Low-Temperature Performance Of RT Na–S Cells For Outdoor Storage
AUG 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
RT Na-S Battery Technology Background and Objectives
Room-temperature sodium-sulfur (RT Na-S) batteries have emerged as a promising energy storage technology over the past decade, evolving from high-temperature Na-S systems that operate at approximately 300-350°C. The development of RT Na-S technology represents a significant breakthrough in addressing the limitations of traditional Na-S batteries, particularly the safety concerns and complex thermal management requirements associated with high operating temperatures.
The evolution of Na-S battery technology began in the 1960s at Ford Motor Company, with commercial deployment primarily focused on grid-scale applications by NGK Insulators since the 1980s. However, these systems required high operating temperatures to maintain sodium in a molten state. The shift toward room-temperature operation began gaining momentum in the early 2010s, driven by the need for safer, more accessible energy storage solutions.
Current RT Na-S batteries leverage the abundant and low-cost nature of both sodium and sulfur, offering theoretical energy densities of approximately 760 Wh/kg. This positions them as potential alternatives to lithium-ion batteries, especially for stationary storage applications where energy density requirements are less stringent than cost considerations.
The primary technical objective in RT Na-S battery development is to enhance low-temperature performance for outdoor storage applications. Outdoor energy storage systems must maintain functionality across a wide temperature range, particularly in cold climates where temperatures can drop significantly below freezing. Current RT Na-S cells experience substantial capacity fading and increased internal resistance at low temperatures, limiting their practical deployment in outdoor environments.
Specific technical goals include improving ionic conductivity of electrolytes at sub-zero temperatures, developing electrode materials with enhanced kinetics for low-temperature operation, and designing cell architectures that minimize temperature-dependent performance variations. Researchers aim to achieve stable cycling at temperatures as low as -20°C while maintaining at least 80% of room-temperature capacity.
The advancement of low-temperature performance in RT Na-S cells aligns with broader energy transition objectives, including grid resilience, renewable energy integration, and reduced dependence on critical materials. By enabling reliable outdoor storage capabilities, RT Na-S technology could significantly contribute to decentralized energy systems and microgrids in diverse geographical locations.
Recent research trends indicate growing interest in novel electrolyte formulations, including polymer-based and hybrid electrolytes, as well as advanced sulfur host materials to address the shuttle effect that becomes more pronounced at lower temperatures. These developments suggest promising pathways toward achieving the technical objectives for outdoor-capable RT Na-S energy storage systems.
The evolution of Na-S battery technology began in the 1960s at Ford Motor Company, with commercial deployment primarily focused on grid-scale applications by NGK Insulators since the 1980s. However, these systems required high operating temperatures to maintain sodium in a molten state. The shift toward room-temperature operation began gaining momentum in the early 2010s, driven by the need for safer, more accessible energy storage solutions.
Current RT Na-S batteries leverage the abundant and low-cost nature of both sodium and sulfur, offering theoretical energy densities of approximately 760 Wh/kg. This positions them as potential alternatives to lithium-ion batteries, especially for stationary storage applications where energy density requirements are less stringent than cost considerations.
The primary technical objective in RT Na-S battery development is to enhance low-temperature performance for outdoor storage applications. Outdoor energy storage systems must maintain functionality across a wide temperature range, particularly in cold climates where temperatures can drop significantly below freezing. Current RT Na-S cells experience substantial capacity fading and increased internal resistance at low temperatures, limiting their practical deployment in outdoor environments.
Specific technical goals include improving ionic conductivity of electrolytes at sub-zero temperatures, developing electrode materials with enhanced kinetics for low-temperature operation, and designing cell architectures that minimize temperature-dependent performance variations. Researchers aim to achieve stable cycling at temperatures as low as -20°C while maintaining at least 80% of room-temperature capacity.
The advancement of low-temperature performance in RT Na-S cells aligns with broader energy transition objectives, including grid resilience, renewable energy integration, and reduced dependence on critical materials. By enabling reliable outdoor storage capabilities, RT Na-S technology could significantly contribute to decentralized energy systems and microgrids in diverse geographical locations.
Recent research trends indicate growing interest in novel electrolyte formulations, including polymer-based and hybrid electrolytes, as well as advanced sulfur host materials to address the shuttle effect that becomes more pronounced at lower temperatures. These developments suggest promising pathways toward achieving the technical objectives for outdoor-capable RT Na-S energy storage systems.
