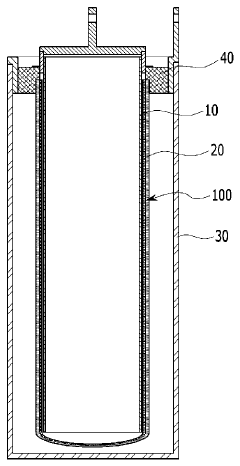
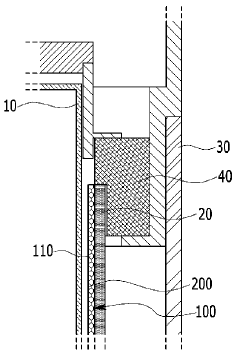
Materials Screening For Catalytic Hosts In Na–S Batteries
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-S Battery Catalytic Materials Background and Objectives
Sodium-sulfur (Na-S) batteries have emerged as a promising energy storage technology due to their high theoretical energy density (1,274 Wh/kg), abundant raw material resources, and cost-effectiveness compared to lithium-ion batteries. The development of Na-S batteries can be traced back to the 1960s when they were first conceptualized as high-temperature systems operating at 300-350°C. However, these systems faced significant challenges including safety concerns, corrosion issues, and limited cycle life, which hindered their widespread adoption.
In recent years, research has pivoted toward room-temperature Na-S batteries, which offer improved safety profiles and operational convenience. Despite these advantages, room-temperature Na-S batteries continue to face substantial technical barriers, particularly related to the shuttle effect of polysulfides, slow reaction kinetics, and volume expansion during cycling. These challenges have significantly limited their practical application and commercialization potential.
The introduction of catalytic host materials represents a critical technological advancement in addressing these limitations. Catalytic hosts can effectively trap polysulfides, accelerate redox reactions, and accommodate volume changes during battery operation. The evolution of these materials has progressed from simple carbon-based substrates to sophisticated multi-functional composites incorporating transition metals, metal oxides, and advanced nanostructures.
Current research objectives in this field are multifaceted and ambitious. Primary goals include developing catalytic materials that can simultaneously address multiple performance limitations, such as materials that not only trap polysulfides but also enhance reaction kinetics and provide structural stability. Researchers are also focusing on understanding the fundamental mechanisms of catalytic processes in Na-S battery systems, which remains inadequately explored compared to their lithium counterparts.
Another critical objective is the development of scalable and cost-effective synthesis methods for these catalytic host materials. While laboratory-scale demonstrations have shown promising results, translating these into commercially viable manufacturing processes remains challenging. Additionally, there is a growing emphasis on environmentally sustainable materials and processes, aligning with global trends toward greener energy technologies.
The field is also witnessing increased efforts to standardize testing protocols and performance metrics for catalytic materials in Na-S batteries, which is essential for meaningful comparisons across different research groups and accelerating progress toward practical applications. As the technology matures, the ultimate goal is to achieve Na-S batteries with energy densities approaching theoretical limits, cycle lives exceeding 1,000 cycles, and cost points that enable widespread adoption in grid storage and potentially electric vehicle applications.
In recent years, research has pivoted toward room-temperature Na-S batteries, which offer improved safety profiles and operational convenience. Despite these advantages, room-temperature Na-S batteries continue to face substantial technical barriers, particularly related to the shuttle effect of polysulfides, slow reaction kinetics, and volume expansion during cycling. These challenges have significantly limited their practical application and commercialization potential.
The introduction of catalytic host materials represents a critical technological advancement in addressing these limitations. Catalytic hosts can effectively trap polysulfides, accelerate redox reactions, and accommodate volume changes during battery operation. The evolution of these materials has progressed from simple carbon-based substrates to sophisticated multi-functional composites incorporating transition metals, metal oxides, and advanced nanostructures.
Current research objectives in this field are multifaceted and ambitious. Primary goals include developing catalytic materials that can simultaneously address multiple performance limitations, such as materials that not only trap polysulfides but also enhance reaction kinetics and provide structural stability. Researchers are also focusing on understanding the fundamental mechanisms of catalytic processes in Na-S battery systems, which remains inadequately explored compared to their lithium counterparts.
