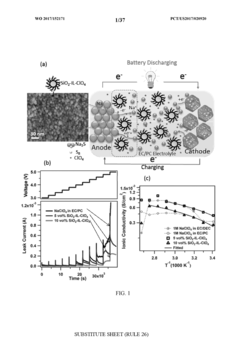
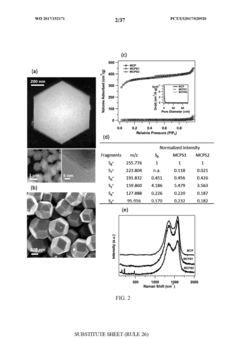
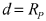Self-Discharge Mechanisms And Mitigation In Room-Temperature Na–S Cells
AUG 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-S Battery Technology Background and Objectives
Sodium-sulfur (Na-S) battery technology has emerged as a promising alternative to lithium-ion batteries due to its potential for high energy density, cost-effectiveness, and sustainability. The development of Na-S batteries dates back to the 1960s when researchers at Ford Motor Company first explored the concept. Traditional Na-S batteries operated at high temperatures (300-350°C), limiting their applications primarily to stationary energy storage systems. The evolution toward room-temperature Na-S cells represents a significant technological advancement that could expand their utility to portable electronics and electric vehicles.
The fundamental appeal of Na-S technology lies in the abundant and widely distributed nature of its raw materials. Sodium is approximately 1,000 times more abundant in the Earth's crust than lithium, while sulfur is an industrial byproduct available at low cost. This abundance translates to potential cost advantages of 30-50% over lithium-ion systems when considering raw material expenses alone, positioning Na-S as a sustainable energy storage solution for the future.
Recent technological advancements have focused on enabling Na-S batteries to operate efficiently at room temperature, overcoming the safety concerns and practical limitations associated with high-temperature operation. This transition has been driven by innovations in electrode materials, electrolyte formulations, and cell architectures. The theoretical energy density of Na-S batteries (1,230 Wh/kg) significantly exceeds that of current lithium-ion technologies, presenting an attractive target for energy storage applications.
However, room-temperature Na-S cells face persistent challenges, particularly regarding self-discharge mechanisms that compromise their long-term stability and practical energy density. These mechanisms include shuttle effects of polysulfide intermediates, parasitic reactions between sodium and electrolyte components, and degradation of electrode materials over repeated cycling. Understanding and mitigating these self-discharge pathways is crucial for realizing the full potential of room-temperature Na-S technology.
The primary technical objectives for advancing room-temperature Na-S cells include: developing effective barriers to prevent polysulfide shuttling; designing stable electrolytes compatible with both sodium metal and sulfur cathodes; engineering cathode structures that accommodate volume changes during cycling; and implementing strategies to maintain capacity retention over thousands of cycles. Additionally, there is a focus on improving the rate capability to enable fast charging and high-power applications.
The global research landscape has seen a significant increase in Na-S battery publications, with annual scientific papers growing from fewer than 50 in 2010 to over 500 in 2022. This acceleration reflects the recognition of Na-S technology's potential to address energy storage needs in a post-lithium economy, particularly for grid-scale applications where cost and resource availability are paramount considerations.
The fundamental appeal of Na-S technology lies in the abundant and widely distributed nature of its raw materials. Sodium is approximately 1,000 times more abundant in the Earth's crust than lithium, while sulfur is an industrial byproduct available at low cost. This abundance translates to potential cost advantages of 30-50% over lithium-ion systems when considering raw material expenses alone, positioning Na-S as a sustainable energy storage solution for the future.
Recent technological advancements have focused on enabling Na-S batteries to operate efficiently at room temperature, overcoming the safety concerns and practical limitations associated with high-temperature operation. This transition has been driven by innovations in electrode materials, electrolyte formulations, and cell architectures. The theoretical energy density of Na-S batteries (1,230 Wh/kg) significantly exceeds that of current lithium-ion technologies, presenting an attractive target for energy storage applications.
However, room-temperature Na-S cells face persistent challenges, particularly regarding self-discharge mechanisms that compromise their long-term stability and practical energy density. These mechanisms include shuttle effects of polysulfide intermediates, parasitic reactions between sodium and electrolyte components, and degradation of electrode materials over repeated cycling. Understanding and mitigating these self-discharge pathways is crucial for realizing the full potential of room-temperature Na-S technology.