Market Analysis for Low-Temperature Energy Storage Solutions
The global market for low-temperature energy storage solutions has witnessed significant growth in recent years, driven by the increasing demand for reliable energy storage systems capable of operating in diverse environmental conditions. Room Temperature Sodium-Sulfur (RT Na-S) cells represent a promising technology in this sector, particularly for outdoor storage applications where temperature fluctuations pose considerable challenges.
Market research indicates that the energy storage market is projected to grow at a compound annual growth rate of 20-25% through 2030, with low-temperature solutions becoming increasingly important as renewable energy deployment expands into colder regions. The outdoor energy storage segment specifically is expected to reach substantial market value as grid stabilization needs increase in areas with extreme weather conditions.
Consumer demand for RT Na-S cells is primarily driven by their potential cost advantages over lithium-ion alternatives, with raw material costs approximately 30-40% lower. Additionally, the absence of critical rare earth materials in Na-S chemistry provides significant supply chain advantages, particularly as geopolitical tensions affect traditional battery material markets.
Regional analysis reveals varying adoption patterns, with Nordic countries, Canada, and northern regions of the United States showing the strongest interest in low-temperature energy storage solutions. These markets prioritize systems that maintain efficiency at temperatures below -20°C, a range where conventional lithium-ion technologies experience significant performance degradation.
Industry surveys indicate that utility companies and renewable energy developers rank low-temperature performance as the third most important criterion when selecting energy storage systems, following only cost and cycle life. This represents a shift from previous years when low-temperature capability was considered a specialized requirement rather than a mainstream feature.
The competitive landscape for low-temperature energy storage is currently fragmented, with several technology approaches competing for market share. RT Na-S cells face competition from advanced lithium-ion formulations with specialized electrolytes, flow batteries with antifreeze additives, and emerging solid-state technologies. Market penetration will depend heavily on demonstrating reliable performance in real-world cold weather conditions.
Customer willingness-to-pay analysis suggests a premium of 15-20% for energy storage systems with verified low-temperature performance, particularly in critical infrastructure applications where system failure could have significant consequences. This premium is expected to decrease as the technology matures and manufacturing scales.
Market forecasts indicate that outdoor energy storage applications will represent approximately one-quarter of the total stationary storage market by 2028, with low-temperature performance becoming a standard specification rather than a specialized feature. RT Na-S technology is well-positioned to capture a significant portion of this growing market segment if current technical challenges can be overcome.
Market research indicates that the energy storage market is projected to grow at a compound annual growth rate of 20-25% through 2030, with low-temperature solutions becoming increasingly important as renewable energy deployment expands into colder regions. The outdoor energy storage segment specifically is expected to reach substantial market value as grid stabilization needs increase in areas with extreme weather conditions.
Consumer demand for RT Na-S cells is primarily driven by their potential cost advantages over lithium-ion alternatives, with raw material costs approximately 30-40% lower. Additionally, the absence of critical rare earth materials in Na-S chemistry provides significant supply chain advantages, particularly as geopolitical tensions affect traditional battery material markets.
Regional analysis reveals varying adoption patterns, with Nordic countries, Canada, and northern regions of the United States showing the strongest interest in low-temperature energy storage solutions. These markets prioritize systems that maintain efficiency at temperatures below -20°C, a range where conventional lithium-ion technologies experience significant performance degradation.
Industry surveys indicate that utility companies and renewable energy developers rank low-temperature performance as the third most important criterion when selecting energy storage systems, following only cost and cycle life. This represents a shift from previous years when low-temperature capability was considered a specialized requirement rather than a mainstream feature.
The competitive landscape for low-temperature energy storage is currently fragmented, with several technology approaches competing for market share. RT Na-S cells face competition from advanced lithium-ion formulations with specialized electrolytes, flow batteries with antifreeze additives, and emerging solid-state technologies. Market penetration will depend heavily on demonstrating reliable performance in real-world cold weather conditions.
Customer willingness-to-pay analysis suggests a premium of 15-20% for energy storage systems with verified low-temperature performance, particularly in critical infrastructure applications where system failure could have significant consequences. This premium is expected to decrease as the technology matures and manufacturing scales.
