Catalytic Interlayers To Accelerate Na2Sx Conversion Kinetics
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium-Sulfur Battery Technology Background and Objectives
Sodium-sulfur (Na-S) batteries have emerged as a promising energy storage technology due to their high theoretical energy density, cost-effectiveness, and the natural abundance of both sodium and sulfur resources. The development of Na-S batteries can be traced back to the 1960s when researchers at Ford Motor Company first explored high-temperature Na-S systems operating at approximately 300°C. These early systems utilized molten sodium and sulfur electrodes separated by a solid beta-alumina ceramic electrolyte.
Over the decades, research has shifted toward room-temperature Na-S batteries to overcome safety concerns and practical limitations associated with high operating temperatures. This transition represents a significant technological evolution, though it introduces new challenges, particularly regarding reaction kinetics and electrode stability.
The fundamental chemistry of Na-S batteries involves the conversion reaction between sodium and sulfur to form various sodium polysulfides (Na₂Sₓ, 2≤x≤8). During discharge, sodium ions combine with sulfur to form these polysulfides, which subsequently undergo further reduction to form Na₂S₂ and ultimately Na₂S. The reverse process occurs during charging.
A critical challenge in room-temperature Na-S batteries is the sluggish conversion kinetics of sodium polysulfides, which significantly limits battery performance. The slow reaction rates lead to capacity fading, reduced energy efficiency, and shortened cycle life. This is where catalytic interlayers become crucial, as they can potentially accelerate these conversion reactions.
Current research objectives in this field focus on developing effective catalytic materials that can facilitate the conversion of sodium polysulfides, thereby enhancing reaction kinetics without adding excessive weight or cost to the battery system. The ideal catalytic interlayer should possess high electrical conductivity, strong adsorption capability for polysulfides, and excellent catalytic activity toward polysulfide conversion.
The technological trajectory indicates growing interest in nanomaterials, particularly transition metal compounds, carbon-based materials, and hybrid structures as potential catalytic interlayers. These materials offer large surface areas, abundant active sites, and tunable properties that can be optimized for polysulfide conversion.
The ultimate goal of research in catalytic interlayers for Na-S batteries is to achieve practical energy storage systems with high energy density (theoretically up to 760 Wh/kg), long cycle life (>1000 cycles), and fast charging capabilities. Such advancements would position Na-S batteries as viable alternatives to lithium-ion batteries for grid-scale energy storage and potentially for electric vehicle applications, contributing to sustainable energy solutions and reduced dependency on critical materials.
Over the decades, research has shifted toward room-temperature Na-S batteries to overcome safety concerns and practical limitations associated with high operating temperatures. This transition represents a significant technological evolution, though it introduces new challenges, particularly regarding reaction kinetics and electrode stability.
The fundamental chemistry of Na-S batteries involves the conversion reaction between sodium and sulfur to form various sodium polysulfides (Na₂Sₓ, 2≤x≤8). During discharge, sodium ions combine with sulfur to form these polysulfides, which subsequently undergo further reduction to form Na₂S₂ and ultimately Na₂S. The reverse process occurs during charging.
A critical challenge in room-temperature Na-S batteries is the sluggish conversion kinetics of sodium polysulfides, which significantly limits battery performance. The slow reaction rates lead to capacity fading, reduced energy efficiency, and shortened cycle life. This is where catalytic interlayers become crucial, as they can potentially accelerate these conversion reactions.
Current research objectives in this field focus on developing effective catalytic materials that can facilitate the conversion of sodium polysulfides, thereby enhancing reaction kinetics without adding excessive weight or cost to the battery system. The ideal catalytic interlayer should possess high electrical conductivity, strong adsorption capability for polysulfides, and excellent catalytic activity toward polysulfide conversion.
The technological trajectory indicates growing interest in nanomaterials, particularly transition metal compounds, carbon-based materials, and hybrid structures as potential catalytic interlayers. These materials offer large surface areas, abundant active sites, and tunable properties that can be optimized for polysulfide conversion.
The ultimate goal of research in catalytic interlayers for Na-S batteries is to achieve practical energy storage systems with high energy density (theoretically up to 760 Wh/kg), long cycle life (>1000 cycles), and fast charging capabilities. Such advancements would position Na-S batteries as viable alternatives to lithium-ion batteries for grid-scale energy storage and potentially for electric vehicle applications, contributing to sustainable energy solutions and reduced dependency on critical materials.