The primary technical objectives for advancing room-temperature Na-S cells include: developing effective barriers to prevent polysulfide shuttling; designing stable electrolytes compatible with both sodium metal and sulfur cathodes; engineering cathode structures that accommodate volume changes during cycling; and implementing strategies to maintain capacity retention over thousands of cycles. Additionally, there is a focus on improving the rate capability to enable fast charging and high-power applications.
The global research landscape has seen a significant increase in Na-S battery publications, with annual scientific papers growing from fewer than 50 in 2010 to over 500 in 2022. This acceleration reflects the recognition of Na-S technology's potential to address energy storage needs in a post-lithium economy, particularly for grid-scale applications where cost and resource availability are paramount considerations.
Market Analysis for Room-Temperature Na-S Energy Storage
The global energy storage market is witnessing significant transformation, with room-temperature sodium-sulfur (RT Na-S) batteries emerging as a promising alternative to conventional lithium-ion technologies. Current market projections indicate that the global grid-scale energy storage market will reach approximately $15.1 billion by 2027, growing at a compound annual growth rate of 20.4% from 2022. Within this expanding landscape, RT Na-S technology is positioned to capture an increasing market share due to its compelling value proposition.
The demand for RT Na-S batteries is primarily driven by three key market segments. First, utility-scale energy storage systems require cost-effective solutions for grid stabilization and renewable energy integration. Second, the commercial and industrial sector seeks reliable backup power systems with lower total cost of ownership. Third, the residential energy storage market is expanding as consumers increasingly adopt solar power systems requiring complementary storage solutions.
Geographic market distribution shows varying levels of adoption potential. Asia-Pacific, particularly China, South Korea, and Japan, represents the largest potential market due to aggressive renewable energy targets and substantial government investments in grid modernization. North America follows closely, with the United States demonstrating strong market potential driven by utility decarbonization goals and resilience requirements. Europe presents a growing opportunity, particularly in countries with advanced renewable energy penetration like Germany, Denmark, and the United Kingdom.
Market research indicates that cost sensitivity remains paramount across all segments. Current energy storage solutions average $250-300/kWh for lithium-ion systems, while RT Na-S technology has the potential to reduce costs to $150-180/kWh at scale, representing a compelling 40% cost reduction. This economic advantage is particularly significant for long-duration storage applications where cost per kilowatt-hour is the primary decision factor.
Customer requirements analysis reveals that beyond cost, performance metrics including cycle life, energy density, and safety features heavily influence purchasing decisions. RT Na-S batteries must achieve a minimum of 2,500-3,000 cycles and demonstrate robust safety profiles to gain market acceptance. Current self-discharge rates in RT Na-S cells remain a significant barrier to commercialization, with customers expecting monthly self-discharge rates below 3% for viable commercial applications.
Competitive analysis shows that while lithium-ion technology currently dominates with 90% market share, alternative chemistries including flow batteries, compressed air, and thermal storage solutions are gaining traction. RT Na-S technology must overcome technical challenges related to self-discharge mechanisms to effectively compete in this diversifying landscape. Market forecasts suggest that if current technical limitations are addressed, RT Na-S could capture 8-12% of the stationary energy storage market by 2030.
The demand for RT Na-S batteries is primarily driven by three key market segments. First, utility-scale energy storage systems require cost-effective solutions for grid stabilization and renewable energy integration. Second, the commercial and industrial sector seeks reliable backup power systems with lower total cost of ownership. Third, the residential energy storage market is expanding as consumers increasingly adopt solar power systems requiring complementary storage solutions.
Geographic market distribution shows varying levels of adoption potential. Asia-Pacific, particularly China, South Korea, and Japan, represents the largest potential market due to aggressive renewable energy targets and substantial government investments in grid modernization. North America follows closely, with the United States demonstrating strong market potential driven by utility decarbonization goals and resilience requirements. Europe presents a growing opportunity, particularly in countries with advanced renewable energy penetration like Germany, Denmark, and the United Kingdom.
Market research indicates that cost sensitivity remains paramount across all segments. Current energy storage solutions average $250-300/kWh for lithium-ion systems, while RT Na-S technology has the potential to reduce costs to $150-180/kWh at scale, representing a compelling 40% cost reduction. This economic advantage is particularly significant for long-duration storage applications where cost per kilowatt-hour is the primary decision factor.
