Scalable Sulfur Infusion Methods For High-Loading Na–S Electrodes
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sulfur Infusion Technology Background and Objectives
Sodium-sulfur (Na-S) battery technology has emerged as a promising alternative to lithium-ion batteries due to its potential for high energy density, low cost, and the abundance of raw materials. The evolution of this technology can be traced back to the 1960s when Ford Motor Company first developed high-temperature Na-S batteries operating at 300-350°C. These early systems, while functional, faced significant challenges related to safety, durability, and practical implementation.
In recent years, room-temperature Na-S batteries have gained substantial attention as they eliminate many of the safety concerns associated with high-temperature operation. However, the development of efficient room-temperature Na-S batteries faces several technical hurdles, particularly regarding sulfur utilization and electrode stability. The shuttle effect of polysulfides, low electronic conductivity of sulfur, and volume expansion during cycling remain critical challenges.
The sulfur infusion process represents a crucial aspect of Na-S battery fabrication, directly impacting electrode performance and battery life. Traditional methods of sulfur loading, such as mechanical mixing or melt diffusion, often result in non-uniform distribution and poor sulfur utilization. These limitations have prompted extensive research into advanced sulfur infusion techniques that can achieve high sulfur loading while maintaining structural integrity and electrochemical performance.
Current technological objectives in this field focus on developing scalable methods for uniform sulfur infusion into various host materials, particularly carbon-based matrices. The ideal infusion process should enable high sulfur loading (>70 wt%) while ensuring intimate contact between sulfur and the conductive host material. Additionally, the process must be compatible with large-scale manufacturing to facilitate commercial viability.
The advancement of sulfur infusion technology aims to address several key performance metrics: increasing the energy density to >500 Wh/kg at the cell level, extending cycle life to >1000 cycles with minimal capacity fade, and improving rate capability for faster charging and discharging. These objectives align with the broader goal of developing sustainable and cost-effective energy storage solutions for grid applications and electric vehicles.
Recent technological trends indicate a shift toward hierarchical porous structures as sulfur hosts, combined with novel infusion techniques that leverage physical and chemical interactions to trap sulfur within the host material. Vapor-phase infiltration, solution-based methods, and in-situ sulfur formation approaches are being explored as potential pathways to achieve optimal sulfur distribution and confinement.
The ultimate goal of sulfur infusion technology development is to establish manufacturing processes that are not only technically effective but also economically viable and environmentally sustainable, paving the way for widespread adoption of Na-S batteries in various applications.
In recent years, room-temperature Na-S batteries have gained substantial attention as they eliminate many of the safety concerns associated with high-temperature operation. However, the development of efficient room-temperature Na-S batteries faces several technical hurdles, particularly regarding sulfur utilization and electrode stability. The shuttle effect of polysulfides, low electronic conductivity of sulfur, and volume expansion during cycling remain critical challenges.
The sulfur infusion process represents a crucial aspect of Na-S battery fabrication, directly impacting electrode performance and battery life. Traditional methods of sulfur loading, such as mechanical mixing or melt diffusion, often result in non-uniform distribution and poor sulfur utilization. These limitations have prompted extensive research into advanced sulfur infusion techniques that can achieve high sulfur loading while maintaining structural integrity and electrochemical performance.
Current technological objectives in this field focus on developing scalable methods for uniform sulfur infusion into various host materials, particularly carbon-based matrices. The ideal infusion process should enable high sulfur loading (>70 wt%) while ensuring intimate contact between sulfur and the conductive host material. Additionally, the process must be compatible with large-scale manufacturing to facilitate commercial viability.
The advancement of sulfur infusion technology aims to address several key performance metrics: increasing the energy density to >500 Wh/kg at the cell level, extending cycle life to >1000 cycles with minimal capacity fade, and improving rate capability for faster charging and discharging. These objectives align with the broader goal of developing sustainable and cost-effective energy storage solutions for grid applications and electric vehicles.
Recent technological trends indicate a shift toward hierarchical porous structures as sulfur hosts, combined with novel infusion techniques that leverage physical and chemical interactions to trap sulfur within the host material. Vapor-phase infiltration, solution-based methods, and in-situ sulfur formation approaches are being explored as potential pathways to achieve optimal sulfur distribution and confinement.