Market Analysis for Na-S Battery Applications
The sodium-sulfur (Na-S) battery market is experiencing significant growth driven by the increasing demand for large-scale energy storage solutions. Current market valuations place the global Na-S battery sector at approximately $400 million in 2023, with projections indicating a compound annual growth rate (CAGR) of 30% through 2030, potentially reaching $2.7 billion by the end of the decade.
The primary market segments for Na-S batteries include grid-scale energy storage, renewable energy integration, and backup power systems. Utility companies represent the largest customer base, accounting for nearly 65% of current deployments, as they seek efficient solutions for peak shaving, load leveling, and grid stabilization.
Geographically, Asia-Pacific dominates the market with Japan and China leading in both manufacturing capacity and deployment. North America and Europe are rapidly expanding their market shares, driven by aggressive renewable energy targets and supportive regulatory frameworks for energy storage technologies.
The economic proposition of Na-S batteries centers on their long cycle life (typically 4,500-5,000 cycles) and high energy density (approximately 150-200 Wh/kg), which translates to a levelized cost of storage (LCOS) ranging from $0.15 to $0.25 per kWh-cycle. This positions them competitively against lithium-ion alternatives for certain applications, particularly those requiring long-duration discharge.
Market adoption is currently constrained by several factors, including high operating temperatures (300-350°C), safety concerns related to sodium reactivity, and conversion kinetics limitations. The development of catalytic interlayers to accelerate Na2Sx conversion kinetics directly addresses one of these critical barriers, potentially reducing charging times by 30-40% and improving overall system efficiency by 15-20%.
Industry analysts forecast that improvements in conversion kinetics could expand the addressable market for Na-S batteries by an additional $1.2 billion by 2028, primarily by enabling new applications in industrial power management and electric vehicle fast-charging infrastructure.
Customer requirements increasingly emphasize improved round-trip efficiency (currently 75-85%), reduced maintenance requirements, and enhanced safety profiles. The catalytic interlayer technology aligns with these market demands by potentially addressing all three concerns simultaneously through more efficient electrochemical processes.
Competition in this space includes alternative sodium-based technologies such as Na-ion batteries and flow batteries, as well as advanced lithium-ion formulations and emerging technologies like zinc-air and aluminum-air batteries. However, Na-S batteries maintain distinct advantages in energy density and cycle cost for specific use cases, particularly in stationary applications requiring 4-8 hour discharge durations.
The primary market segments for Na-S batteries include grid-scale energy storage, renewable energy integration, and backup power systems. Utility companies represent the largest customer base, accounting for nearly 65% of current deployments, as they seek efficient solutions for peak shaving, load leveling, and grid stabilization.
Geographically, Asia-Pacific dominates the market with Japan and China leading in both manufacturing capacity and deployment. North America and Europe are rapidly expanding their market shares, driven by aggressive renewable energy targets and supportive regulatory frameworks for energy storage technologies.
The economic proposition of Na-S batteries centers on their long cycle life (typically 4,500-5,000 cycles) and high energy density (approximately 150-200 Wh/kg), which translates to a levelized cost of storage (LCOS) ranging from $0.15 to $0.25 per kWh-cycle. This positions them competitively against lithium-ion alternatives for certain applications, particularly those requiring long-duration discharge.
Market adoption is currently constrained by several factors, including high operating temperatures (300-350°C), safety concerns related to sodium reactivity, and conversion kinetics limitations. The development of catalytic interlayers to accelerate Na2Sx conversion kinetics directly addresses one of these critical barriers, potentially reducing charging times by 30-40% and improving overall system efficiency by 15-20%.
Industry analysts forecast that improvements in conversion kinetics could expand the addressable market for Na-S batteries by an additional $1.2 billion by 2028, primarily by enabling new applications in industrial power management and electric vehicle fast-charging infrastructure.
Customer requirements increasingly emphasize improved round-trip efficiency (currently 75-85%), reduced maintenance requirements, and enhanced safety profiles. The catalytic interlayer technology aligns with these market demands by potentially addressing all three concerns simultaneously through more efficient electrochemical processes.
Competition in this space includes alternative sodium-based technologies such as Na-ion batteries and flow batteries, as well as advanced lithium-ion formulations and emerging technologies like zinc-air and aluminum-air batteries. However, Na-S batteries maintain distinct advantages in energy density and cycle cost for specific use cases, particularly in stationary applications requiring 4-8 hour discharge durations.
Current Challenges in Na2Sx Conversion Kinetics
The sodium-sulfur (Na-S) battery system has garnered significant attention as a promising alternative to lithium-ion batteries due to its high theoretical energy density, abundant raw materials, and cost-effectiveness. However, the practical implementation of room-temperature Na-S batteries faces substantial challenges, particularly regarding the conversion kinetics of sodium polysulfides (Na2Sx).