Customer requirements analysis reveals that beyond cost, performance metrics including cycle life, energy density, and safety features heavily influence purchasing decisions. RT Na-S batteries must achieve a minimum of 2,500-3,000 cycles and demonstrate robust safety profiles to gain market acceptance. Current self-discharge rates in RT Na-S cells remain a significant barrier to commercialization, with customers expecting monthly self-discharge rates below 3% for viable commercial applications.
Competitive analysis shows that while lithium-ion technology currently dominates with 90% market share, alternative chemistries including flow batteries, compressed air, and thermal storage solutions are gaining traction. RT Na-S technology must overcome technical challenges related to self-discharge mechanisms to effectively compete in this diversifying landscape. Market forecasts suggest that if current technical limitations are addressed, RT Na-S could capture 8-12% of the stationary energy storage market by 2030.
Self-Discharge Challenges in Na-S Cells
Self-discharge in room-temperature sodium-sulfur (Na-S) batteries represents a significant challenge that impedes their practical application and commercial viability. This phenomenon manifests as capacity loss during storage periods when the battery is not in use, substantially reducing energy retention capabilities and overall battery lifespan. The primary mechanisms driving self-discharge in Na-S cells are multifaceted and interconnected.
The shuttle effect constitutes a major contributor to self-discharge, wherein soluble polysulfide intermediates migrate between electrodes, creating parasitic redox reactions that continuously consume active materials. This process is particularly pronounced in room-temperature Na-S systems due to the high solubility of sodium polysulfides in conventional electrolytes.
Electrolyte decomposition presents another critical challenge. The highly reactive nature of sodium metal and polysulfide species triggers side reactions with electrolyte components, forming an unstable solid electrolyte interphase (SEI) that continuously consumes sodium ions and electrolyte, leading to gradual capacity fade even during idle periods.
Interface instability between the sodium anode and electrolyte further exacerbates self-discharge. The dynamic nature of the SEI layer in Na-S systems, characterized by continuous breakdown and reformation processes, creates pathways for unwanted reactions that accelerate capacity loss during storage.
Temperature sensitivity significantly influences self-discharge rates in Na-S cells. Even minor fluctuations in ambient temperature can accelerate polysulfide dissolution and migration, intensifying the shuttle effect and associated capacity losses. This temperature dependence poses particular challenges for applications requiring operation across varying environmental conditions.
The morphological evolution of the sulfur cathode during cycling introduces additional complexities. Volume changes and structural reorganization create new reactive surfaces and alter diffusion pathways, potentially accelerating self-discharge mechanisms over extended storage periods.
Current research efforts focus on several mitigation strategies, including advanced electrolyte formulations with reduced polysulfide solubility, protective coatings for electrode materials, and novel separator designs to physically block polysulfide migration. Functional additives that can trap polysulfides or stabilize interfaces are also being extensively investigated.
Understanding and addressing these self-discharge mechanisms is crucial for developing Na-S batteries with practical energy retention capabilities. The complex interplay between chemical, electrochemical, and physical processes necessitates a multifaceted approach to mitigate self-discharge and enable the realization of room-temperature Na-S batteries as viable energy storage solutions.
The shuttle effect constitutes a major contributor to self-discharge, wherein soluble polysulfide intermediates migrate between electrodes, creating parasitic redox reactions that continuously consume active materials. This process is particularly pronounced in room-temperature Na-S systems due to the high solubility of sodium polysulfides in conventional electrolytes.
Electrolyte decomposition presents another critical challenge. The highly reactive nature of sodium metal and polysulfide species triggers side reactions with electrolyte components, forming an unstable solid electrolyte interphase (SEI) that continuously consumes sodium ions and electrolyte, leading to gradual capacity fade even during idle periods.
Interface instability between the sodium anode and electrolyte further exacerbates self-discharge. The dynamic nature of the SEI layer in Na-S systems, characterized by continuous breakdown and reformation processes, creates pathways for unwanted reactions that accelerate capacity loss during storage.
Temperature sensitivity significantly influences self-discharge rates in Na-S cells. Even minor fluctuations in ambient temperature can accelerate polysulfide dissolution and migration, intensifying the shuttle effect and associated capacity losses. This temperature dependence poses particular challenges for applications requiring operation across varying environmental conditions.