Market forecasts indicate that outdoor energy storage applications will represent approximately one-quarter of the total stationary storage market by 2028, with low-temperature performance becoming a standard specification rather than a specialized feature. RT Na-S technology is well-positioned to capture a significant portion of this growing market segment if current technical challenges can be overcome.
Current Challenges in Low-Temperature Na-S Cell Performance
Despite the promising potential of room temperature (RT) sodium-sulfur (Na-S) batteries for outdoor energy storage applications, their performance at low temperatures remains a significant challenge. The ionic conductivity of electrolytes decreases substantially as temperature drops, leading to increased internal resistance and reduced power output. This phenomenon is particularly problematic in outdoor storage scenarios where batteries may be exposed to temperatures well below freezing in many regions.
The sluggish kinetics of sodium ion transport at low temperatures creates a bottleneck in the electrochemical reactions. Studies have shown that below 0°C, the capacity of RT Na-S cells can decrease by up to 70% compared to their performance at room temperature. This dramatic reduction severely limits their practical utility in cold climate regions where reliable energy storage is often most needed during winter months.
Another critical challenge is the increased dendrite formation risk at low temperatures. The uneven deposition of sodium metal at the anode becomes more pronounced in cold conditions, potentially leading to internal short circuits and safety hazards. This issue is exacerbated by the higher viscosity of electrolytes at low temperatures, which further impedes uniform ion distribution.
The solid-electrolyte interphase (SEI) layer, crucial for battery stability, exhibits altered formation dynamics and properties at low temperatures. This can lead to less effective passivation of the electrode surfaces, accelerating capacity fade and reducing cycle life. Research indicates that the composition and structure of the SEI layer formed at low temperatures differ significantly from those formed under standard conditions.
Sulfur utilization, already a challenge in Na-S systems, becomes even more problematic at reduced temperatures. The conversion reactions between sodium and sulfur species slow down considerably, leading to incomplete utilization of active materials and reduced energy density. The precipitation of sodium polysulfides becomes less controlled, exacerbating the notorious "shuttle effect" that plagues sulfur-based battery systems.
Material degradation processes are also accelerated under low-temperature cycling. Thermal stress from temperature fluctuations can cause mechanical failures in electrode materials, while the increased viscosity of electrolytes may lead to poor wetting of electrode surfaces. These factors collectively contribute to faster capacity degradation and shorter battery lifespan when operated in cold environments.
Current electrolyte formulations for RT Na-S batteries are not optimized for low-temperature performance. Most conventional electrolytes exhibit poor ionic conductivity below 0°C, creating a fundamental limitation that cannot be overcome through electrode design alone. This highlights the need for specialized electrolyte systems specifically engineered for cold-weather operation.
The sluggish kinetics of sodium ion transport at low temperatures creates a bottleneck in the electrochemical reactions. Studies have shown that below 0°C, the capacity of RT Na-S cells can decrease by up to 70% compared to their performance at room temperature. This dramatic reduction severely limits their practical utility in cold climate regions where reliable energy storage is often most needed during winter months.
Another critical challenge is the increased dendrite formation risk at low temperatures. The uneven deposition of sodium metal at the anode becomes more pronounced in cold conditions, potentially leading to internal short circuits and safety hazards. This issue is exacerbated by the higher viscosity of electrolytes at low temperatures, which further impedes uniform ion distribution.
The solid-electrolyte interphase (SEI) layer, crucial for battery stability, exhibits altered formation dynamics and properties at low temperatures. This can lead to less effective passivation of the electrode surfaces, accelerating capacity fade and reducing cycle life. Research indicates that the composition and structure of the SEI layer formed at low temperatures differ significantly from those formed under standard conditions.
Sulfur utilization, already a challenge in Na-S systems, becomes even more problematic at reduced temperatures. The conversion reactions between sodium and sulfur species slow down considerably, leading to incomplete utilization of active materials and reduced energy density. The precipitation of sodium polysulfides becomes less controlled, exacerbating the notorious "shuttle effect" that plagues sulfur-based battery systems.
Material degradation processes are also accelerated under low-temperature cycling. Thermal stress from temperature fluctuations can cause mechanical failures in electrode materials, while the increased viscosity of electrolytes may lead to poor wetting of electrode surfaces. These factors collectively contribute to faster capacity degradation and shorter battery lifespan when operated in cold environments.