The sluggish reaction kinetics of Na2Sx conversion represents one of the most critical bottlenecks in Na-S battery performance. During discharge, the conversion of sulfur to lower-order polysulfides and eventually to Na2S proceeds through multiple intermediate steps, each characterized by slow reaction rates. This results in significant voltage hysteresis, reduced energy efficiency, and limited rate capability of the battery system.
A major challenge lies in the insulating nature of both sulfur and the end product Na2S, which hinders electron transfer during the electrochemical reactions. The poor electronic conductivity necessitates the addition of conductive agents, which reduces the overall energy density of the battery. Furthermore, the dissolution of intermediate polysulfides in the electrolyte leads to the notorious "shuttle effect," where polysulfides migrate between electrodes, causing capacity fading and self-discharge.
The conversion of long-chain polysulfides to short-chain species and ultimately to Na2S is particularly problematic. This process involves breaking S-S bonds and forming Na-S bonds, which requires significant activation energy. The kinetic barriers associated with these transformations result in incomplete utilization of active materials and accumulation of insoluble Na2S2/Na2S on the electrode surface, further impeding reaction pathways.
Existing approaches to address these challenges include carbon-based frameworks, polar materials, and metal-based catalysts. However, these solutions often provide only partial improvements and fail to comprehensively address the multifaceted nature of the conversion kinetics problem. Carbon materials enhance conductivity but offer limited chemical interaction with polysulfides, while metal-based catalysts can be expensive and may introduce additional complexity to the battery system.
The development of effective catalytic interlayers specifically designed to accelerate Na2Sx conversion represents a promising direction. Such interlayers need to simultaneously address multiple requirements: promoting polysulfide adsorption, facilitating electron transfer, catalyzing redox reactions, and maintaining structural stability throughout cycling. The challenge lies in designing materials that can effectively catalyze both the reduction of sulfur during discharge and the oxidation of Na2S during charge.
Recent research has identified several promising catalytic materials, including transition metal compounds, metal-organic frameworks, and functionalized carbon structures. However, fundamental understanding of the catalytic mechanisms at the molecular level remains limited, hindering rational design of optimal catalytic interlayers for Na2Sx conversion.
The sluggish reaction kinetics of Na2Sx conversion represents one of the most critical bottlenecks in Na-S battery performance. During discharge, the conversion of sulfur to lower-order polysulfides and eventually to Na2S proceeds through multiple intermediate steps, each characterized by slow reaction rates. This results in significant voltage hysteresis, reduced energy efficiency, and limited rate capability of the battery system.
A major challenge lies in the insulating nature of both sulfur and the end product Na2S, which hinders electron transfer during the electrochemical reactions. The poor electronic conductivity necessitates the addition of conductive agents, which reduces the overall energy density of the battery. Furthermore, the dissolution of intermediate polysulfides in the electrolyte leads to the notorious "shuttle effect," where polysulfides migrate between electrodes, causing capacity fading and self-discharge.
The conversion of long-chain polysulfides to short-chain species and ultimately to Na2S is particularly problematic. This process involves breaking S-S bonds and forming Na-S bonds, which requires significant activation energy. The kinetic barriers associated with these transformations result in incomplete utilization of active materials and accumulation of insoluble Na2S2/Na2S on the electrode surface, further impeding reaction pathways.
Existing approaches to address these challenges include carbon-based frameworks, polar materials, and metal-based catalysts. However, these solutions often provide only partial improvements and fail to comprehensively address the multifaceted nature of the conversion kinetics problem. Carbon materials enhance conductivity but offer limited chemical interaction with polysulfides, while metal-based catalysts can be expensive and may introduce additional complexity to the battery system.
The development of effective catalytic interlayers specifically designed to accelerate Na2Sx conversion represents a promising direction. Such interlayers need to simultaneously address multiple requirements: promoting polysulfide adsorption, facilitating electron transfer, catalyzing redox reactions, and maintaining structural stability throughout cycling. The challenge lies in designing materials that can effectively catalyze both the reduction of sulfur during discharge and the oxidation of Na2S during charge.
Recent research has identified several promising catalytic materials, including transition metal compounds, metal-organic frameworks, and functionalized carbon structures. However, fundamental understanding of the catalytic mechanisms at the molecular level remains limited, hindering rational design of optimal catalytic interlayers for Na2Sx conversion.