The ultimate goal of sulfur infusion technology development is to establish manufacturing processes that are not only technically effective but also economically viable and environmentally sustainable, paving the way for widespread adoption of Na-S batteries in various applications.
Market Analysis for Na-S Battery Applications
The sodium-sulfur (Na-S) battery market is experiencing significant growth potential due to the increasing demand for large-scale energy storage solutions. Current market projections indicate that the global Na-S battery market is expected to reach $500 million by 2025, with a compound annual growth rate of 12% from 2020 to 2025. This growth is primarily driven by the expanding renewable energy sector, which requires efficient and cost-effective energy storage systems to manage intermittent power generation.
The utility sector represents the largest application segment for Na-S batteries, accounting for approximately 70% of the total market share. Grid stabilization and load leveling applications are particularly prominent, as power companies seek solutions to balance supply and demand fluctuations. The renewable energy integration segment is growing at the fastest rate, as countries worldwide increase their renewable energy capacity and require complementary storage technologies.
Geographically, Asia Pacific dominates the Na-S battery market, with Japan and South Korea leading in both production and deployment. This regional dominance is attributed to the strong presence of key manufacturers and supportive government policies promoting clean energy technologies. North America and Europe are emerging as significant markets, driven by increasing investments in renewable energy infrastructure and grid modernization initiatives.
Cost considerations remain a critical factor influencing market adoption. Traditional high-temperature Na-S batteries have faced challenges due to their high manufacturing and operational costs. However, the development of room-temperature Na-S batteries with high-loading electrodes presents a promising opportunity to reduce costs by up to 40% compared to conventional lithium-ion systems, potentially expanding market penetration across various sectors.
Customer segments for Na-S batteries include utility companies, renewable energy developers, industrial facilities, and telecommunications providers. Each segment has distinct requirements regarding capacity, discharge duration, and cycle life. The utility segment typically demands large-scale systems with capacities exceeding 1 MWh, while industrial applications focus on medium-sized installations ranging from 100 kWh to 1 MWh.
Market barriers include competition from alternative energy storage technologies such as lithium-ion batteries, flow batteries, and compressed air energy storage. However, Na-S batteries with improved sulfur loading methods offer competitive advantages in terms of energy density, cycle life, and raw material availability, potentially overcoming these barriers as the technology matures.
Future market growth will likely be influenced by technological advancements in electrode design, manufacturing scalability, and system integration. As scalable sulfur infusion methods for high-loading electrodes continue to improve, the economic proposition of Na-S batteries is expected to strengthen, potentially capturing market share from incumbent technologies in the energy storage landscape.
The utility sector represents the largest application segment for Na-S batteries, accounting for approximately 70% of the total market share. Grid stabilization and load leveling applications are particularly prominent, as power companies seek solutions to balance supply and demand fluctuations. The renewable energy integration segment is growing at the fastest rate, as countries worldwide increase their renewable energy capacity and require complementary storage technologies.
Geographically, Asia Pacific dominates the Na-S battery market, with Japan and South Korea leading in both production and deployment. This regional dominance is attributed to the strong presence of key manufacturers and supportive government policies promoting clean energy technologies. North America and Europe are emerging as significant markets, driven by increasing investments in renewable energy infrastructure and grid modernization initiatives.
Cost considerations remain a critical factor influencing market adoption. Traditional high-temperature Na-S batteries have faced challenges due to their high manufacturing and operational costs. However, the development of room-temperature Na-S batteries with high-loading electrodes presents a promising opportunity to reduce costs by up to 40% compared to conventional lithium-ion systems, potentially expanding market penetration across various sectors.
Customer segments for Na-S batteries include utility companies, renewable energy developers, industrial facilities, and telecommunications providers. Each segment has distinct requirements regarding capacity, discharge duration, and cycle life. The utility segment typically demands large-scale systems with capacities exceeding 1 MWh, while industrial applications focus on medium-sized installations ranging from 100 kWh to 1 MWh.
Market barriers include competition from alternative energy storage technologies such as lithium-ion batteries, flow batteries, and compressed air energy storage. However, Na-S batteries with improved sulfur loading methods offer competitive advantages in terms of energy density, cycle life, and raw material availability, potentially overcoming these barriers as the technology matures.
