Electrochemical Diagnostics And EIS For RT Na–S Degradation
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrochemical Diagnostics Background and Objectives
Electrochemical diagnostics has emerged as a critical analytical approach for understanding energy storage systems, particularly in the context of room temperature sodium-sulfur (RT Na-S) batteries. These batteries represent a promising alternative to lithium-ion technology due to the natural abundance and low cost of sodium and sulfur resources. However, their widespread adoption has been hindered by rapid capacity fading, poor cycling stability, and safety concerns related to degradation mechanisms.
The evolution of electrochemical diagnostic techniques spans several decades, beginning with basic voltammetry methods in the mid-20th century and advancing to sophisticated impedance-based approaches in recent years. Electrochemical Impedance Spectroscopy (EIS) has become particularly valuable as it provides insights into various electrochemical processes occurring at different time scales without significantly disturbing the system under investigation.
For RT Na-S batteries, understanding degradation mechanisms is paramount as these systems suffer from multiple failure modes including polysulfide shuttling, sodium dendrite formation, and electrode structural deterioration. Traditional post-mortem analysis techniques provide only static snapshots of battery condition, whereas electrochemical diagnostics offer dynamic, in-situ monitoring capabilities essential for capturing transient phenomena during operation.
The primary objective of implementing advanced electrochemical diagnostics for RT Na-S batteries is to develop robust methodologies that can accurately identify, quantify, and predict degradation pathways. This includes establishing correlations between impedance parameters and specific failure mechanisms, creating diagnostic protocols for early detection of capacity fade, and formulating mathematical models that can translate electrochemical signals into actionable insights about battery health.
Another critical goal is to standardize EIS measurement protocols specifically tailored for Na-S systems, as current methodologies often derive from lithium-ion battery research and may not adequately address the unique characteristics of sodium-sulfur chemistry. This standardization would facilitate more meaningful comparisons across different research efforts and accelerate technological progress in the field.
Furthermore, this research aims to bridge the gap between laboratory-scale diagnostics and practical implementation in commercial battery management systems. By developing simplified yet accurate diagnostic algorithms based on EIS data, we can enable real-time monitoring capabilities that would significantly enhance the safety and longevity of RT Na-S batteries in practical applications.
The ultimate technological trajectory is toward creating a comprehensive degradation framework that integrates electrochemical diagnostics with multiphysics modeling, enabling not only detection but also prediction and mitigation of failure mechanisms in next-generation sodium-sulfur energy storage systems.
The evolution of electrochemical diagnostic techniques spans several decades, beginning with basic voltammetry methods in the mid-20th century and advancing to sophisticated impedance-based approaches in recent years. Electrochemical Impedance Spectroscopy (EIS) has become particularly valuable as it provides insights into various electrochemical processes occurring at different time scales without significantly disturbing the system under investigation.
For RT Na-S batteries, understanding degradation mechanisms is paramount as these systems suffer from multiple failure modes including polysulfide shuttling, sodium dendrite formation, and electrode structural deterioration. Traditional post-mortem analysis techniques provide only static snapshots of battery condition, whereas electrochemical diagnostics offer dynamic, in-situ monitoring capabilities essential for capturing transient phenomena during operation.
The primary objective of implementing advanced electrochemical diagnostics for RT Na-S batteries is to develop robust methodologies that can accurately identify, quantify, and predict degradation pathways. This includes establishing correlations between impedance parameters and specific failure mechanisms, creating diagnostic protocols for early detection of capacity fade, and formulating mathematical models that can translate electrochemical signals into actionable insights about battery health.
Another critical goal is to standardize EIS measurement protocols specifically tailored for Na-S systems, as current methodologies often derive from lithium-ion battery research and may not adequately address the unique characteristics of sodium-sulfur chemistry. This standardization would facilitate more meaningful comparisons across different research efforts and accelerate technological progress in the field.