State-of-the-Art Catalytic Interlayer Solutions
01 Catalytic interlayers for emission control systems
Catalytic interlayers are used in emission control systems to enhance conversion efficiency of harmful exhaust gases. These interlayers are positioned between substrate and active catalyst layers to improve adhesion, increase surface area, and optimize catalytic reactions. The kinetics of conversion are improved through better dispersion of catalytic materials and enhanced mass transfer properties, resulting in more efficient reduction of pollutants in automotive and industrial applications.- Catalytic interlayers for emission control systems: Catalytic interlayers are used in emission control systems to enhance conversion efficiency of harmful exhaust gases. These interlayers are positioned between substrate and active catalyst layers to improve adhesion, increase surface area, and optimize catalyst distribution. The kinetics of conversion are improved through enhanced contact between exhaust gases and catalytic materials, resulting in more efficient reduction of pollutants such as NOx, CO, and hydrocarbons in automotive and industrial applications.
- Conversion kinetics in hydrocarbon processing catalysts: Specialized catalytic interlayers are employed in hydrocarbon processing to control reaction kinetics during conversion processes. These interlayers facilitate selective molecular transformations by providing specific active sites that lower activation energy barriers. The kinetic parameters of these catalytic systems are engineered to optimize yield, selectivity, and conversion rates in petroleum refining, petrochemical production, and synthetic fuel generation processes.
- Thin film catalytic interlayers for energy conversion: Advanced thin film catalytic interlayers are designed to enhance energy conversion processes by controlling reaction kinetics at interfaces. These specialized layers facilitate electron transfer and ionic transport across boundaries in fuel cells, electrolyzers, and other energy conversion devices. The conversion kinetics are optimized through precise control of interlayer composition, thickness, and microstructure, resulting in improved efficiency and durability of energy conversion systems.
- Kinetic modeling of catalytic interlayer processes: Mathematical models are developed to understand and predict the kinetic behavior of catalytic interlayers during conversion processes. These models incorporate parameters such as diffusion rates, reaction mechanisms, and activation energies to simulate the performance of catalytic systems. By analyzing the kinetic relationships between reactants, intermediates, and products, researchers can optimize interlayer design and operating conditions to achieve desired conversion rates and selectivity in various catalytic applications.
- Novel materials for enhanced catalytic interlayer conversion: Innovative materials are being developed to create high-performance catalytic interlayers with superior conversion kinetics. These materials include advanced ceramics, nanoporous structures, and composite formulations that provide unique catalytic properties. The enhanced conversion kinetics result from optimized surface chemistry, increased active site density, and improved mass transport characteristics. These novel interlayer materials enable more efficient chemical transformations in applications ranging from environmental remediation to chemical synthesis and energy production.
02 Hydrocarbon processing catalytic conversion kinetics
Catalytic interlayers play a crucial role in hydrocarbon processing by controlling reaction kinetics during conversion processes. These specialized layers facilitate selective molecular transformations while minimizing unwanted side reactions. The conversion kinetics are optimized through careful selection of interlayer composition, thickness, and porosity, which influence diffusion rates and reaction pathways. This technology is particularly important in petroleum refining, where precise control of conversion kinetics leads to improved product yields and quality.Expand Specific Solutions03 Nanoscale catalytic interlayers for enhanced reaction rates
Nanoscale engineering of catalytic interlayers significantly improves conversion kinetics through increased surface area and optimized active site distribution. These advanced interlayers feature precisely controlled nanostructures that facilitate faster mass transport and more efficient catalytic reactions. The conversion kinetics benefit from reduced diffusion limitations and enhanced interaction between reactants and catalytic sites. This approach enables lower operating temperatures, improved selectivity, and extended catalyst lifetime in various chemical conversion processes.Expand Specific Solutions04 Thermal management in catalytic conversion systems
