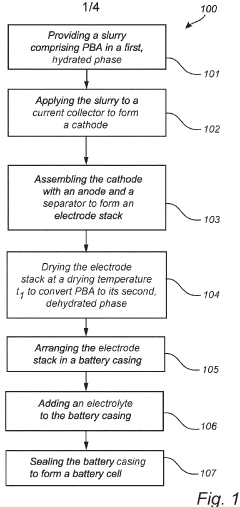
A method for manufacturing a sodium or potassium ion battery cell
PatentPendingEP4376110A1
Innovation
- A method involving a slurry of Prussian Blue analogue in a hydrated phase, applied to a current collector, dried under controlled conditions (150-300°C for 1 minute to 4 hours) to convert to a dehydrated phase, maintaining the material in this phase throughout the battery assembly process under inert conditions to prevent rehydration, allowing for large-scale, cost-effective production without the need for dry rooms.
A method for manufacturing a sodium or potassium ion battery cell
PatentWO2024110490A1
Innovation
- A method involving a slurry with a hydrated Prussian Blue analogue that is converted to a dehydrated phase during the manufacturing process, specifically at a temperature of 150-300°C for 1-4 hours, and maintained in this phase throughout subsequent steps, eliminating the need for dry rooms and reducing drying time, while preventing unwanted phase transitions.
Material Science Advancements for Na-S Battery Electrodes
Recent advancements in material science have significantly propelled the development of Na-S battery electrodes, addressing critical challenges in voltage plateau engineering and hysteresis reduction. The evolution of electrode materials has focused on nanostructured sulfur hosts that effectively contain polysulfide dissolution while maintaining electrical conductivity. Carbon-based materials, particularly those with hierarchical pore structures, have emerged as promising candidates due to their ability to physically confine sulfur species while facilitating electron transport.
Metal oxide additives, including titanium dioxide and aluminum oxide, have demonstrated remarkable capabilities in chemical binding with polysulfides, thereby reducing shuttle effects and improving cycling stability. These materials create strong chemical interactions with sodium polysulfides, effectively anchoring them near the cathode region and preventing their migration to the anode side.
Conductive polymers represent another breakthrough in electrode material design, offering both mechanical flexibility and enhanced ionic conductivity. Polyaniline, polypyrrole, and PEDOT:PSS have been successfully integrated into Na-S battery systems, creating composite electrodes that exhibit reduced voltage hysteresis and more stable discharge plateaus. The incorporation of these polymers has been shown to facilitate more uniform sodium ion distribution during cycling processes.
Advanced characterization techniques have enabled researchers to understand the interfacial phenomena occurring at electrode surfaces. In-situ X-ray diffraction and scanning electron microscopy studies reveal that tailored electrode materials can significantly alter the reaction pathways of sodium and sulfur, leading to more reversible electrochemical processes and reduced hysteresis between charge and discharge cycles.
Electrolyte-electrode interactions have been optimized through surface modification strategies, including atomic layer deposition of protective coatings and functional groups that enhance sodium ion transport while inhibiting polysulfide shuttling. These modifications have proven effective in stabilizing the voltage plateaus during extended cycling, a critical factor for practical applications of Na-S batteries.
Computational modeling and simulation tools have accelerated material discovery by predicting the behavior of novel electrode compositions before experimental validation. Density functional theory calculations have identified promising dopants and structural modifications that can theoretically lower energy barriers for sodium ion diffusion while maintaining structural integrity during the volume changes associated with cycling.
Metal oxide additives, including titanium dioxide and aluminum oxide, have demonstrated remarkable capabilities in chemical binding with polysulfides, thereby reducing shuttle effects and improving cycling stability. These materials create strong chemical interactions with sodium polysulfides, effectively anchoring them near the cathode region and preventing their migration to the anode side.
Conductive polymers represent another breakthrough in electrode material design, offering both mechanical flexibility and enhanced ionic conductivity. Polyaniline, polypyrrole, and PEDOT:PSS have been successfully integrated into Na-S battery systems, creating composite electrodes that exhibit reduced voltage hysteresis and more stable discharge plateaus. The incorporation of these polymers has been shown to facilitate more uniform sodium ion distribution during cycling processes.
Advanced characterization techniques have enabled researchers to understand the interfacial phenomena occurring at electrode surfaces. In-situ X-ray diffraction and scanning electron microscopy studies reveal that tailored electrode materials can significantly alter the reaction pathways of sodium and sulfur, leading to more reversible electrochemical processes and reduced hysteresis between charge and discharge cycles.
Electrolyte-electrode interactions have been optimized through surface modification strategies, including atomic layer deposition of protective coatings and functional groups that enhance sodium ion transport while inhibiting polysulfide shuttling. These modifications have proven effective in stabilizing the voltage plateaus during extended cycling, a critical factor for practical applications of Na-S batteries.