Another critical objective is the development of scalable and cost-effective synthesis methods for these catalytic host materials. While laboratory-scale demonstrations have shown promising results, translating these into commercially viable manufacturing processes remains challenging. Additionally, there is a growing emphasis on environmentally sustainable materials and processes, aligning with global trends toward greener energy technologies.
The field is also witnessing increased efforts to standardize testing protocols and performance metrics for catalytic materials in Na-S batteries, which is essential for meaningful comparisons across different research groups and accelerating progress toward practical applications. As the technology matures, the ultimate goal is to achieve Na-S batteries with energy densities approaching theoretical limits, cycle lives exceeding 1,000 cycles, and cost points that enable widespread adoption in grid storage and potentially electric vehicle applications.
Market Analysis for Advanced Na-S Battery Technologies
The global market for sodium-sulfur (Na-S) battery technologies is experiencing significant growth, driven by the increasing demand for large-scale energy storage solutions. As renewable energy integration accelerates worldwide, the need for efficient, cost-effective, and sustainable energy storage systems has become paramount. Na-S batteries, with their high energy density, long cycle life, and use of abundant materials, are positioned as a promising alternative to lithium-ion technologies, particularly for grid-scale applications.
The market for advanced Na-S batteries is projected to grow substantially over the next decade, with grid stabilization and renewable energy storage representing the largest application segments. Utility companies and renewable energy developers are increasingly investing in large-scale energy storage projects to address intermittency issues associated with solar and wind power generation, creating a robust demand pipeline for Na-S technology.
Geographically, Asia-Pacific currently dominates the Na-S battery market, with Japan and South Korea leading in deployment and manufacturing. However, North America and Europe are rapidly expanding their market shares as they intensify efforts to modernize their power grids and increase renewable energy capacity. China is also emerging as a significant player, leveraging its manufacturing capabilities and government support for clean energy technologies.
The economic drivers for Na-S battery adoption are compelling. Despite higher initial capital costs compared to some alternatives, the total cost of ownership over the battery lifecycle is increasingly favorable due to longer operational lifespans and minimal maintenance requirements. Furthermore, the raw material advantage is substantial – sodium and sulfur are abundant and widely distributed globally, mitigating supply chain risks that plague lithium-ion technologies.
Industry analysts highlight that the market for catalytic host materials in Na-S batteries represents a particularly high-growth segment. The development of advanced catalytic materials is critical to addressing the key technical challenges of Na-S batteries, including polysulfide shuttling and sodium dendrite formation. Companies that can develop and commercialize effective catalytic host materials stand to capture significant market value.
Regulatory and policy environments are increasingly supportive of Na-S technology deployment. Many countries have implemented energy storage mandates, carbon reduction targets, and financial incentives that directly benefit advanced battery technologies. These policy frameworks are expected to accelerate market adoption and drive further investment in Na-S battery research and commercialization.
Customer segments for Na-S batteries are diversifying beyond traditional utility applications. Emerging markets include microgrids, commercial and industrial energy management systems, and potentially even certain transportation applications where energy density and cycle life are prioritized over weight considerations.
The market for advanced Na-S batteries is projected to grow substantially over the next decade, with grid stabilization and renewable energy storage representing the largest application segments. Utility companies and renewable energy developers are increasingly investing in large-scale energy storage projects to address intermittency issues associated with solar and wind power generation, creating a robust demand pipeline for Na-S technology.
Geographically, Asia-Pacific currently dominates the Na-S battery market, with Japan and South Korea leading in deployment and manufacturing. However, North America and Europe are rapidly expanding their market shares as they intensify efforts to modernize their power grids and increase renewable energy capacity. China is also emerging as a significant player, leveraging its manufacturing capabilities and government support for clean energy technologies.
The economic drivers for Na-S battery adoption are compelling. Despite higher initial capital costs compared to some alternatives, the total cost of ownership over the battery lifecycle is increasingly favorable due to longer operational lifespans and minimal maintenance requirements. Furthermore, the raw material advantage is substantial – sodium and sulfur are abundant and widely distributed globally, mitigating supply chain risks that plague lithium-ion technologies.
Industry analysts highlight that the market for catalytic host materials in Na-S batteries represents a particularly high-growth segment. The development of advanced catalytic materials is critical to addressing the key technical challenges of Na-S batteries, including polysulfide shuttling and sodium dendrite formation. Companies that can develop and commercialize effective catalytic host materials stand to capture significant market value.