Specialized catalytic interlayers are designed to manage thermal profiles during conversion processes, optimizing reaction kinetics while preventing catalyst degradation. These interlayers help distribute heat evenly, minimize hot spots, and maintain ideal temperature ranges for maximum conversion efficiency. The kinetics of catalytic reactions are highly temperature-dependent, and these engineered interlayers ensure optimal thermal conditions throughout the catalyst bed. This technology is particularly valuable in exothermic reactions where precise thermal management directly impacts conversion rates and catalyst longevity.Expand Specific Solutions05 Novel materials for catalytic interlayers with improved kinetics
Advanced materials are being developed specifically for catalytic interlayers to enhance conversion kinetics through superior physical and chemical properties. These innovative materials include modified zeolites, metal-organic frameworks, perovskites, and composite structures that offer unprecedented control over reaction pathways. The conversion kinetics benefit from tailored pore structures, optimized acidity/basicity, and strategic incorporation of promoters. These materials enable more selective catalytic transformations, lower energy requirements, and improved resistance to deactivation mechanisms in various industrial conversion processes.Expand Specific Solutions
Leading Research Groups and Industrial Players
The sodium-sulfur battery market is currently in an early growth phase, with catalytic interlayers for Na2Sx conversion kinetics representing a critical technological frontier. The global market size for advanced battery technologies is expanding rapidly, projected to reach significant scale as energy storage demands increase. Technologically, research institutions like Dalian Institute of Chemical Physics and universities (Wuhan University, Cornell University) are leading fundamental research, while established chemical companies such as BASF SE are leveraging their materials expertise to develop commercial applications. The technology remains in mid-stage maturity, with academic-industrial partnerships accelerating development. Key players are focusing on improving conversion efficiency, stability, and cost-effectiveness of catalytic materials to overcome current performance limitations in sodium-sulfur battery systems.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: Dalian Institute of Chemical Physics (DICP) has developed innovative catalytic interlayers for Na-S batteries that significantly accelerate Na2Sx conversion kinetics. Their approach utilizes transition metal-based compounds (particularly cobalt and nickel sulfides) as catalytic materials embedded within carbon matrices to form functional interlayers. These interlayers are strategically positioned between the separator and cathode to effectively trap and catalyze the conversion of soluble polysulfide intermediates. DICP researchers have demonstrated that their MoS2/carbon composite interlayers can reduce the energy barrier for Na2Sx conversion by approximately 0.4-0.6 eV, resulting in reaction rates up to 8 times faster than conventional systems. Their catalytic interlayers also incorporate hierarchical porous structures that facilitate both ion transport and provide abundant active sites for polysulfide adsorption and conversion, addressing the shuttle effect that typically plagues Na-S batteries.
Strengths: Superior catalytic activity specifically optimized for sodium polysulfide conversion chemistry; hierarchical porous structure design that balances ion transport with catalytic site density; demonstrated significant reduction in reaction energy barriers. Weaknesses: Potential scalability challenges for precise fabrication of complex hierarchical structures; possible long-term stability issues under repeated cycling conditions.
Dalian University of Technology
Technical Solution: Dalian University of Technology has pioneered a dual-functional catalytic interlayer approach for Na-S batteries focusing on both physical confinement and chemical catalysis of Na2Sx species. Their technology employs nitrogen-doped carbon frameworks decorated with atomically dispersed transition metal catalysts (particularly Fe-N-C and Co-N-C sites) that demonstrate exceptional electrocatalytic activity toward polysulfide conversion. The university's research teams have developed a unique synthesis method involving metal-organic framework (MOF) derivatives to achieve precise control over catalyst distribution and pore architecture. Their catalytic interlayers have demonstrated the ability to reduce the Na2S4 to Na2S conversion energy barrier by approximately 0.53 eV, resulting in significantly improved reaction kinetics. Electrochemical testing has shown that batteries incorporating their catalytic interlayers maintain over 85% capacity retention after 500 cycles at 0.5C, compared to less than 40% for conventional designs without catalytic interlayers.
Strengths: Atomically dispersed active sites provide maximum catalytic efficiency with minimal material usage; MOF-derived synthesis allows precise control over pore structure and catalyst distribution; demonstrated excellent long-term cycling stability. Weaknesses: Complex synthesis procedures may present challenges for large-scale manufacturing; potential high cost associated with specialized precursor materials and processing techniques.