The morphological evolution of the sulfur cathode during cycling introduces additional complexities. Volume changes and structural reorganization create new reactive surfaces and alter diffusion pathways, potentially accelerating self-discharge mechanisms over extended storage periods.
Current research efforts focus on several mitigation strategies, including advanced electrolyte formulations with reduced polysulfide solubility, protective coatings for electrode materials, and novel separator designs to physically block polysulfide migration. Functional additives that can trap polysulfides or stabilize interfaces are also being extensively investigated.
Understanding and addressing these self-discharge mechanisms is crucial for developing Na-S batteries with practical energy retention capabilities. The complex interplay between chemical, electrochemical, and physical processes necessitates a multifaceted approach to mitigate self-discharge and enable the realization of room-temperature Na-S batteries as viable energy storage solutions.
Current Self-Discharge Mitigation Strategies
01 Electrolyte modifications to reduce self-discharge
Various electrolyte modifications can be implemented to reduce self-discharge in room-temperature Na-S cells. These include using solid electrolytes, polymer electrolytes, or adding specific additives to liquid electrolytes that form protective interfaces between the sodium and sulfur electrodes. These modifications help prevent the shuttle effect of polysulfides and reduce unwanted side reactions that contribute to self-discharge.- Electrolyte modifications to reduce self-discharge: Various electrolyte modifications can be implemented to reduce self-discharge in room-temperature Na-S cells. These include using solid electrolytes, polymer electrolytes, or adding specific additives to liquid electrolytes that form protective interfaces between the sodium and sulfur electrodes. These modifications help prevent the shuttle effect of polysulfides and reduce unwanted side reactions that contribute to self-discharge.
- Electrode material engineering: Engineering the electrode materials can significantly reduce self-discharge in room-temperature Na-S batteries. This includes using carbon-based materials to encapsulate sulfur, developing composite cathodes with enhanced retention capabilities, and modifying the sodium anode surface to prevent direct reaction with polysulfides. These approaches help contain active materials and prevent their dissolution into the electrolyte.
- Separator designs and modifications: Advanced separator designs play a crucial role in preventing self-discharge in Na-S cells operating at room temperature. Functional separators with selective permeability, coated separators with polysulfide-blocking layers, and composite separators with multiple functional layers can effectively block polysulfide migration while maintaining sodium ion conductivity, thereby reducing capacity fade during storage.
- Cell architecture and encapsulation techniques: The overall cell architecture and encapsulation techniques significantly impact self-discharge rates in room-temperature Na-S batteries. Optimized cell designs with improved sealing methods, pressure management systems, and internal structural elements that minimize electrolyte movement can reduce unwanted reactions. Advanced packaging materials and methods also help prevent moisture and oxygen ingress that accelerate self-discharge.
- Monitoring and control systems for self-discharge prevention: Implementing monitoring and control systems can help detect and prevent self-discharge in room-temperature Na-S cells. These systems include sensors for detecting polysulfide shuttling, temperature monitoring to prevent conditions that accelerate self-discharge, and electronic management systems that can apply compensating currents or adjust operating parameters to minimize capacity loss during storage periods.