Future market growth will likely be influenced by technological advancements in electrode design, manufacturing scalability, and system integration. As scalable sulfur infusion methods for high-loading electrodes continue to improve, the economic proposition of Na-S batteries is expected to strengthen, potentially capturing market share from incumbent technologies in the energy storage landscape.
Current Challenges in Sulfur Loading Techniques
Despite the promising theoretical energy density of sodium-sulfur (Na-S) batteries, achieving high sulfur loading in electrodes remains one of the most significant technical bottlenecks. Current sulfur infusion methods face substantial scalability challenges when attempting to increase sulfur content beyond conventional levels. The primary obstacle lies in the inherent electrical insulating properties of sulfur, which severely limits electron transport within the electrode structure when loading exceeds certain thresholds.
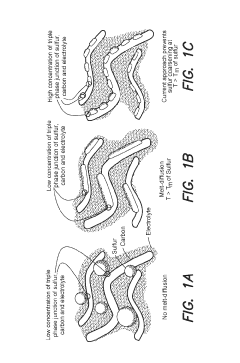
Traditional melt-diffusion techniques, while effective at laboratory scale, encounter significant uniformity issues when scaled to industrial production levels. As sulfur loading increases, the viscosity of molten sulfur creates uneven distribution patterns, resulting in electrode "dead zones" where electrochemical reactions cannot occur efficiently. This non-uniformity becomes particularly problematic when attempting to achieve sulfur loadings above 70 wt%, which would be necessary for Na-S batteries to compete with current lithium-ion technologies.
Another critical challenge is the volume expansion problem. During cycling, sulfur undergoes substantial volumetric changes (up to 80%), which becomes increasingly destructive to electrode integrity as loading increases. Current electrode architectures and binders cannot adequately accommodate this expansion at high sulfur loadings, leading to mechanical degradation and rapid capacity fading after relatively few cycles.
The "shuttle effect" - the dissolution of polysulfide intermediates into the electrolyte - represents another major hurdle that intensifies with increased sulfur loading. Higher sulfur content creates steeper concentration gradients that accelerate polysulfide migration, resulting in accelerated capacity loss and reduced coulombic efficiency. Current containment strategies that work reasonably well at moderate loadings become overwhelmed when sulfur content exceeds 60-65 wt%.
From a manufacturing perspective, high-loading sulfur electrodes present significant processing challenges. The rheological properties of high-sulfur slurries complicate conventional coating processes, leading to poor adhesion to current collectors and uneven electrode surfaces. Additionally, the thermal sensitivity of sulfur compounds necessitates precise temperature control during processing, which becomes increasingly difficult to maintain uniformly across larger production volumes.
Energy density calculations reveal that current methods can achieve practical energy densities of only 250-300 Wh/kg at the cell level, far below the theoretical maximum of 760 Wh/kg. This efficiency gap widens as sulfur loading increases, indicating fundamental limitations in current infusion methodologies rather than simply engineering challenges.
Traditional melt-diffusion techniques, while effective at laboratory scale, encounter significant uniformity issues when scaled to industrial production levels. As sulfur loading increases, the viscosity of molten sulfur creates uneven distribution patterns, resulting in electrode "dead zones" where electrochemical reactions cannot occur efficiently. This non-uniformity becomes particularly problematic when attempting to achieve sulfur loadings above 70 wt%, which would be necessary for Na-S batteries to compete with current lithium-ion technologies.
Another critical challenge is the volume expansion problem. During cycling, sulfur undergoes substantial volumetric changes (up to 80%), which becomes increasingly destructive to electrode integrity as loading increases. Current electrode architectures and binders cannot adequately accommodate this expansion at high sulfur loadings, leading to mechanical degradation and rapid capacity fading after relatively few cycles.
The "shuttle effect" - the dissolution of polysulfide intermediates into the electrolyte - represents another major hurdle that intensifies with increased sulfur loading. Higher sulfur content creates steeper concentration gradients that accelerate polysulfide migration, resulting in accelerated capacity loss and reduced coulombic efficiency. Current containment strategies that work reasonably well at moderate loadings become overwhelmed when sulfur content exceeds 60-65 wt%.
From a manufacturing perspective, high-loading sulfur electrodes present significant processing challenges. The rheological properties of high-sulfur slurries complicate conventional coating processes, leading to poor adhesion to current collectors and uneven electrode surfaces. Additionally, the thermal sensitivity of sulfur compounds necessitates precise temperature control during processing, which becomes increasingly difficult to maintain uniformly across larger production volumes.