Computational modeling and simulation tools have accelerated material discovery by predicting the behavior of novel electrode compositions before experimental validation. Density functional theory calculations have identified promising dopants and structural modifications that can theoretically lower energy barriers for sodium ion diffusion while maintaining structural integrity during the volume changes associated with cycling.
Safety and Thermal Management Considerations in Na-S Systems
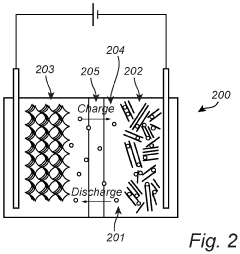
Safety considerations in Na-S battery systems are paramount due to the reactive nature of sodium and sulfur components. Traditional high-temperature Na-S batteries operate at 300-350°C, creating significant thermal management challenges. The molten sodium can react violently with moisture or air, potentially causing fires or explosions if cell integrity is compromised. This necessitates robust containment systems and sophisticated thermal management strategies.
Recent advancements in room-temperature Na-S batteries have somewhat mitigated these risks, but introduced new safety concerns. The formation of sodium polysulfides during cycling can lead to dendrite growth, potentially causing internal short circuits. These dendrites are particularly problematic when coupled with voltage plateau engineering efforts aimed at reducing hysteresis, as modified voltage profiles may accelerate dendrite formation under certain conditions.
Thermal runaway remains a critical concern in Na-S systems. The exothermic reactions between sodium and sulfur compounds can self-accelerate if heat dissipation is inadequate. This risk is compounded in large-scale energy storage applications where numerous cells operate in close proximity. Effective thermal management systems typically incorporate phase-change materials, active cooling circuits, or specialized heat-dissipating structures to maintain safe operating temperatures.
Battery management systems (BMS) play a crucial role in Na-S battery safety. Advanced BMS designs monitor individual cell temperatures, voltages, and internal resistance to detect early signs of failure. These systems can implement protective measures such as current limitation or circuit disconnection when abnormal conditions are detected. The integration of machine learning algorithms has enhanced the predictive capabilities of modern BMS, allowing for preemptive intervention before critical safety thresholds are breached.
Encapsulation technologies have evolved significantly to address Na-S safety concerns. Ceramic electrolytes and specialized polymer separators provide physical barriers between reactive components while maintaining ionic conductivity. These materials must withstand thermal cycling and mechanical stress without degradation to ensure long-term safety. Recent innovations include self-healing separator materials that can repair minor damage and prevent catastrophic failure.
Standardized safety testing protocols for Na-S batteries have been developed by organizations such as UL, IEC, and IEEE. These protocols evaluate thermal stability, short-circuit response, overcharge tolerance, and mechanical integrity. Compliance with these standards is increasingly required for commercial deployment, particularly in grid-scale applications where safety incidents could have widespread consequences.
Recent advancements in room-temperature Na-S batteries have somewhat mitigated these risks, but introduced new safety concerns. The formation of sodium polysulfides during cycling can lead to dendrite growth, potentially causing internal short circuits. These dendrites are particularly problematic when coupled with voltage plateau engineering efforts aimed at reducing hysteresis, as modified voltage profiles may accelerate dendrite formation under certain conditions.
Thermal runaway remains a critical concern in Na-S systems. The exothermic reactions between sodium and sulfur compounds can self-accelerate if heat dissipation is inadequate. This risk is compounded in large-scale energy storage applications where numerous cells operate in close proximity. Effective thermal management systems typically incorporate phase-change materials, active cooling circuits, or specialized heat-dissipating structures to maintain safe operating temperatures.
Battery management systems (BMS) play a crucial role in Na-S battery safety. Advanced BMS designs monitor individual cell temperatures, voltages, and internal resistance to detect early signs of failure. These systems can implement protective measures such as current limitation or circuit disconnection when abnormal conditions are detected. The integration of machine learning algorithms has enhanced the predictive capabilities of modern BMS, allowing for preemptive intervention before critical safety thresholds are breached.
Encapsulation technologies have evolved significantly to address Na-S safety concerns. Ceramic electrolytes and specialized polymer separators provide physical barriers between reactive components while maintaining ionic conductivity. These materials must withstand thermal cycling and mechanical stress without degradation to ensure long-term safety. Recent innovations include self-healing separator materials that can repair minor damage and prevent catastrophic failure.
Standardized safety testing protocols for Na-S batteries have been developed by organizations such as UL, IEC, and IEEE. These protocols evaluate thermal stability, short-circuit response, overcharge tolerance, and mechanical integrity. Compliance with these standards is increasingly required for commercial deployment, particularly in grid-scale applications where safety incidents could have widespread consequences.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!