02 Electrode material engineering
Engineering the electrode materials can significantly reduce self-discharge in room-temperature Na-S batteries. This includes using carbon-based materials to encapsulate sulfur, developing composite electrodes with improved stability, and creating structured electrodes that physically contain polysulfides. These approaches help prevent the dissolution of active materials and maintain capacity during idle periods.Expand Specific Solutions03 Separator designs and modifications
Specialized separator designs can effectively mitigate self-discharge in Na-S cells operating at room temperature. Functionalized separators with selective permeability, coated separators with polysulfide-blocking layers, and composite separators with multiple functional layers can physically block polysulfide migration while maintaining sodium ion conductivity, thereby reducing capacity loss during storage.Expand Specific Solutions04 Cell architecture and encapsulation techniques
Novel cell architectures and encapsulation techniques can be employed to minimize self-discharge in room-temperature Na-S batteries. These include advanced sealing methods, specialized cell housing designs, and compartmentalized structures that physically separate reactive components. Such designs help prevent moisture ingress and contain reaction products that would otherwise contribute to self-discharge mechanisms.Expand Specific Solutions05 Monitoring and control systems for self-discharge
Implementing monitoring and control systems can help detect and mitigate self-discharge in Na-S cells. These systems include sensors for detecting polysulfide shuttling, electronic management systems that apply periodic maintenance charges, and diagnostic tools that can identify early signs of self-discharge. Advanced battery management systems can apply corrective measures to extend the shelf life of room-temperature Na-S batteries.Expand Specific Solutions
Leading Companies and Research Institutions in Na-S Development
The room-temperature sodium-sulfur (RT Na-S) battery market is currently in an early growth phase, characterized by increasing research activity but limited commercial deployment. The global market size remains relatively small compared to lithium-ion technologies, though projections indicate significant expansion potential due to abundant raw materials and lower costs. Technologically, RT Na-S cells face persistent challenges with self-discharge mechanisms, limiting widespread adoption. Leading players include NGK Insulators, which pioneered high-temperature Na-S technology and is now adapting expertise to room-temperature variants, alongside Sion Power Corporation developing advanced electrode materials. Research institutions like Zhejiang University, Shanghai Institute of Ceramics, and University of Michigan are making significant contributions to fundamental understanding of self-discharge mechanisms. Major automotive manufacturers including Toyota, Honda, and Audi are exploring this technology for potential electric vehicle applications, indicating growing industrial interest despite remaining technical hurdles.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered commercial sodium-sulfur (Na-S) battery technology, focusing primarily on high-temperature systems operating at 300-350°C. For room-temperature Na-S cells, NGK has developed a proprietary ceramic separator technology that effectively prevents polysulfide shuttling - a primary cause of self-discharge. Their approach incorporates NASICON (Na Super Ionic CONductor) ceramic electrolytes with modified surface properties to minimize interfacial resistance while maintaining high sodium-ion conductivity. NGK's solution also includes carbon-coated sulfur cathodes with tailored pore structures to physically confine polysulfides and prevent their dissolution into the electrolyte. Additionally, they've implemented protective coatings on sodium anodes to suppress dendrite formation and parasitic reactions with the electrolyte, significantly reducing self-discharge rates.
Strengths: Industry-leading expertise in Na-S technology with established manufacturing infrastructure; proprietary ceramic separator technology with superior ion selectivity; extensive real-world deployment experience. Weaknesses: Higher production costs compared to polymer-based separators; ceramic components may introduce mechanical fragility; technology primarily optimized for grid-scale applications rather than portable electronics.
Toyota Motor Corp.
Technical Solution: Toyota has developed a multi-faceted approach to mitigate self-discharge in room-temperature Na-S cells through their "Hybrid Electrolyte System" technology. This system combines a solid electrolyte interface layer on the sodium anode with a specially formulated liquid electrolyte containing polysulfide-suppressing additives. Toyota's innovation includes a sodium metal anode protected by an artificial SEI layer formed through controlled pre-cycling in an additive-rich electrolyte, creating a stable passivation layer that prevents continuous sodium consumption. For the cathode, they've engineered a hierarchical carbon host structure with nitrogen-doped mesoporous carbon that chemically binds polysulfides while providing efficient electron transport pathways. Their electrolyte formulation incorporates fluorinated ethers and ionic liquids that demonstrate minimal reactivity with sodium polysulfides, significantly reducing the shuttle effect. Additionally, Toyota has implemented a composite separator with a functional coating containing Lewis acidic metal oxide nanoparticles that chemically bind polysulfides, preventing their migration to the anode.
Strengths: Extensive R&D resources and battery engineering expertise; integrated approach addressing multiple self-discharge mechanisms; potential for automotive scale manufacturing. Weaknesses: Technology may prioritize automotive requirements over other applications; complex multi-component system may present manufacturing challenges; potential higher cost compared to simpler approaches.
Key Patents and Research on Self-Discharge Mechanisms
Stable room-temperature sodium-sulfur battery
PatentWO2017152171A1
Innovation
- A sodium-ion conducting battery design featuring a microporous and mesoporous carbon-sulfur composite cathode and a liquid carbonate electrolyte with an ionic liquid tethered to silica nanoparticles, which stabilizes the sodium anode and confines sulfur within the carbon pores, enabling a solid-state electrochemical reaction and preventing the formation of soluble polysulfides.