Current electrolyte formulations for RT Na-S batteries are not optimized for low-temperature performance. Most conventional electrolytes exhibit poor ionic conductivity below 0°C, creating a fundamental limitation that cannot be overcome through electrode design alone. This highlights the need for specialized electrolyte systems specifically engineered for cold-weather operation.
Technical Solutions for Cold Environment Battery Operation
01 Electrolyte modifications for low-temperature performance
Various electrolyte modifications can enhance the low-temperature performance of room temperature sodium-sulfur (RT Na-S) cells. These include using polymer electrolytes, ionic liquids, or gel electrolytes with additives that maintain ionic conductivity at lower temperatures. Such modifications reduce the freezing point of the electrolyte and maintain sufficient Na+ ion mobility even at reduced temperatures, ensuring the battery can operate efficiently in cold environments.- Electrolyte modifications for low-temperature performance: Various electrolyte modifications can enhance the low-temperature performance of room temperature sodium-sulfur (RT Na-S) cells. These include using polymer electrolytes, ionic liquids, or gel electrolytes that maintain ionic conductivity at lower temperatures. Additives that prevent electrolyte freezing or improve ion transport at low temperatures are also effective. These modifications help maintain cell functionality and capacity retention when operating below standard room temperature.
- Electrode material engineering for cold environments: Engineering electrode materials specifically for low-temperature operation improves RT Na-S cell performance. This includes developing sulfur cathodes with enhanced reaction kinetics at low temperatures, using carbon-based materials with optimized pore structures, and incorporating conductive additives. Modified sodium anodes with reduced activation energy for ion transfer also contribute to better cold-weather performance. These approaches address the sluggish electrochemical reactions that typically occur at lower temperatures.
- Cell structure and design optimization: Optimizing the physical structure and design of RT Na-S cells can significantly improve their low-temperature performance. This includes reducing electrode spacing to minimize ion diffusion distances, using thinner separators with higher porosity, and implementing advanced cell packaging that provides thermal insulation. Some designs incorporate internal heating elements or thermal management systems that maintain optimal operating temperature even in cold environments.
- Interface engineering and protective layers: Interface engineering and the application of protective layers enhance RT Na-S cell performance at low temperatures. This includes developing solid electrolyte interphase (SEI) layers that remain stable and conductive at low temperatures, using interface modifiers to reduce charge transfer resistance, and applying protective coatings on electrodes to prevent side reactions. These approaches maintain efficient ion transport across interfaces even when thermal energy is reduced.
- Composite and hybrid systems for temperature resilience: Composite and hybrid systems can be designed to enhance the temperature resilience of RT Na-S cells. These include combining Na-S chemistry with other battery technologies that perform well at low temperatures, using phase-change materials for thermal regulation, and developing multi-layer electrode structures with complementary properties. Some approaches incorporate temperature-responsive components that adapt their properties based on environmental conditions, ensuring consistent performance across a wide temperature range.
02 Cathode material engineering for cold environment operation
Engineering sulfur-based cathode materials with specific structures and compositions can improve the low-temperature performance of RT Na-S cells. Approaches include using carbon-sulfur composites, sulfur polymers, or metal sulfides that maintain electrochemical activity at lower temperatures. These materials provide better reaction kinetics and sulfur utilization at reduced temperatures, minimizing capacity loss and performance degradation in cold environments.Expand Specific Solutions03 Anode design strategies for low-temperature applications
Specialized anode designs can enhance the low-temperature performance of RT Na-S batteries. These include using sodium metal anodes with protective coatings, sodium alloys with lower melting points, or carbon-based materials that can effectively store and release sodium ions at reduced temperatures. Such anode modifications improve the charge transfer kinetics and reduce polarization at the anode-electrolyte interface during low-temperature operation.Expand Specific Solutions04 Cell structure and component optimization
Optimizing the overall cell structure and components can significantly improve the low-temperature performance of RT Na-S batteries. This includes designing specialized separators with controlled porosity, using thermal management systems, adjusting electrode thickness, and implementing pressure regulation mechanisms. These structural optimizations help maintain proper ion transport pathways and reaction kinetics even when temperatures drop below optimal operating conditions.Expand Specific Solutions05 Additives and interface engineering