Energy density calculations reveal that current methods can achieve practical energy densities of only 250-300 Wh/kg at the cell level, far below the theoretical maximum of 760 Wh/kg. This efficiency gap widens as sulfur loading increases, indicating fundamental limitations in current infusion methodologies rather than simply engineering challenges.
Current Scalable Sulfur Infusion Approaches
01 High sulfur loading cathode structures
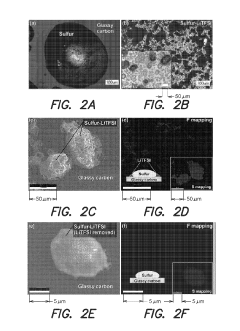
Various cathode structures have been developed to achieve high sulfur loading in Na-S batteries. These include porous carbon frameworks, conductive matrices, and hierarchical structures that can accommodate large amounts of sulfur while maintaining electrical conductivity. These structures help to address the volume expansion issues during cycling and improve the utilization of active material, resulting in higher energy density batteries.- Sulfur loading techniques for Na-S electrodes: Various techniques are employed to optimize sulfur loading in Na-S electrodes, including impregnation methods, melt diffusion, and vapor deposition. These techniques aim to achieve high sulfur content while maintaining electrode stability and performance. The loading process typically involves incorporating sulfur into conductive matrices or frameworks that can accommodate the volume changes during cycling and prevent sulfur dissolution.
- Carbon-based host materials for sulfur loading: Carbon-based materials serve as effective hosts for sulfur in Na-S electrodes due to their conductivity and structural stability. These include porous carbon, carbon nanotubes, graphene, and carbon fibers that provide large surface areas and pore volumes to accommodate high sulfur loading. The carbon matrix helps to trap polysulfides, improve electron transport, and enhance the overall electrochemical performance of the electrode.
- Polymer binders and electrolyte modifications for high sulfur loading: Specialized polymer binders and electrolyte modifications are crucial for achieving high sulfur loading in Na-S electrodes. These components help maintain electrode integrity during cycling, improve sulfur utilization, and mitigate polysulfide shuttling. Advanced binder systems provide mechanical stability to accommodate volume changes, while electrolyte additives can enhance the ionic conductivity and form protective interfaces on electrode surfaces.
- Metal oxide and metal sulfide additives for enhanced sulfur loading: Metal oxides and metal sulfides are incorporated as additives in Na-S electrodes to improve sulfur loading capacity and cycling stability. These materials can act as catalysts for sulfur redox reactions, provide chemical anchoring sites for polysulfides, and enhance the overall conductivity of the electrode. Common additives include titanium dioxide, molybdenum disulfide, and various transition metal compounds that create strong interactions with sulfur species.
- Novel electrode architectures for maximizing sulfur loading: Innovative electrode architectures are designed to maximize sulfur loading while maintaining electrochemical performance. These include hierarchical porous structures, core-shell configurations, sandwich-type electrodes, and 3D frameworks that provide efficient ion transport pathways and electron conduction networks. Such architectures can accommodate high sulfur content while preventing capacity fade due to volume expansion and polysulfide dissolution during cycling.