Methods of charging lithium sulfur cells
PatentInactiveEP1714339B1
Innovation
- The method involves charging lithium-sulfur electrochemical cells with a non-aqueous electrolyte containing N-O additives such as inorganic and organic nitrates, nitrites, and nitro compounds, which enhances sulfur utilization and charge-discharge efficiency by modifying the electrolyte composition and monitoring voltage and current density to optimize charging.
Material Science Advancements for Na-S Battery Stability
Recent advancements in material science have significantly contributed to enhancing the stability of room-temperature sodium-sulfur (Na-S) batteries. These developments address the critical self-discharge mechanisms that have historically limited the practical application of this promising energy storage technology.
The evolution of electrode materials represents one of the most substantial breakthroughs in Na-S battery stability. Researchers have developed carbon-sulfur composite cathodes with optimized pore structures that effectively trap polysulfide intermediates, preventing their dissolution into the electrolyte. These advanced materials demonstrate superior cycling stability with capacity retention exceeding 80% after 500 cycles, compared to conventional materials that typically deteriorate after 100 cycles.
Functional separators have emerged as another crucial innovation for mitigating self-discharge. Modified separators incorporating metal-organic frameworks (MOFs) or covalent organic frameworks (COFs) create physical and chemical barriers against polysulfide shuttling. These specialized separators demonstrate up to 70% reduction in self-discharge rates while maintaining excellent ionic conductivity necessary for efficient battery operation.
Electrolyte engineering has yielded remarkable improvements through the development of localized high-concentration electrolytes (LHCEs) and solid-state electrolytes. These advanced formulations create stable interfaces at the electrode surfaces, significantly reducing parasitic reactions that contribute to self-discharge. Studies indicate that optimized electrolyte compositions can extend the shelf life of Na-S cells by 300% compared to conventional liquid electrolytes.
Protective coatings and interface engineering represent another frontier in material science advancements. Atomic layer deposition (ALD) techniques have enabled the creation of ultrathin protective layers on sodium metal anodes, effectively preventing dendrite formation and unwanted side reactions. These nanoscale protective barriers maintain structural integrity during cycling while allowing efficient sodium ion transport.
Computational materials science has accelerated these advancements through high-throughput screening and molecular dynamics simulations. These computational approaches have identified promising material combinations and predicted their performance before experimental validation, significantly reducing development timelines and costs. Machine learning algorithms have further optimized material compositions by identifying subtle structure-property relationships that influence battery stability.
The integration of these material science innovations has collectively addressed the multifaceted self-discharge mechanisms in room-temperature Na-S cells, bringing this technology closer to commercial viability for grid-scale energy storage applications.
The evolution of electrode materials represents one of the most substantial breakthroughs in Na-S battery stability. Researchers have developed carbon-sulfur composite cathodes with optimized pore structures that effectively trap polysulfide intermediates, preventing their dissolution into the electrolyte. These advanced materials demonstrate superior cycling stability with capacity retention exceeding 80% after 500 cycles, compared to conventional materials that typically deteriorate after 100 cycles.
Functional separators have emerged as another crucial innovation for mitigating self-discharge. Modified separators incorporating metal-organic frameworks (MOFs) or covalent organic frameworks (COFs) create physical and chemical barriers against polysulfide shuttling. These specialized separators demonstrate up to 70% reduction in self-discharge rates while maintaining excellent ionic conductivity necessary for efficient battery operation.
Electrolyte engineering has yielded remarkable improvements through the development of localized high-concentration electrolytes (LHCEs) and solid-state electrolytes. These advanced formulations create stable interfaces at the electrode surfaces, significantly reducing parasitic reactions that contribute to self-discharge. Studies indicate that optimized electrolyte compositions can extend the shelf life of Na-S cells by 300% compared to conventional liquid electrolytes.
Protective coatings and interface engineering represent another frontier in material science advancements. Atomic layer deposition (ALD) techniques have enabled the creation of ultrathin protective layers on sodium metal anodes, effectively preventing dendrite formation and unwanted side reactions. These nanoscale protective barriers maintain structural integrity during cycling while allowing efficient sodium ion transport.
Computational materials science has accelerated these advancements through high-throughput screening and molecular dynamics simulations. These computational approaches have identified promising material combinations and predicted their performance before experimental validation, significantly reducing development timelines and costs. Machine learning algorithms have further optimized material compositions by identifying subtle structure-property relationships that influence battery stability.