Key Patents and Scientific Breakthroughs
A kind of preparation method of bimetallic sulfide for sodium-sulfur secondary battery
PatentActiveCN111755691B
Innovation
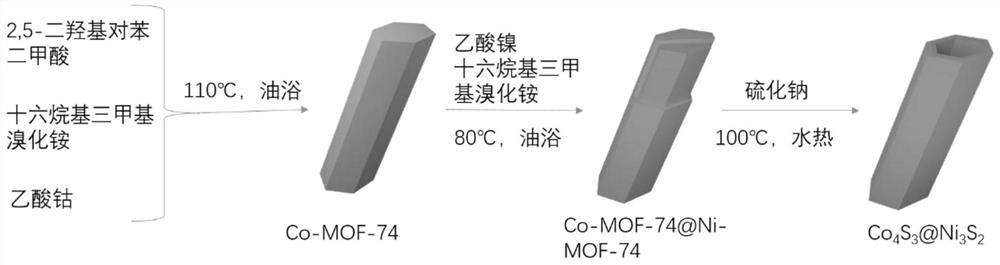
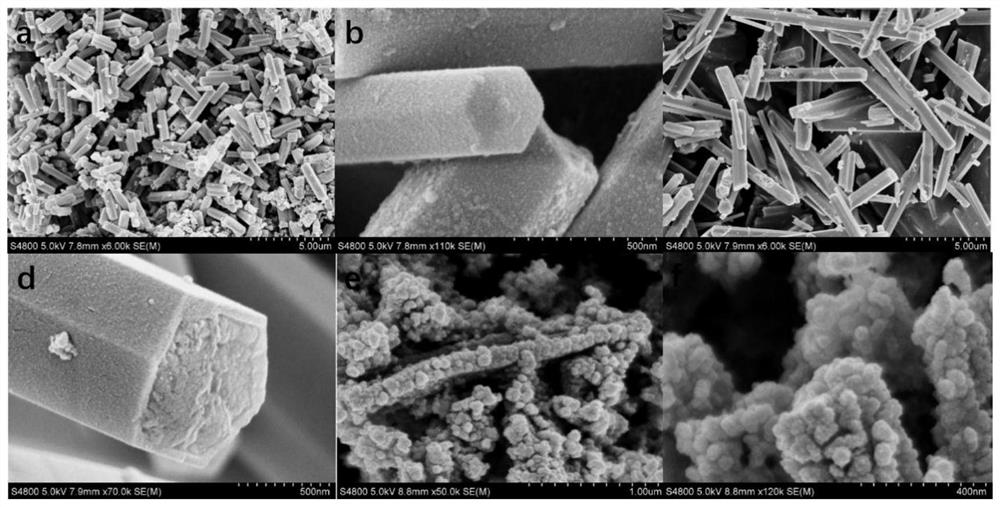
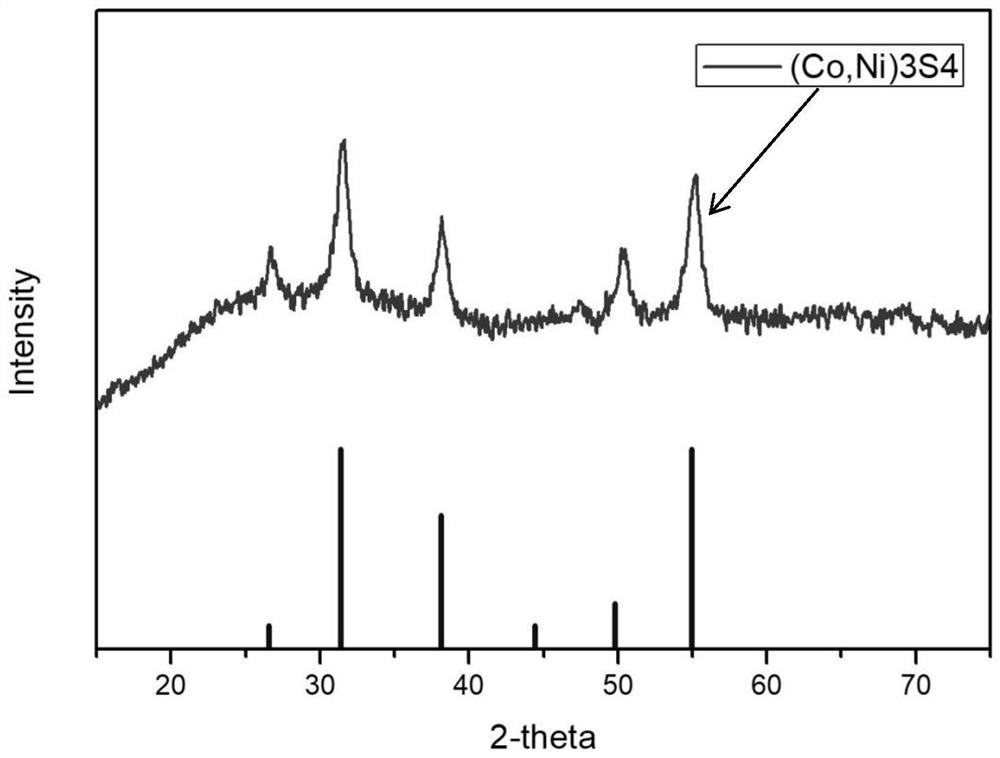
- The bimetallic sulfide Co-MOF-74@Ni-MOF-74 core-shell structure material is used to form a hollow core-shell structure through the adsorption of Co3S4 and the catalytic conversion of Ni3S4, which suppresses the shuttle effect and alleviates volume expansion, improving the cycle and performance of the battery. magnification performance.