Furthermore, this research aims to bridge the gap between laboratory-scale diagnostics and practical implementation in commercial battery management systems. By developing simplified yet accurate diagnostic algorithms based on EIS data, we can enable real-time monitoring capabilities that would significantly enhance the safety and longevity of RT Na-S batteries in practical applications.
The ultimate technological trajectory is toward creating a comprehensive degradation framework that integrates electrochemical diagnostics with multiphysics modeling, enabling not only detection but also prediction and mitigation of failure mechanisms in next-generation sodium-sulfur energy storage systems.
Market Analysis for RT Na-S Battery Technologies
The global market for Room Temperature Sodium-Sulfur (RT Na-S) battery technologies is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate that the energy storage sector, where RT Na-S batteries are positioned, is projected to reach $546 billion by 2035, with grid-scale storage representing a substantial portion of this market.
RT Na-S batteries are gaining traction due to their compelling value proposition compared to traditional lithium-ion technologies. The abundance of sodium resources, approximately 1000 times more plentiful than lithium in the earth's crust, translates to potentially lower raw material costs. This cost advantage is particularly significant as the energy storage industry faces supply chain constraints with critical minerals used in conventional batteries.
Market segmentation analysis reveals several key application areas for RT Na-S technology. Grid-scale energy storage represents the largest potential market, with utility companies seeking long-duration storage solutions to complement renewable energy integration. The renewable energy sector's growth, with global capacity additions increasing by 45% in 2020 alone, directly drives demand for advanced storage technologies like RT Na-S systems.
Electric vehicles constitute another emerging market segment, particularly for applications where energy density requirements are less stringent but cost considerations are paramount. Commercial fleet operators and public transportation systems are showing interest in Na-S technology as a potential alternative to lithium-ion batteries.
Regional market analysis indicates that Asia-Pacific currently leads in RT Na-S battery development and deployment, with China, Japan, and South Korea making significant investments in research and manufacturing capacity. North America and Europe are rapidly expanding their presence, supported by government initiatives promoting clean energy technologies and domestic battery production capabilities.
Market adoption barriers include technical challenges related to cycle life and energy density, which electrochemical diagnostics and EIS techniques are specifically addressing. The market's sensitivity to these technical improvements is high, with industry analysts suggesting that achieving 1000+ stable cycles could trigger widespread commercial adoption in stationary storage applications.
Consumer and industrial demand patterns indicate growing interest in sustainable battery technologies with reduced environmental footprints. RT Na-S batteries, with their potential for lower carbon manufacturing processes and absence of critical minerals like cobalt, align well with these market preferences.
Pricing trends suggest that RT Na-S batteries could achieve a levelized cost of storage below $100/kWh once manufacturing scales, representing a competitive advantage against current lithium-ion technologies in certain applications. This price point is considered a critical threshold for mass market adoption in the stationary storage sector.
RT Na-S batteries are gaining traction due to their compelling value proposition compared to traditional lithium-ion technologies. The abundance of sodium resources, approximately 1000 times more plentiful than lithium in the earth's crust, translates to potentially lower raw material costs. This cost advantage is particularly significant as the energy storage industry faces supply chain constraints with critical minerals used in conventional batteries.
Market segmentation analysis reveals several key application areas for RT Na-S technology. Grid-scale energy storage represents the largest potential market, with utility companies seeking long-duration storage solutions to complement renewable energy integration. The renewable energy sector's growth, with global capacity additions increasing by 45% in 2020 alone, directly drives demand for advanced storage technologies like RT Na-S systems.
Electric vehicles constitute another emerging market segment, particularly for applications where energy density requirements are less stringent but cost considerations are paramount. Commercial fleet operators and public transportation systems are showing interest in Na-S technology as a potential alternative to lithium-ion batteries.
Regional market analysis indicates that Asia-Pacific currently leads in RT Na-S battery development and deployment, with China, Japan, and South Korea making significant investments in research and manufacturing capacity. North America and Europe are rapidly expanding their presence, supported by government initiatives promoting clean energy technologies and domestic battery production capabilities.