The integration of these material science innovations has collectively addressed the multifaceted self-discharge mechanisms in room-temperature Na-S cells, bringing this technology closer to commercial viability for grid-scale energy storage applications.
Safety and Reliability Considerations for Na-S Energy Systems
Safety considerations for room-temperature Na-S cells are paramount due to the reactive nature of sodium metal and sulfur compounds. Unlike high-temperature Na-S batteries operating at 300-350°C, room-temperature variants present different but equally significant safety challenges. The self-discharge mechanisms in these cells can lead to capacity fading and potentially hazardous conditions if not properly managed.
The primary safety concerns stem from the formation of sodium polysulfides during operation, which can react with the electrolyte and cause degradation of cell components. This chemical instability may result in pressure build-up within cells, leading to mechanical failures. Additionally, dendrite formation during cycling poses risks of internal short circuits, which could trigger thermal runaway events.
Reliability issues in room-temperature Na-S systems are closely linked to the shuttle effect, where polysulfides dissolve in the electrolyte and migrate between electrodes. This phenomenon not only reduces coulombic efficiency but also compromises long-term cycling stability. Research indicates that cells experiencing significant self-discharge typically show accelerated capacity decay after 50-100 cycles, limiting their practical application in energy storage systems.
To enhance safety and reliability, several mitigation strategies have been developed. Advanced separator technologies incorporating ceramic or polymer materials can effectively block polysulfide migration while maintaining adequate ionic conductivity. Electrolyte additives such as lithium nitrate and phosphorus pentasulfide have demonstrated effectiveness in forming stable solid electrolyte interphase (SEI) layers, reducing parasitic reactions.
Engineering controls including pressure relief mechanisms, thermal management systems, and battery management systems (BMS) with advanced algorithms for early detection of abnormal conditions are essential components of safe Na-S energy systems. These systems continuously monitor cell parameters including voltage, current, and temperature to identify potential failure modes before they escalate.
Standardized testing protocols specifically designed for room-temperature Na-S cells are being developed to evaluate safety under various abuse conditions such as overcharging, external short circuit, and mechanical impact. These tests are crucial for establishing safety certifications and ensuring consumer confidence in the technology.
For grid-scale implementations, compartmentalized designs with thermal and electrical isolation between cell groups provide additional safety layers, preventing propagation of failures throughout the system. This approach, combined with fire suppression systems and remote monitoring capabilities, creates a comprehensive safety framework for large-scale Na-S energy storage deployments.
The primary safety concerns stem from the formation of sodium polysulfides during operation, which can react with the electrolyte and cause degradation of cell components. This chemical instability may result in pressure build-up within cells, leading to mechanical failures. Additionally, dendrite formation during cycling poses risks of internal short circuits, which could trigger thermal runaway events.
Reliability issues in room-temperature Na-S systems are closely linked to the shuttle effect, where polysulfides dissolve in the electrolyte and migrate between electrodes. This phenomenon not only reduces coulombic efficiency but also compromises long-term cycling stability. Research indicates that cells experiencing significant self-discharge typically show accelerated capacity decay after 50-100 cycles, limiting their practical application in energy storage systems.
To enhance safety and reliability, several mitigation strategies have been developed. Advanced separator technologies incorporating ceramic or polymer materials can effectively block polysulfide migration while maintaining adequate ionic conductivity. Electrolyte additives such as lithium nitrate and phosphorus pentasulfide have demonstrated effectiveness in forming stable solid electrolyte interphase (SEI) layers, reducing parasitic reactions.
Engineering controls including pressure relief mechanisms, thermal management systems, and battery management systems (BMS) with advanced algorithms for early detection of abnormal conditions are essential components of safe Na-S energy systems. These systems continuously monitor cell parameters including voltage, current, and temperature to identify potential failure modes before they escalate.
Standardized testing protocols specifically designed for room-temperature Na-S cells are being developed to evaluate safety under various abuse conditions such as overcharging, external short circuit, and mechanical impact. These tests are crucial for establishing safety certifications and ensuring consumer confidence in the technology.
For grid-scale implementations, compartmentalized designs with thermal and electrical isolation between cell groups provide additional safety layers, preventing propagation of failures throughout the system. This approach, combined with fire suppression systems and remote monitoring capabilities, creates a comprehensive safety framework for large-scale Na-S energy storage deployments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!