Regulatory and policy environments are increasingly supportive of Na-S technology deployment. Many countries have implemented energy storage mandates, carbon reduction targets, and financial incentives that directly benefit advanced battery technologies. These policy frameworks are expected to accelerate market adoption and drive further investment in Na-S battery research and commercialization.
Customer segments for Na-S batteries are diversifying beyond traditional utility applications. Emerging markets include microgrids, commercial and industrial energy management systems, and potentially even certain transportation applications where energy density and cycle life are prioritized over weight considerations.
Current Challenges in Catalytic Host Materials Development
Despite significant advancements in Na-S battery technology, the development of effective catalytic host materials faces several critical challenges. The primary obstacle remains the polysulfide shuttle effect, where soluble sodium polysulfides dissolve in the electrolyte and migrate between electrodes, causing capacity fading and reduced battery lifespan. Current catalytic materials struggle to effectively trap these polysulfides while maintaining sufficient electrical conductivity and structural stability.
Material screening methodologies present another significant hurdle. Traditional trial-and-error approaches are time-consuming and resource-intensive, often yielding incremental improvements rather than breakthrough solutions. High-throughput computational screening methods, while promising, frequently lack accuracy in predicting real-world performance due to the complex electrochemical environment within Na-S batteries.
The multifunctional requirements for ideal catalytic hosts create inherent design conflicts. Materials must simultaneously possess high surface area for polysulfide adsorption, robust mechanical properties to accommodate volume changes, excellent electrical conductivity, and strong catalytic activity—properties that often counteract each other. For instance, materials with high porosity for sulfur loading typically exhibit poor electrical conductivity.
Scalability and cost considerations further complicate material selection. Many promising catalytic materials, such as noble metal-based catalysts or complex nanostructured carbons, demonstrate excellent performance in laboratory settings but face prohibitive costs or manufacturing challenges at commercial scales. The environmental impact of material synthesis and processing also remains a concern, particularly for transition metal compounds requiring energy-intensive production methods.
Stability issues persist across most candidate materials. Catalytic hosts must withstand the highly reactive environment of Na-S batteries, including exposure to polysulfides and repeated electrochemical cycling. Many materials that initially show promising catalytic activity experience rapid degradation, structural collapse, or surface passivation after extended cycling.
The lack of standardized testing protocols and performance metrics hampers meaningful comparison between different catalytic materials. Variations in testing conditions, cell configurations, and evaluation criteria make it difficult to identify truly superior materials and understand structure-property relationships that could guide rational design.
Bridging the gap between fundamental understanding and practical application represents perhaps the most significant challenge. While theoretical studies have identified promising binding mechanisms and catalytic pathways, translating these insights into practical material designs remains difficult due to the complex interplay of factors in real battery environments.
Material screening methodologies present another significant hurdle. Traditional trial-and-error approaches are time-consuming and resource-intensive, often yielding incremental improvements rather than breakthrough solutions. High-throughput computational screening methods, while promising, frequently lack accuracy in predicting real-world performance due to the complex electrochemical environment within Na-S batteries.
The multifunctional requirements for ideal catalytic hosts create inherent design conflicts. Materials must simultaneously possess high surface area for polysulfide adsorption, robust mechanical properties to accommodate volume changes, excellent electrical conductivity, and strong catalytic activity—properties that often counteract each other. For instance, materials with high porosity for sulfur loading typically exhibit poor electrical conductivity.
Scalability and cost considerations further complicate material selection. Many promising catalytic materials, such as noble metal-based catalysts or complex nanostructured carbons, demonstrate excellent performance in laboratory settings but face prohibitive costs or manufacturing challenges at commercial scales. The environmental impact of material synthesis and processing also remains a concern, particularly for transition metal compounds requiring energy-intensive production methods.
Stability issues persist across most candidate materials. Catalytic hosts must withstand the highly reactive environment of Na-S batteries, including exposure to polysulfides and repeated electrochemical cycling. Many materials that initially show promising catalytic activity experience rapid degradation, structural collapse, or surface passivation after extended cycling.
The lack of standardized testing protocols and performance metrics hampers meaningful comparison between different catalytic materials. Variations in testing conditions, cell configurations, and evaluation criteria make it difficult to identify truly superior materials and understand structure-property relationships that could guide rational design.