Market adoption barriers include technical challenges related to cycle life and energy density, which electrochemical diagnostics and EIS techniques are specifically addressing. The market's sensitivity to these technical improvements is high, with industry analysts suggesting that achieving 1000+ stable cycles could trigger widespread commercial adoption in stationary storage applications.
Consumer and industrial demand patterns indicate growing interest in sustainable battery technologies with reduced environmental footprints. RT Na-S batteries, with their potential for lower carbon manufacturing processes and absence of critical minerals like cobalt, align well with these market preferences.
Pricing trends suggest that RT Na-S batteries could achieve a levelized cost of storage below $100/kWh once manufacturing scales, representing a competitive advantage against current lithium-ion technologies in certain applications. This price point is considered a critical threshold for mass market adoption in the stationary storage sector.
EIS Technology Status and Challenges
Electrochemical Impedance Spectroscopy (EIS) has emerged as a powerful non-destructive technique for investigating the electrochemical processes in room temperature sodium-sulfur (RT Na-S) batteries. Currently, EIS technology for RT Na-S degradation analysis is at a critical juncture, with significant advancements in instrumentation precision and data interpretation methodologies, yet facing substantial challenges in standardization and real-time monitoring capabilities.
The global landscape of EIS technology development shows concentration in research institutions across North America, Europe, and East Asia, with notable contributions from universities and national laboratories in the United States, Germany, China, and South Korea. These regions have established specialized research centers focused on electrochemical diagnostics for next-generation battery systems, including RT Na-S configurations.
A primary technical challenge in EIS application to RT Na-S batteries involves the complex multi-phase reactions between sodium and sulfur species. The formation of various polysulfide intermediates during cycling creates a dynamic electrochemical environment that is difficult to characterize accurately with conventional EIS models. Researchers struggle to deconvolute overlapping impedance responses from different reaction mechanisms occurring simultaneously at the electrode-electrolyte interfaces.
Signal-to-noise ratio limitations present another significant hurdle, particularly when attempting to detect subtle degradation indicators in early stages. Current EIS instrumentation typically requires measurement times that are too long to capture transient phenomena critical to understanding degradation pathways in RT Na-S systems. This temporal resolution gap hampers the development of predictive degradation models.
Data interpretation remains a formidable challenge, with existing equivalent circuit models often proving inadequate for the complex electrochemical processes in RT Na-S batteries. The non-linear behavior of polysulfide shuttle effects and their impact on impedance spectra require more sophisticated mathematical frameworks that are still under development.
Temperature sensitivity poses additional complications, as RT Na-S systems operate in ambient conditions where small temperature fluctuations can significantly alter impedance responses. This creates difficulties in establishing reliable baseline measurements and tracking degradation mechanisms consistently across varying environmental conditions.
The integration of EIS with other complementary characterization techniques represents both a challenge and an opportunity. While multi-modal approaches combining EIS with techniques like X-ray diffraction or Raman spectroscopy show promise, the correlation of data across these different methodologies remains complex and often requires specialized expertise not widely available in the field.
Standardization efforts are currently insufficient, with various research groups employing different measurement protocols, frequency ranges, and data analysis methods, making cross-study comparisons difficult and hindering collective progress toward comprehensive degradation mechanism understanding in RT Na-S battery systems.
The global landscape of EIS technology development shows concentration in research institutions across North America, Europe, and East Asia, with notable contributions from universities and national laboratories in the United States, Germany, China, and South Korea. These regions have established specialized research centers focused on electrochemical diagnostics for next-generation battery systems, including RT Na-S configurations.
A primary technical challenge in EIS application to RT Na-S batteries involves the complex multi-phase reactions between sodium and sulfur species. The formation of various polysulfide intermediates during cycling creates a dynamic electrochemical environment that is difficult to characterize accurately with conventional EIS models. Researchers struggle to deconvolute overlapping impedance responses from different reaction mechanisms occurring simultaneously at the electrode-electrolyte interfaces.