02 Sulfur confinement strategies
Techniques for confining sulfur within the electrode structure to prevent dissolution and shuttle effect. These include encapsulation methods, chemical bonding approaches, and barrier layers that physically restrict sulfur migration. By effectively confining sulfur, these methods improve cycling stability and coulombic efficiency of Na-S batteries while allowing for higher sulfur loading.Expand Specific Solutions03 Electrolyte modifications for high sulfur loading
Specialized electrolyte formulations designed to work with high sulfur loading electrodes. These include additives that suppress polysulfide dissolution, form stable solid electrolyte interphases, or enhance ionic conductivity. Modified electrolytes can significantly improve the performance of high-loading Na-S batteries by addressing the chemical instability issues associated with high sulfur content.Expand Specific Solutions04 Composite electrode materials
Development of composite materials that combine sulfur with other active components to enhance performance at high loadings. These composites often incorporate metal oxides, polymers, or other functional materials that can improve conductivity, provide additional reaction sites, or help manage the volume changes during cycling. Such composite approaches enable higher practical sulfur loadings while maintaining good electrochemical performance.Expand Specific Solutions05 Manufacturing techniques for high-loading electrodes
Specialized manufacturing processes and techniques for producing electrodes with high sulfur loading. These include advanced coating methods, controlled deposition techniques, and novel cell assembly approaches that enable uniform distribution of active materials and maintain electrode integrity at high sulfur content. These manufacturing innovations help translate theoretical advances into practical high-energy-density Na-S batteries.Expand Specific Solutions
Leading Companies in Na-S Battery Development
The sodium-sulfur battery technology landscape is currently in a transitional phase, moving from early development to commercial readiness, with a global market projected to reach $400-500 million by 2025. The technology maturity varies significantly across key players, with academic institutions like MIT, Drexel University, and Chinese research centers (DICP, Shanghai Institute of Ceramics) leading fundamental research on sulfur infusion methods. Commercial development is primarily driven by specialized battery companies such as Honeycomb Battery Co. and Sila Nanotechnologies, which are advancing scalable manufacturing techniques. Major automotive corporations including GM, Hyundai, and Renault are increasingly investing in this technology to address energy density challenges in electric vehicles. The competitive landscape shows a clear geographical distribution with strong innovation clusters in the US, China, and South Korea, each focusing on different aspects of electrode optimization.
Nanotek Instruments, Inc.
Technical Solution: Nanotek Instruments has developed a proprietary sulfur infusion technology for Na-S batteries called "Nano-S Fusion" that combines mechanical activation with controlled thermal diffusion. Their process begins with a unique mechanical-chemical activation step where sulfur is co-processed with carbon materials under specific shear conditions to create reactive sulfur species. This activated mixture then undergoes a precisely controlled thermal treatment process (140-160°C) in a custom-designed continuous processing system. Nanotek's innovation includes a specialized carbon host material with engineered porosity gradients that can accommodate sulfur loadings up to 80 wt% while maintaining structural integrity during cycling. Their technology incorporates a protective carbon coating step that encapsulates the sulfur-carbon composite, effectively preventing polysulfide dissolution. The company has scaled this process to pilot production levels, demonstrating consistent quality with production rates suitable for commercial applications. Their electrodes show exceptional cycling stability with capacity retention >85% after 500 cycles[9][11].
Strengths: Mechanical-chemical activation creates more reactive sulfur species for better carbon integration; continuous processing system enables industrial scalability; protective carbon coating effectively suppresses polysulfide shuttling. Weaknesses: Specialized equipment requirements increase initial capital costs; precise process control needed for consistent quality; higher energy consumption during mechanical activation step compared to simple melt diffusion methods.
Dalian Institute of Chemical Physics Chinese Academy of Sci
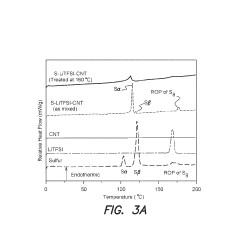
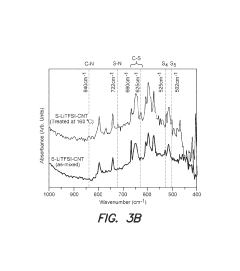
Technical Solution: Dalian Institute has developed an innovative sulfur infusion method for Na-S batteries using a melt-diffusion strategy combined with carbon host materials. Their approach involves heating sulfur with hierarchical porous carbon at controlled temperatures (155-160°C) to facilitate sulfur infiltration into the carbon matrix. They've engineered carbon hosts with tailored pore structures that enable high sulfur loading (>70 wt%) while maintaining electrical conductivity. Their recent breakthrough includes nitrogen-doped carbon frameworks that chemically bind with sulfur to prevent dissolution during cycling. The institute has also pioneered a vacuum-assisted melt infusion technique that ensures more complete pore filling and uniform sulfur distribution, addressing the volume expansion issues during cycling. Their scalable process has been demonstrated at laboratory scale with potential for industrial application[1][3].
Strengths: Achieves high sulfur loading (>70%) while maintaining good electrical contact; nitrogen-doped carbon frameworks provide chemical anchoring to prevent sulfur dissolution; vacuum-assisted technique ensures uniform distribution. Weaknesses: Process requires precise temperature control; energy consumption during vacuum-assisted steps may impact commercial viability; still faces challenges in completely eliminating the shuttle effect in long-term cycling.