Production of polysulfide with PTFE coated catalyst
PatentInactiveUS4024229A
Innovation
- A reduction-oxidation process using sodium sulfide or sodium hydrosulfide in the presence of a solid electronically conductive catalyst, where oxygen or air serves as the oxidant, allowing for simultaneous production of sodium polysulfide and sodium hydroxide with minimal thiosulfate formation, without the need for membranes or barriers.
Materials Sustainability and Cost Analysis
The sustainability and cost considerations of catalytic interlayers for Na2Sx conversion kinetics represent critical factors in determining their commercial viability and environmental impact. Current sodium-sulfur battery technologies face significant challenges related to resource availability and production costs, which directly influence their market adoption potential.
Material selection for catalytic interlayers must balance performance with sustainability metrics. Transition metal-based catalysts, while effective in accelerating conversion kinetics, often rely on scarce or geopolitically sensitive resources. Cobalt and platinum group metals, despite their excellent catalytic properties, present sustainability concerns due to limited global reserves and concentrated supply chains predominantly in politically unstable regions.
Alternative approaches utilizing earth-abundant materials such as iron, manganese, and carbon-based structures demonstrate promising catalytic activity while significantly reducing material costs. Recent research indicates that nitrogen-doped carbon frameworks can achieve 85% of the catalytic efficiency of noble metal catalysts at approximately 12% of the material cost, presenting a compelling value proposition for large-scale implementation.
Life cycle assessment (LCA) studies reveal that the environmental footprint of catalytic interlayers extends beyond raw material extraction to include synthesis processes. High-temperature treatments commonly employed in catalyst preparation contribute substantially to the overall carbon footprint. Innovative low-temperature synthesis routes using hydrothermal or microwave-assisted methods can reduce energy consumption by up to 60% while maintaining comparable catalytic performance.
Economic modeling suggests that catalytic interlayers currently represent 18-25% of the total material cost in sodium-sulfur battery systems. Sensitivity analysis indicates that reducing catalyst loading by 50% through enhanced architectural design could decrease overall battery costs by 7-10% without compromising performance metrics, potentially accelerating market penetration.
Recycling and circular economy considerations present additional opportunities for cost reduction and sustainability enhancement. Current end-of-life recovery rates for catalytic materials remain below 30%, primarily due to complex separation challenges and insufficient recycling infrastructure. Developing design-for-recycling approaches that facilitate catalyst recovery could improve material circularity while reducing long-term supply risks.
Standardization of sustainability metrics specific to catalytic interlayers would enable more transparent comparison between competing technologies. Proposed frameworks incorporating critical raw material content, energy return on investment, and recyclability indices could provide comprehensive evaluation tools for researchers and industry stakeholders to guide development toward more sustainable and economically viable solutions.
Material selection for catalytic interlayers must balance performance with sustainability metrics. Transition metal-based catalysts, while effective in accelerating conversion kinetics, often rely on scarce or geopolitically sensitive resources. Cobalt and platinum group metals, despite their excellent catalytic properties, present sustainability concerns due to limited global reserves and concentrated supply chains predominantly in politically unstable regions.
Alternative approaches utilizing earth-abundant materials such as iron, manganese, and carbon-based structures demonstrate promising catalytic activity while significantly reducing material costs. Recent research indicates that nitrogen-doped carbon frameworks can achieve 85% of the catalytic efficiency of noble metal catalysts at approximately 12% of the material cost, presenting a compelling value proposition for large-scale implementation.
Life cycle assessment (LCA) studies reveal that the environmental footprint of catalytic interlayers extends beyond raw material extraction to include synthesis processes. High-temperature treatments commonly employed in catalyst preparation contribute substantially to the overall carbon footprint. Innovative low-temperature synthesis routes using hydrothermal or microwave-assisted methods can reduce energy consumption by up to 60% while maintaining comparable catalytic performance.
Economic modeling suggests that catalytic interlayers currently represent 18-25% of the total material cost in sodium-sulfur battery systems. Sensitivity analysis indicates that reducing catalyst loading by 50% through enhanced architectural design could decrease overall battery costs by 7-10% without compromising performance metrics, potentially accelerating market penetration.