Signal-to-noise ratio limitations present another significant hurdle, particularly when attempting to detect subtle degradation indicators in early stages. Current EIS instrumentation typically requires measurement times that are too long to capture transient phenomena critical to understanding degradation pathways in RT Na-S systems. This temporal resolution gap hampers the development of predictive degradation models.
Data interpretation remains a formidable challenge, with existing equivalent circuit models often proving inadequate for the complex electrochemical processes in RT Na-S batteries. The non-linear behavior of polysulfide shuttle effects and their impact on impedance spectra require more sophisticated mathematical frameworks that are still under development.
Temperature sensitivity poses additional complications, as RT Na-S systems operate in ambient conditions where small temperature fluctuations can significantly alter impedance responses. This creates difficulties in establishing reliable baseline measurements and tracking degradation mechanisms consistently across varying environmental conditions.
The integration of EIS with other complementary characterization techniques represents both a challenge and an opportunity. While multi-modal approaches combining EIS with techniques like X-ray diffraction or Raman spectroscopy show promise, the correlation of data across these different methodologies remains complex and often requires specialized expertise not widely available in the field.
Standardization efforts are currently insufficient, with various research groups employing different measurement protocols, frequency ranges, and data analysis methods, making cross-study comparisons difficult and hindering collective progress toward comprehensive degradation mechanism understanding in RT Na-S battery systems.
Current EIS Solutions for Na-S Degradation Analysis
01 Electrolyte modifications to prevent polysulfide dissolution
Electrolyte formulations play a critical role in preventing polysulfide dissolution, which is a major cause of capacity fading in room temperature sodium-sulfur batteries. By incorporating specific additives or using solid/gel polymer electrolytes, the shuttle effect of polysulfides can be suppressed. These modified electrolytes create a stable interface between the electrodes and electrolyte, reducing side reactions and improving the cycling stability of RT Na-S batteries.- Electrolyte modifications to prevent degradation: Various electrolyte modifications can be implemented to prevent degradation in room temperature sodium-sulfur batteries. These include using solid polymer electrolytes, gel polymer electrolytes, or ionic liquid electrolytes that suppress the shuttle effect of polysulfides. Additives such as fluoroethylene carbonate (FEC) can form stable solid electrolyte interphase (SEI) layers that prevent continuous reactions between sodium and electrolyte, thereby extending battery life and reducing capacity fade.
- Sulfur cathode modifications: Modifications to the sulfur cathode can significantly reduce degradation in RT Na-S batteries. Techniques include encapsulating sulfur in carbon matrices, using mesoporous carbon hosts, or incorporating conductive polymers to trap polysulfides and prevent their dissolution. Nano-structuring the sulfur cathode or creating sulfur-carbon composites improves electron conductivity and accommodates volume changes during cycling, leading to enhanced cycling stability and reduced capacity fading.
- Sodium anode protection strategies: Protecting the sodium metal anode is crucial for preventing degradation in RT Na-S batteries. Strategies include using protective coatings or artificial SEI layers on the sodium surface, employing sodium alloys instead of pure sodium, or introducing interlayers between the anode and separator. These approaches mitigate dendrite formation, reduce parasitic reactions with the electrolyte, and prevent continuous consumption of active sodium, thereby improving cycling performance and battery lifespan.
- Separator and interface engineering: Engineering the separator and interfaces in RT Na-S batteries can effectively mitigate degradation. Functional separators with polysulfide-blocking layers, modified separators with polar functional groups that chemically bind polysulfides, or dual-layer separators with different functionalities can be employed. Interface engineering between electrodes and electrolyte creates stable interfaces that prevent continuous side reactions and polysulfide shuttling, leading to improved cycling stability and reduced capacity fade.