Bridging the gap between fundamental understanding and practical application represents perhaps the most significant challenge. While theoretical studies have identified promising binding mechanisms and catalytic pathways, translating these insights into practical material designs remains difficult due to the complex interplay of factors in real battery environments.
Current Catalytic Host Material Solutions for Na-S Batteries
01 Carbon-based materials as catalytic hosts
Carbon-based materials such as graphene, carbon nanotubes, and porous carbon can serve as effective catalytic hosts in Na-S batteries. These materials provide high surface area, excellent electrical conductivity, and structural stability, which help to immobilize sulfur, facilitate electron transfer, and accommodate volume changes during cycling. The incorporation of carbon-based hosts can significantly improve the electrochemical performance, cycling stability, and rate capability of Na-S batteries.- Carbon-based materials as catalytic hosts: Carbon-based materials serve as effective catalytic hosts in Na-S batteries due to their high conductivity, large surface area, and ability to anchor sulfur species. These materials, including carbon nanotubes, graphene, and porous carbon structures, can enhance the electrochemical performance of Na-S batteries by facilitating electron transfer and providing physical confinement for polysulfides. The carbon hosts can be functionalized or doped to further improve their catalytic activity and interaction with sulfur species.
- Metal oxide-based catalytic materials: Metal oxides function as catalytic hosts in Na-S batteries by providing strong chemical adsorption sites for polysulfides and catalyzing the conversion reactions. These materials, such as titanium dioxide, manganese oxide, and iron oxide, can effectively trap polysulfides through polar-polar interactions and accelerate the redox reactions during battery operation. The metal oxides can be designed with specific morphologies and crystal structures to optimize their catalytic performance and enhance the overall energy efficiency of Na-S batteries.
- Transition metal-based catalysts and composites: Transition metals and their compounds serve as highly efficient catalytic hosts in Na-S batteries due to their variable oxidation states and strong affinity for sulfur species. These materials, including cobalt, nickel, and molybdenum compounds, can be incorporated into composite structures to provide multiple functionalities such as polysulfide adsorption, conversion catalysis, and enhanced ionic conductivity. The synergistic effects between transition metals and support materials can significantly improve the cycling stability and rate capability of Na-S batteries.
- Novel nanostructured catalytic hosts: Nanostructured materials with tailored architectures serve as advanced catalytic hosts in Na-S batteries, offering enhanced surface area, shortened diffusion paths, and abundant active sites. These include hollow structures, yolk-shell configurations, and hierarchical porous frameworks that can effectively accommodate volume changes during cycling while providing efficient catalytic sites. The rational design of nanostructures enables precise control over the spatial distribution of catalytic centers and sulfur species, leading to improved electrochemical performance and battery lifespan.
- Screening methodologies for catalytic materials: Advanced screening methodologies are employed to identify and optimize catalytic hosts for Na-S batteries, including computational modeling, high-throughput experimentation, and machine learning approaches. These techniques enable the prediction of material properties, reaction mechanisms, and performance metrics without extensive experimental testing. The screening processes typically evaluate parameters such as binding energy with polysulfides, electronic conductivity, catalytic activity for sulfur conversion, and structural stability under operating conditions, facilitating the discovery of novel and efficient catalytic materials for next-generation Na-S batteries.