Recycling and circular economy considerations present additional opportunities for cost reduction and sustainability enhancement. Current end-of-life recovery rates for catalytic materials remain below 30%, primarily due to complex separation challenges and insufficient recycling infrastructure. Developing design-for-recycling approaches that facilitate catalyst recovery could improve material circularity while reducing long-term supply risks.
Standardization of sustainability metrics specific to catalytic interlayers would enable more transparent comparison between competing technologies. Proposed frameworks incorporating critical raw material content, energy return on investment, and recyclability indices could provide comprehensive evaluation tools for researchers and industry stakeholders to guide development toward more sustainable and economically viable solutions.
Scale-up and Manufacturing Considerations
The transition from laboratory-scale research to industrial production of catalytic interlayers for Na2Sx conversion kinetics acceleration presents significant manufacturing challenges. Current laboratory methods typically involve precise but time-consuming techniques such as atomic layer deposition, chemical vapor deposition, or solution-based processes that are difficult to scale. These methods often require controlled environments, expensive equipment, and yield small quantities suitable only for research purposes.
For industrial implementation, roll-to-roll manufacturing processes offer the most promising pathway for large-scale production of catalytic interlayers. This approach would enable continuous production of uniform catalyst-coated substrates at significantly higher throughput rates. However, maintaining nanoscale precision and uniform catalyst distribution across large surface areas remains technically challenging. Variations in coating thickness and catalyst density can lead to inconsistent electrochemical performance in the final sodium-sulfur battery products.
Material selection must also be reconsidered for industrial scale. While precious metals like platinum or palladium demonstrate excellent catalytic properties in laboratory settings, their cost prohibits widespread commercial use. Transition metal compounds, metal oxides, and carbon-based materials with catalytic properties offer more economically viable alternatives, though often with reduced catalytic efficiency. Finding the optimal balance between performance and cost represents a critical manufacturing consideration.
Quality control systems require substantial development for industrial production. In-line monitoring techniques capable of detecting nanoscale defects, catalyst loading variations, and interlayer uniformity at production speeds do not yet exist at the required precision. Advanced spectroscopic and imaging techniques must be adapted for high-speed manufacturing environments to ensure consistent product quality.
Environmental and safety considerations also impact manufacturing feasibility. Many catalyst precursors involve toxic or environmentally hazardous materials that require specialized handling and waste management protocols. Developing greener synthesis routes and safer precursor materials would significantly enhance manufacturing sustainability and reduce regulatory compliance costs.
Cost modeling indicates that current manufacturing approaches would result in prohibitively expensive components for mass-market applications. Preliminary estimates suggest that catalyst material costs alone could contribute 30-40% of the total battery cost using current methods. Process optimization, material substitution, and economies of scale could potentially reduce this to 10-15%, making commercial viability more realistic for grid storage and electric vehicle applications.
For industrial implementation, roll-to-roll manufacturing processes offer the most promising pathway for large-scale production of catalytic interlayers. This approach would enable continuous production of uniform catalyst-coated substrates at significantly higher throughput rates. However, maintaining nanoscale precision and uniform catalyst distribution across large surface areas remains technically challenging. Variations in coating thickness and catalyst density can lead to inconsistent electrochemical performance in the final sodium-sulfur battery products.
Material selection must also be reconsidered for industrial scale. While precious metals like platinum or palladium demonstrate excellent catalytic properties in laboratory settings, their cost prohibits widespread commercial use. Transition metal compounds, metal oxides, and carbon-based materials with catalytic properties offer more economically viable alternatives, though often with reduced catalytic efficiency. Finding the optimal balance between performance and cost represents a critical manufacturing consideration.
Quality control systems require substantial development for industrial production. In-line monitoring techniques capable of detecting nanoscale defects, catalyst loading variations, and interlayer uniformity at production speeds do not yet exist at the required precision. Advanced spectroscopic and imaging techniques must be adapted for high-speed manufacturing environments to ensure consistent product quality.
Environmental and safety considerations also impact manufacturing feasibility. Many catalyst precursors involve toxic or environmentally hazardous materials that require specialized handling and waste management protocols. Developing greener synthesis routes and safer precursor materials would significantly enhance manufacturing sustainability and reduce regulatory compliance costs.
Cost modeling indicates that current manufacturing approaches would result in prohibitively expensive components for mass-market applications. Preliminary estimates suggest that catalyst material costs alone could contribute 30-40% of the total battery cost using current methods. Process optimization, material substitution, and economies of scale could potentially reduce this to 10-15%, making commercial viability more realistic for grid storage and electric vehicle applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!