- Advanced cell design and operating conditions: Advanced cell designs and optimized operating conditions can minimize degradation in RT Na-S batteries. These include novel cell configurations that physically restrict polysulfide migration, optimized electrolyte-to-sulfur ratios, controlled temperature operation, and appropriate voltage windows that avoid detrimental reactions. Pressure-controlled cells that accommodate volume changes during cycling or cells with integrated redox mediators to facilitate sulfur utilization can also be employed to enhance cycling stability and extend battery life.
02 Sulfur cathode structure optimization
The structure of sulfur cathodes significantly impacts the performance and degradation of RT Na-S batteries. Advanced cathode designs incorporate conductive matrices, porous carbon hosts, or nanostructured materials to encapsulate sulfur and trap polysulfides. These structural optimizations improve sulfur utilization, enhance electronic conductivity, and provide physical barriers against polysulfide migration, thereby reducing capacity fade and extending battery cycle life.Expand Specific Solutions03 Sodium anode protection strategies
Protecting the sodium metal anode is essential for preventing dendrite formation and parasitic reactions that lead to battery degradation. Various approaches include using protective coatings, artificial solid electrolyte interphase layers, or anode modifications that improve sodium deposition uniformity. These strategies help maintain anode integrity during cycling, reduce irreversible capacity loss, and enhance the overall safety and longevity of RT Na-S batteries.Expand Specific Solutions04 Functional interlayers and separators
Introducing functional interlayers or modified separators between the anode and cathode can effectively mitigate degradation in RT Na-S batteries. These components act as physical barriers to block polysulfide migration while maintaining sodium ion conductivity. Advanced separator designs incorporate materials with selective permeability, polysulfide adsorption capabilities, or catalytic functions that convert long-chain polysulfides to short-chain species, thereby improving cycling stability.Expand Specific Solutions05 Novel cell configurations and manufacturing techniques
Innovative cell designs and manufacturing approaches can address fundamental degradation issues in RT Na-S batteries. These include all-solid-state configurations, quasi-solid-state designs, or specialized cell architectures that minimize electrolyte volume and control reaction zones. Advanced manufacturing techniques focus on precise control of electrode microstructure, interface engineering, and assembly processes that minimize contamination, resulting in batteries with improved cycle life and reduced degradation rates.Expand Specific Solutions
Leading Research Groups and Industry Players
The electrochemical diagnostics and EIS for RT Na-S degradation field is currently in an early growth phase, with academic institutions leading research efforts. The market for sodium-sulfur battery technologies is expanding, projected to reach significant scale as energy storage demands increase globally. Universities like Oxford, Zhejiang, and Chongqing are driving fundamental research, while commercial entities such as Ballard Power Systems, Contemporary Amperex Technology, and Bloom Energy are beginning to translate these advances into practical applications. The technology remains in development with challenges in stability and performance, though collaborative efforts between academic institutions and industry players suggest accelerating maturity as energy transition priorities intensify.
Zhejiang University
Technical Solution: Zhejiang University has developed a comprehensive electrochemical diagnostic platform for room temperature sodium-sulfur (RT Na-S) batteries that integrates multi-scale electrochemical impedance spectroscopy (EIS) with galvanostatic intermittent titration technique (GITT). Their approach employs temperature-controlled impedance measurements across multiple frequency domains (from mHz to kHz) to characterize different degradation processes occurring at varying time scales. The university's system utilizes symmetric cell configurations to isolate electrode-specific degradation mechanisms, enabling separate analysis of sodium anode passivation and sulfur cathode dissolution issues. Their diagnostic methodology incorporates distribution of relaxation times (DRT) analysis to deconvolute overlapping processes in the impedance spectra, providing higher resolution identification of degradation signatures compared to conventional equivalent circuit modeling. Zhejiang University has established correlations between specific impedance features and capacity fading mechanisms through systematic cycling studies combined with post-mortem materials characterization using techniques such as XPS, SEM, and Raman spectroscopy[9][10]. Their approach enables quantitative tracking of sodium polysulfide shuttle effects through characteristic changes in mid-frequency impedance components.