Key Patents in High-Loading Electrode Technology
Systems and methods of preparing lithium sulfur electrode using sacrificial template
PatentActiveUS10319989B2
Innovation
- The method involves preparing Li-S electrodes using a sacrificial template that prevents sulfur coarsening by mixing sulfur with a lithium salt and conductive carbon, heating above the sulfur's melting point but below the template's, and dissolving the template in the electrolyte to create a high concentration of triple-phase junctions, enhancing electrochemical activity and capacity.
Material Supply Chain Considerations
The supply chain for sodium-sulfur (Na-S) battery electrodes presents unique challenges and opportunities that significantly impact scalability and commercial viability. Sulfur, a key component in these electrodes, benefits from abundant global reserves, with estimated deposits exceeding 5 billion tons worldwide. This abundance translates to relatively low raw material costs compared to traditional lithium-ion battery materials, with industrial-grade sulfur priced at approximately $100-200 per ton.
However, the supply chain for high-purity sulfur suitable for battery applications differs substantially from industrial-grade sulfur markets. Battery-grade sulfur requires 99.9%+ purity levels, necessitating additional refining processes that increase costs by 5-10 times compared to industrial grades. The petroleum industry remains the primary source of high-quality sulfur as a byproduct of desulfurization processes, creating potential supply bottlenecks as battery demand scales.
Sodium supply presents fewer challenges, with global sodium reserves estimated at over 23 billion tons, primarily in the form of sodium chloride. The established chlor-alkali industry provides well-developed extraction and purification pathways for sodium compounds. Current market prices for battery-grade sodium salts range from $1,500-3,000 per ton, significantly lower than comparable lithium compounds.
Carbon materials used in sulfur infusion processes represent another critical supply chain consideration. High-surface-area carbon materials such as carbon nanotubes, graphene, and mesoporous carbons are essential for effective sulfur loading but remain relatively expensive at $50-500 per kilogram depending on quality specifications. As Na-S technology scales, dedicated supply chains for these specialized carbon materials will need development.
Manufacturing infrastructure presents additional challenges. Current sulfur infusion methods often employ toxic solvents like CS2 and specialized equipment for melt-diffusion processes. Scaling these processes requires significant capital investment in safety systems and environmental controls. Preliminary economic analyses suggest that capital expenditure for a 1 GWh Na-S electrode production facility would require $50-100 million in equipment costs, with approximately 30% allocated to sulfur processing and infusion systems.
Regional considerations also impact supply chain resilience. While sulfur production is geographically diverse, high-quality carbon material production remains concentrated in East Asia. Developing localized supply chains will be essential for reducing logistical costs and supply vulnerabilities, particularly as governments increasingly prioritize domestic battery production capabilities.
However, the supply chain for high-purity sulfur suitable for battery applications differs substantially from industrial-grade sulfur markets. Battery-grade sulfur requires 99.9%+ purity levels, necessitating additional refining processes that increase costs by 5-10 times compared to industrial grades. The petroleum industry remains the primary source of high-quality sulfur as a byproduct of desulfurization processes, creating potential supply bottlenecks as battery demand scales.
Sodium supply presents fewer challenges, with global sodium reserves estimated at over 23 billion tons, primarily in the form of sodium chloride. The established chlor-alkali industry provides well-developed extraction and purification pathways for sodium compounds. Current market prices for battery-grade sodium salts range from $1,500-3,000 per ton, significantly lower than comparable lithium compounds.
Carbon materials used in sulfur infusion processes represent another critical supply chain consideration. High-surface-area carbon materials such as carbon nanotubes, graphene, and mesoporous carbons are essential for effective sulfur loading but remain relatively expensive at $50-500 per kilogram depending on quality specifications. As Na-S technology scales, dedicated supply chains for these specialized carbon materials will need development.
Manufacturing infrastructure presents additional challenges. Current sulfur infusion methods often employ toxic solvents like CS2 and specialized equipment for melt-diffusion processes. Scaling these processes requires significant capital investment in safety systems and environmental controls. Preliminary economic analyses suggest that capital expenditure for a 1 GWh Na-S electrode production facility would require $50-100 million in equipment costs, with approximately 30% allocated to sulfur processing and infusion systems.