Incorporating specific additives and engineering the interfaces between cell components can enhance low-temperature performance of RT Na-S cells. This includes using electrolyte additives that prevent sodium dendrite formation, interface modifiers that reduce charge transfer resistance, and compounds that stabilize the solid electrolyte interphase at low temperatures. These approaches minimize the increase in internal resistance that typically occurs when operating sodium-sulfur batteries in cold environments.Expand Specific Solutions
Key Industry Players in Na-S Battery Development
The room-temperature sodium-sulfur (RT Na-S) battery market is in an early growth phase, characterized by increasing research focus on low-temperature performance challenges. While the global market remains relatively small compared to lithium-ion technologies, it shows promising expansion potential due to sodium's abundance and cost advantages. Technologically, the field is advancing through collaborative efforts between academic institutions and industry players. NGK Insulators leads as the established commercial player, while research institutions like Shanghai Institute of Ceramics and companies including Global Graphene Group, Honeycomb Battery, and Siemens are making significant contributions to solving low-temperature performance issues. The technology is approaching commercial viability, with recent breakthroughs in electrolyte formulations and electrode materials demonstrating improved cold-weather operation, though mass-market adoption remains several years away.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered room-temperature sodium-sulfur (RT Na-S) battery technology specifically addressing low-temperature performance challenges for outdoor storage applications. Their approach involves using a specialized NASICON (Na Super Ionic CONductor) ceramic electrolyte with optimized composition to maintain ionic conductivity at temperatures as low as -20°C. The company has developed a proprietary electrode formulation incorporating carbon nanotubes to enhance electron transfer at low temperatures, while their cell design features a thermal management system that utilizes the battery's internal resistance to generate heat during initial discharge, gradually warming the cell to optimal operating temperature. NGK has also implemented a protective coating on the sodium anode to prevent dendrite formation during low-temperature charging cycles, significantly extending battery life in cold environments. Their latest generation cells maintain approximately 85% of room temperature capacity at 0°C and 70% at -10°C, representing a substantial improvement over conventional Na-S systems that typically lose functionality below 10°C.
Strengths: Industry-leading expertise in ceramic electrolytes; established manufacturing infrastructure for large-scale production; extensive field testing data from real-world deployments. Weaknesses: Higher production costs compared to lithium-ion alternatives; still experiences significant capacity reduction below -15°C; requires specialized battery management systems for optimal low-temperature operation.
Shanghai Institute of Ceramics, Chinese Academy of Sciences
Technical Solution: The Shanghai Institute of Ceramics has developed an innovative approach to low-temperature RT Na-S cell performance through their advanced ceramic electrolyte technology. Their research focuses on a novel composite solid electrolyte system combining NASICON-type materials with polymer components to create a flexible, high-conductivity interface that maintains performance at low temperatures. The institute has engineered a nanostructured sulfur cathode with hierarchical porosity that accommodates the volume changes during cycling while facilitating rapid ion transport even at sub-zero temperatures. Their cells incorporate a specially formulated electrolyte additive that reduces the freezing point and maintains ionic mobility down to -30°C. Additionally, they've developed a carbon-based interfacial layer between the sodium anode and electrolyte that prevents unwanted side reactions at low temperatures while promoting uniform sodium deposition. Testing has demonstrated their cells maintain over 75% capacity at -20°C compared to room temperature performance, with stable cycling for more than 500 cycles under these conditions.
Strengths: Cutting-edge materials science expertise; strong integration of theoretical modeling with experimental validation; excellent low-temperature ionic conductivity in their composite electrolytes. Weaknesses: Technology remains primarily at laboratory scale; manufacturing complexity may limit commercial scalability; requires further optimization for long-term stability in real-world outdoor storage conditions.
Critical Patents and Research on Low-Temperature Electrolytes
Room-temperature sodium-sulfur battery and preparation method thereof
PatentPendingCN118099560A
Innovation
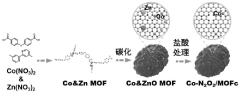
- Metal-organic framework materials (MOF) are used as precursors to synthesize single-atom catalyst Co-N2O2/MOFc composites as sulfur storage materials, which can be used as cathodes of room-temperature sodium-sulfur batteries to improve polysulfide conversion efficiency and prevent shuttle and loss.