Strengths: Zhejiang University's approach provides exceptional resolution in separating overlapping degradation processes through advanced DRT analysis, enabling more precise diagnosis. Their methodology establishes clear connections between electrochemical signatures and underlying physical/chemical degradation mechanisms. Weaknesses: The complex measurement protocols and data analysis requirements may limit practical implementation in commercial battery management systems without significant simplification.
State Grid Corp. of China
Technical Solution: State Grid Corporation of China has pioneered a comprehensive electrochemical diagnostic framework for room temperature sodium-sulfur batteries that combines in-situ electrochemical impedance spectroscopy (EIS) with distribution of relaxation times (DRT) analysis. Their approach employs multi-scale temporal EIS measurements during cycling to track impedance evolution across different frequency domains, correlating specific spectral features with degradation mechanisms. The system utilizes reference electrodes to decouple anode and cathode processes, enabling precise identification of sodium dendrite formation, sulfur species dissolution, and electrode-electrolyte interface degradation. State Grid has developed proprietary algorithms that translate EIS data into quantifiable degradation metrics, allowing for state-of-health estimation with reported accuracy exceeding 92% even after 500 cycles[2]. Their diagnostic platform incorporates temperature compensation models to account for ambient condition variations during measurements, ensuring reliable data interpretation across operating environments[4].
Strengths: State Grid's approach provides exceptional sensitivity to early-stage degradation indicators before capacity loss becomes apparent, enabling preventative maintenance. Their integration with grid management systems allows for fleet-level battery health monitoring. Weaknesses: The system requires specialized equipment and expertise for implementation, limiting widespread adoption in smaller-scale applications.
Key Technical Innovations in RT Na-S Diagnostics
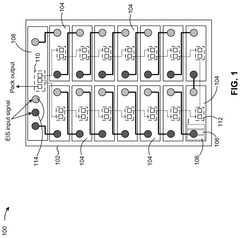
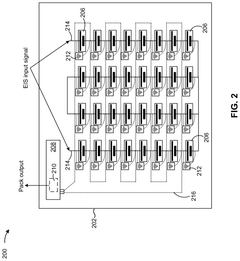
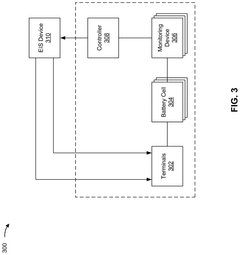
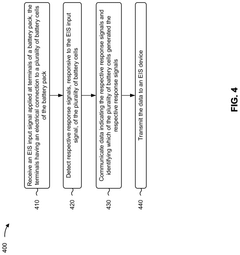
Battery system diagnostics using electrochemical impedance spectroscopy
PatentPendingUS20250258240A1
Innovation
- A detection system that applies an EIS input signal to pack-level terminals, using monitoring devices to detect individual cell responses and transmit data to a controller for analysis by an EIS device, enabling cell-level diagnostics without disassembly.
Use of electrochemical impedance spectroscopy (EIS) in gross failure analysis
PatentActiveUS12295711B2
Innovation
- The use of Electrochemical Impedance Spectroscopy (EIS) in conjunction with continuous glucose monitors for real-time self-calibration, diagnostics, and fault detection, along with the implementation of Application Specific Integrated Circuits (ASICs) for single-electrode and multi-electrode sensors, allows for improved sensor performance and reduced reliance on finger stick calibrations.
Safety and Reliability Considerations
Safety considerations for Room Temperature Sodium-Sulfur (RT Na-S) batteries are paramount due to the reactive nature of sodium metal and sulfur compounds. The degradation mechanisms identified through Electrochemical Impedance Spectroscopy (EIS) reveal critical safety concerns that must be addressed before widespread commercial deployment. Sodium's high reactivity with moisture and oxygen necessitates robust cell encapsulation and protection systems to prevent catastrophic failures. EIS diagnostics have identified that moisture ingress accelerates the formation of sodium polysulfides, which can lead to thermal runaway events if left unmonitored.