Regional considerations also impact supply chain resilience. While sulfur production is geographically diverse, high-quality carbon material production remains concentrated in East Asia. Developing localized supply chains will be essential for reducing logistical costs and supply vulnerabilities, particularly as governments increasingly prioritize domestic battery production capabilities.
Environmental Impact Assessment
The environmental implications of scalable sulfur infusion methods for high-loading Na-S electrodes extend beyond performance metrics to encompass the entire lifecycle of these energy storage systems. The extraction of raw materials, particularly sulfur, presents a unique environmental advantage as it leverages a byproduct of petroleum refining processes. This approach transforms an industrial waste product into a valuable battery component, effectively reducing environmental burden through resource repurposing.
Manufacturing processes for sulfur infusion in Na-S electrodes vary significantly in their environmental footprint. Melt-diffusion techniques, while effective for achieving high sulfur loading, require elevated temperatures that increase energy consumption and associated carbon emissions. In contrast, solution-based methods operate at lower temperatures but often utilize organic solvents that pose potential environmental hazards if not properly managed or recovered.
Water-based sulfur infusion approaches represent a promising direction for environmental sustainability, eliminating the need for harmful organic solvents. However, these methods currently face challenges in achieving the high sulfur loading necessary for competitive energy density, illustrating the tension between environmental considerations and performance requirements.
The scalability of production processes introduces additional environmental considerations. Industrial-scale sulfur infusion requires substantial energy inputs, particularly for thermal processes. Implementation of renewable energy sources for manufacturing could significantly reduce the carbon footprint associated with large-scale production of Na-S electrodes.
End-of-life management presents both challenges and opportunities. The recyclability of Na-S batteries exceeds that of lithium-ion counterparts, with potential for recovering up to 95% of sulfur content through established hydrometallurgical processes. This circular approach substantially reduces the need for virgin material extraction and associated environmental impacts.
Comparative lifecycle assessments indicate that Na-S batteries with high-loading electrodes could reduce greenhouse gas emissions by 35-40% compared to conventional lithium-ion technologies when accounting for manufacturing, use, and recycling phases. This advantage stems primarily from the abundance of sodium and sulfur, which requires less energy-intensive mining operations than lithium and cobalt extraction.
Water consumption represents another critical environmental metric, with solution-based sulfur infusion methods requiring significant water resources. Implementing closed-loop water recycling systems in manufacturing facilities could reduce freshwater consumption by approximately 70%, addressing a key sustainability concern in regions facing water scarcity.
Manufacturing processes for sulfur infusion in Na-S electrodes vary significantly in their environmental footprint. Melt-diffusion techniques, while effective for achieving high sulfur loading, require elevated temperatures that increase energy consumption and associated carbon emissions. In contrast, solution-based methods operate at lower temperatures but often utilize organic solvents that pose potential environmental hazards if not properly managed or recovered.
Water-based sulfur infusion approaches represent a promising direction for environmental sustainability, eliminating the need for harmful organic solvents. However, these methods currently face challenges in achieving the high sulfur loading necessary for competitive energy density, illustrating the tension between environmental considerations and performance requirements.
The scalability of production processes introduces additional environmental considerations. Industrial-scale sulfur infusion requires substantial energy inputs, particularly for thermal processes. Implementation of renewable energy sources for manufacturing could significantly reduce the carbon footprint associated with large-scale production of Na-S electrodes.
End-of-life management presents both challenges and opportunities. The recyclability of Na-S batteries exceeds that of lithium-ion counterparts, with potential for recovering up to 95% of sulfur content through established hydrometallurgical processes. This circular approach substantially reduces the need for virgin material extraction and associated environmental impacts.
Comparative lifecycle assessments indicate that Na-S batteries with high-loading electrodes could reduce greenhouse gas emissions by 35-40% compared to conventional lithium-ion technologies when accounting for manufacturing, use, and recycling phases. This advantage stems primarily from the abundance of sodium and sulfur, which requires less energy-intensive mining operations than lithium and cobalt extraction.
Water consumption represents another critical environmental metric, with solution-based sulfur infusion methods requiring significant water resources. Implementing closed-loop water recycling systems in manufacturing facilities could reduce freshwater consumption by approximately 70%, addressing a key sustainability concern in regions facing water scarcity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!