Material Science Advancements for Sub-Zero Temperature Applications
Recent advancements in material science have significantly contributed to enhancing the low-temperature performance of room temperature sodium-sulfur (RT Na-S) cells for outdoor storage applications. Traditional Na-S batteries typically operate at high temperatures (300-350°C), limiting their practical deployment in variable climate conditions. The development of materials capable of maintaining electrochemical stability and ionic conductivity at sub-zero temperatures represents a critical breakthrough for expanding the application scope of these energy storage systems.
Polymer electrolyte modifications have emerged as a primary focus area, with researchers developing composite electrolytes incorporating ceramic nanoparticles that maintain flexibility while preventing crystallization at low temperatures. These materials demonstrate superior ionic conductivity below 0°C compared to conventional electrolytes, with some advanced formulations maintaining up to 65% of their room temperature performance at -20°C.
Electrode material innovations have also addressed the sluggish kinetics observed in sub-zero conditions. Novel carbon-sulfur composites with hierarchical pore structures facilitate better ion transport and mitigate the volume expansion issues that typically plague sulfur electrodes. Additionally, sodium anode protection strategies using specialized coatings have demonstrated remarkable stability during repeated freeze-thaw cycles, preventing dendrite formation that commonly occurs at low temperatures.
Interface engineering represents another crucial advancement, with researchers developing functional interlayers that maintain intimate contact between electrodes and electrolytes despite thermal contraction at low temperatures. These interlayers, often comprising flexible polymers doped with ionic liquids, ensure continuous ion transport pathways even when thermal stress threatens to create microscopic separations within the cell architecture.
Cryogenic additives have been incorporated into electrolyte formulations to depress freezing points and maintain solution-phase transport. Compounds such as ethylene carbonate derivatives and certain ionic liquids have demonstrated antifreeze properties while remaining electrochemically compatible with Na-S chemistry. Some advanced formulations maintain fluidity down to -40°C, representing a significant improvement over previous generations.
Nanoscale engineering of active materials has yielded sulfur hosts with enhanced thermal conductivity, addressing the challenge of temperature gradients within cells during operation in cold environments. These materials, often incorporating graphene or carbon nanotube networks, facilitate more uniform temperature distribution and prevent localized freezing that can lead to capacity loss and accelerated degradation.
The collective impact of these material science advancements has extended the operational temperature range of RT Na-S cells, making them increasingly viable for outdoor energy storage applications in regions experiencing seasonal temperature variations and extreme cold weather events.
Polymer electrolyte modifications have emerged as a primary focus area, with researchers developing composite electrolytes incorporating ceramic nanoparticles that maintain flexibility while preventing crystallization at low temperatures. These materials demonstrate superior ionic conductivity below 0°C compared to conventional electrolytes, with some advanced formulations maintaining up to 65% of their room temperature performance at -20°C.
Electrode material innovations have also addressed the sluggish kinetics observed in sub-zero conditions. Novel carbon-sulfur composites with hierarchical pore structures facilitate better ion transport and mitigate the volume expansion issues that typically plague sulfur electrodes. Additionally, sodium anode protection strategies using specialized coatings have demonstrated remarkable stability during repeated freeze-thaw cycles, preventing dendrite formation that commonly occurs at low temperatures.
Interface engineering represents another crucial advancement, with researchers developing functional interlayers that maintain intimate contact between electrodes and electrolytes despite thermal contraction at low temperatures. These interlayers, often comprising flexible polymers doped with ionic liquids, ensure continuous ion transport pathways even when thermal stress threatens to create microscopic separations within the cell architecture.
Cryogenic additives have been incorporated into electrolyte formulations to depress freezing points and maintain solution-phase transport. Compounds such as ethylene carbonate derivatives and certain ionic liquids have demonstrated antifreeze properties while remaining electrochemically compatible with Na-S chemistry. Some advanced formulations maintain fluidity down to -40°C, representing a significant improvement over previous generations.
Nanoscale engineering of active materials has yielded sulfur hosts with enhanced thermal conductivity, addressing the challenge of temperature gradients within cells during operation in cold environments. These materials, often incorporating graphene or carbon nanotube networks, facilitate more uniform temperature distribution and prevent localized freezing that can lead to capacity loss and accelerated degradation.
The collective impact of these material science advancements has extended the operational temperature range of RT Na-S cells, making them increasingly viable for outdoor energy storage applications in regions experiencing seasonal temperature variations and extreme cold weather events.