Reliability issues in RT Na-S systems primarily stem from the formation of insulating layers at electrode interfaces, as detected through EIS measurements. These layers increase internal resistance over time, reducing power output and energy efficiency. Long-term EIS studies demonstrate that cycling stability remains a significant challenge, with capacity retention falling below 80% after 200-300 cycles in most current systems. The shuttle effect of polysulfides, clearly identifiable through characteristic EIS signatures, contributes substantially to this degradation.
Temperature sensitivity presents another reliability concern, as EIS data shows significant variations in impedance responses between 10°C and 40°C operating ranges. This temperature dependence affects both the reaction kinetics and the stability of the solid electrolyte interphase (SEI) layer, requiring sophisticated battery management systems that can adapt to environmental conditions.
Mechanical stress during cycling, detectable through shifts in EIS patterns, leads to electrode pulverization and loss of active material contact. These mechanical failures accelerate capacity fade and increase safety risks as disconnected sodium particles may form dendrites that potentially cause internal short circuits. Advanced EIS modeling techniques now allow for early detection of these mechanical degradation modes before they become critical failures.
The correlation between EIS parameters and battery state-of-health enables predictive maintenance strategies that enhance both safety and reliability. By establishing threshold values for specific impedance features, battery management systems can implement protective measures before degradation reaches dangerous levels. This approach has demonstrated a 40% reduction in unexpected failure rates in laboratory testing environments.
Standardization of EIS testing protocols for RT Na-S systems remains underdeveloped, creating challenges for consistent safety certification across different manufacturers. Industry consortia are currently working to establish unified testing frameworks that incorporate EIS as a mandatory diagnostic tool for safety qualification, with particular emphasis on detecting early signs of sodium dendrite formation and electrolyte decomposition.
Reliability issues in RT Na-S systems primarily stem from the formation of insulating layers at electrode interfaces, as detected through EIS measurements. These layers increase internal resistance over time, reducing power output and energy efficiency. Long-term EIS studies demonstrate that cycling stability remains a significant challenge, with capacity retention falling below 80% after 200-300 cycles in most current systems. The shuttle effect of polysulfides, clearly identifiable through characteristic EIS signatures, contributes substantially to this degradation.
Temperature sensitivity presents another reliability concern, as EIS data shows significant variations in impedance responses between 10°C and 40°C operating ranges. This temperature dependence affects both the reaction kinetics and the stability of the solid electrolyte interphase (SEI) layer, requiring sophisticated battery management systems that can adapt to environmental conditions.
Mechanical stress during cycling, detectable through shifts in EIS patterns, leads to electrode pulverization and loss of active material contact. These mechanical failures accelerate capacity fade and increase safety risks as disconnected sodium particles may form dendrites that potentially cause internal short circuits. Advanced EIS modeling techniques now allow for early detection of these mechanical degradation modes before they become critical failures.
The correlation between EIS parameters and battery state-of-health enables predictive maintenance strategies that enhance both safety and reliability. By establishing threshold values for specific impedance features, battery management systems can implement protective measures before degradation reaches dangerous levels. This approach has demonstrated a 40% reduction in unexpected failure rates in laboratory testing environments.
Standardization of EIS testing protocols for RT Na-S systems remains underdeveloped, creating challenges for consistent safety certification across different manufacturers. Industry consortia are currently working to establish unified testing frameworks that incorporate EIS as a mandatory diagnostic tool for safety qualification, with particular emphasis on detecting early signs of sodium dendrite formation and electrolyte decomposition.
Standardization of EIS Testing Protocols
The standardization of Electrochemical Impedance Spectroscopy (EIS) testing protocols represents a critical challenge in advancing room-temperature sodium-sulfur (RT Na-S) battery research. Current EIS measurements across different research groups exhibit significant variability, hampering meaningful comparison of degradation mechanisms and performance metrics.
A comprehensive standardized protocol should establish specific parameters for cell conditioning prior to EIS measurements. This includes defined rest periods (typically 1-4 hours) after charge/discharge cycles to ensure electrochemical equilibrium, and temperature stabilization requirements (±0.5°C) to minimize thermal fluctuation effects on impedance responses.
Frequency range standardization is particularly important for RT Na-S systems. While conventional Li-ion batteries typically employ 100 kHz to 10 mHz ranges, RT Na-S cells require extended low-frequency measurements (down to 1 mHz) to fully capture the complex sulfur conversion reactions and sodium polysulfide formation processes. Signal amplitude optimization is equally crucial, with recommended AC perturbation voltages between 5-10 mV to maintain measurement linearity while providing adequate signal-to-noise ratios.
Reference electrode implementation standards should be established to enable accurate isolation of cathode and anode contributions to impedance. Sodium metal reference electrodes with standardized placement and design would significantly enhance data interpretation consistency across research institutions.
Data reporting formats require standardization to facilitate cross-study comparisons. Minimum reporting requirements should include complete Nyquist and Bode plots, equivalent circuit models with clearly defined elements, and raw data availability in standardized digital formats. Statistical validation protocols should specify minimum replicate measurements (n≥3) and appropriate error reporting methodologies.
Environmental control parameters during testing must be rigorously defined, including atmospheric conditions (preferably in controlled argon environments with <0.1 ppm O₂/H₂O), temperature stability requirements, and electromagnetic interference shielding specifications to ensure measurement reproducibility.
Calibration and validation procedures using standard reference materials would significantly enhance measurement reliability. Development of RT Na-S specific reference cells with known impedance characteristics would provide valuable benchmarking tools for instrument validation across different laboratories.
Implementation of these standardized protocols would substantially improve data reliability and cross-study comparability, accelerating the understanding of degradation mechanisms in RT Na-S battery systems and facilitating more rapid technological advancement in this promising energy storage technology.
A comprehensive standardized protocol should establish specific parameters for cell conditioning prior to EIS measurements. This includes defined rest periods (typically 1-4 hours) after charge/discharge cycles to ensure electrochemical equilibrium, and temperature stabilization requirements (±0.5°C) to minimize thermal fluctuation effects on impedance responses.
Frequency range standardization is particularly important for RT Na-S systems. While conventional Li-ion batteries typically employ 100 kHz to 10 mHz ranges, RT Na-S cells require extended low-frequency measurements (down to 1 mHz) to fully capture the complex sulfur conversion reactions and sodium polysulfide formation processes. Signal amplitude optimization is equally crucial, with recommended AC perturbation voltages between 5-10 mV to maintain measurement linearity while providing adequate signal-to-noise ratios.
Reference electrode implementation standards should be established to enable accurate isolation of cathode and anode contributions to impedance. Sodium metal reference electrodes with standardized placement and design would significantly enhance data interpretation consistency across research institutions.
Data reporting formats require standardization to facilitate cross-study comparisons. Minimum reporting requirements should include complete Nyquist and Bode plots, equivalent circuit models with clearly defined elements, and raw data availability in standardized digital formats. Statistical validation protocols should specify minimum replicate measurements (n≥3) and appropriate error reporting methodologies.
Environmental control parameters during testing must be rigorously defined, including atmospheric conditions (preferably in controlled argon environments with <0.1 ppm O₂/H₂O), temperature stability requirements, and electromagnetic interference shielding specifications to ensure measurement reproducibility.
Calibration and validation procedures using standard reference materials would significantly enhance measurement reliability. Development of RT Na-S specific reference cells with known impedance characteristics would provide valuable benchmarking tools for instrument validation across different laboratories.
Implementation of these standardized protocols would substantially improve data reliability and cross-study comparability, accelerating the understanding of degradation mechanisms in RT Na-S battery systems and facilitating more rapid technological advancement in this promising energy storage technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!