Environmental Impact and Sustainability of Na-S Battery Systems
The environmental impact and sustainability of Na-S battery systems represent critical considerations in their development and deployment, particularly when examining low-temperature performance for outdoor storage applications. Room temperature (RT) Na-S cells offer significant environmental advantages compared to traditional high-temperature sodium-sulfur batteries and lithium-ion alternatives.
Sodium-sulfur battery systems utilize abundant raw materials, with sodium being the sixth most common element in the Earth's crust. This abundance translates to reduced environmental strain from mining operations compared to lithium-ion batteries that require scarce materials like cobalt and lithium. The extraction processes for sodium are generally less environmentally damaging, resulting in lower carbon footprints during the manufacturing phase.
When considering outdoor storage applications, the improved low-temperature performance of RT Na-S cells contributes to sustainability by reducing the need for energy-intensive heating systems that would otherwise be required to maintain operational temperatures. This energy efficiency translates directly to reduced greenhouse gas emissions during operation, particularly in cold climate regions where energy demands for battery temperature management can be substantial.
The life cycle assessment of RT Na-S batteries reveals favorable environmental metrics. These systems demonstrate lower embodied energy in production and potentially longer service lives when properly engineered for low-temperature resilience. The reduced degradation at lower temperatures, when achieved through proper electrolyte and electrode design, extends battery lifespan and decreases replacement frequency, thereby minimizing waste generation.
End-of-life considerations also favor Na-S systems. The materials in sodium-sulfur batteries are more readily recyclable than those in competing technologies. Sodium compounds can be recovered and repurposed with relatively straightforward processes, while sulfur is already managed in established recycling streams. This recyclability reduces the environmental burden associated with battery disposal and supports circular economy principles.
Water consumption represents another environmental advantage of Na-S battery production. Manufacturing processes for these batteries typically require significantly less water than lithium-ion alternatives, an increasingly important consideration as water scarcity becomes a pressing global issue. This reduced water footprint enhances the overall sustainability profile of Na-S technology for grid-scale storage applications.
The environmental benefits extend to safety considerations as well. RT Na-S cells designed for low-temperature performance typically incorporate safer electrolytes and containment systems than high-temperature versions, reducing the risk of environmentally harmful leaks or thermal events during outdoor storage in variable climate conditions.
Sodium-sulfur battery systems utilize abundant raw materials, with sodium being the sixth most common element in the Earth's crust. This abundance translates to reduced environmental strain from mining operations compared to lithium-ion batteries that require scarce materials like cobalt and lithium. The extraction processes for sodium are generally less environmentally damaging, resulting in lower carbon footprints during the manufacturing phase.
When considering outdoor storage applications, the improved low-temperature performance of RT Na-S cells contributes to sustainability by reducing the need for energy-intensive heating systems that would otherwise be required to maintain operational temperatures. This energy efficiency translates directly to reduced greenhouse gas emissions during operation, particularly in cold climate regions where energy demands for battery temperature management can be substantial.
The life cycle assessment of RT Na-S batteries reveals favorable environmental metrics. These systems demonstrate lower embodied energy in production and potentially longer service lives when properly engineered for low-temperature resilience. The reduced degradation at lower temperatures, when achieved through proper electrolyte and electrode design, extends battery lifespan and decreases replacement frequency, thereby minimizing waste generation.
End-of-life considerations also favor Na-S systems. The materials in sodium-sulfur batteries are more readily recyclable than those in competing technologies. Sodium compounds can be recovered and repurposed with relatively straightforward processes, while sulfur is already managed in established recycling streams. This recyclability reduces the environmental burden associated with battery disposal and supports circular economy principles.
Water consumption represents another environmental advantage of Na-S battery production. Manufacturing processes for these batteries typically require significantly less water than lithium-ion alternatives, an increasingly important consideration as water scarcity becomes a pressing global issue. This reduced water footprint enhances the overall sustainability profile of Na-S technology for grid-scale storage applications.
The environmental benefits extend to safety considerations as well. RT Na-S cells designed for low-temperature performance typically incorporate safer electrolytes and containment systems than high-temperature versions, reducing the risk of environmentally harmful leaks or thermal events during outdoor storage in variable climate conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!