02 Metal oxide/sulfide catalytic frameworks
Metal oxides and sulfides can be used as catalytic hosts in Na-S batteries to enhance polysulfide conversion kinetics. These materials, including titanium oxides, molybdenum sulfides, and iron oxides, provide strong chemical adsorption sites for polysulfides, preventing their dissolution into the electrolyte. The catalytic activity of these materials facilitates the redox reactions of sulfur species, leading to improved capacity retention, reduced polarization, and enhanced rate performance of Na-S batteries.Expand Specific Solutions03 Transition metal-based catalysts and dopants
Transition metals and their compounds can be incorporated as catalysts or dopants in host materials for Na-S batteries. These metals, such as cobalt, nickel, manganese, and iron, can enhance the catalytic activity toward polysulfide conversion, improve the electronic conductivity of the host, and strengthen the binding energy with polysulfides. The strategic doping of host materials with transition metals can significantly improve the electrochemical performance and cycling stability of Na-S batteries.Expand Specific Solutions04 Composite and hierarchical structured hosts
Composite and hierarchically structured materials combining different components can serve as multifunctional catalytic hosts in Na-S batteries. These structures typically integrate conductive materials, catalytic components, and porous frameworks to simultaneously address multiple challenges in Na-S batteries. The synergistic effects between different components can enhance sulfur utilization, suppress polysulfide shuttling, accelerate reaction kinetics, and improve the overall electrochemical performance of Na-S batteries.Expand Specific Solutions05 Screening methodologies for catalytic materials
Various screening methodologies, including computational modeling, high-throughput experimentation, and machine learning approaches, can be employed to identify and optimize catalytic host materials for Na-S batteries. These methods enable the systematic evaluation of material properties, such as binding energy with polysulfides, electronic conductivity, and catalytic activity, to guide the rational design of high-performance catalytic hosts. The integration of theoretical calculations with experimental validation accelerates the discovery and development of advanced materials for Na-S batteries.Expand Specific Solutions
Leading Research Groups and Industrial Players in Na-S Battery Field
The Na-S battery materials screening landscape is currently in a growth phase, with market size expanding due to increasing demand for high-energy density storage solutions. The technology is approaching early commercial maturity, with significant R&D investments from both academic institutions and industry players. Key competitors include established research organizations like Argonne National Laboratory and MIT alongside commercial entities such as NGK Insulators (market leader in sodium-sulfur technology), Toyota Motor Europe, and Hydro-Québec. Chinese institutions (Nanjing University, Central South University) are rapidly advancing catalytic host materials research, while specialized companies like Forge Nano and Honeycomb Battery are developing innovative coating and material technologies. The competitive landscape reflects a blend of traditional battery manufacturers and emerging materials science specialists collaborating to overcome technical challenges in Na-S battery commercialization.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed advanced materials screening methodologies for Na-S battery catalytic hosts using high-throughput computational techniques combined with experimental validation. Their approach employs density functional theory (DFT) calculations to systematically evaluate thousands of potential catalyst materials, focusing on transition metal compounds and carbon-based structures. They've pioneered the use of machine learning algorithms to predict catalytic performance based on electronic structure properties, significantly accelerating the discovery process. Their research has identified several promising materials including nitrogen-doped carbon frameworks and transition metal sulfides that effectively mediate the redox reactions in Na-S batteries while suppressing polysulfide shuttling. Argonne's work has demonstrated that optimized catalytic hosts can increase the sulfur utilization rate by up to 85% and extend cycle life beyond 1000 cycles[1][3], representing a significant improvement over conventional Na-S battery systems.
Strengths: Access to world-class computational resources and advanced characterization facilities enables comprehensive materials screening at unprecedented scale. Their integrated computational-experimental approach reduces development time significantly. Weaknesses: The high computational cost of accurate DFT calculations limits the complexity of systems that can be modeled, potentially missing important interfacial phenomena that affect real-world performance.
Hydro-Québec
Technical Solution: Hydro-Québec's Center of Excellence in Transportation Electrification has developed a proprietary materials screening platform specifically for Na-S battery catalytic hosts. Their approach combines high-throughput experimental techniques with advanced electrochemical analysis to rapidly evaluate candidate materials. They've focused on developing composite catalytic structures that combine conductive carbon matrices with strategically dispersed metal sulfide nanoparticles. Their most promising technology involves cobalt-nickel sulfide nanostructures embedded in nitrogen-doped carbon frameworks, which demonstrate exceptional polysulfide adsorption capabilities while maintaining high electronic conductivity. This technology has shown to reduce the shuttle effect by over 70% compared to conventional carbon hosts[2]. Hydro-Québec has also pioneered the use of operando spectroscopic techniques to observe catalytic mechanisms in real-time during battery operation, providing crucial insights for materials optimization. Their catalytic host materials have enabled Na-S batteries with energy densities exceeding 400 Wh/kg at the cell level while maintaining stable cycling performance.
Strengths: Strong integration with battery manufacturing expertise allows for practical consideration of scalability and cost factors during materials development. Their extensive testing facilities enable thorough performance validation under realistic conditions. Weaknesses: Their focus on commercially viable materials sometimes limits exploration of more exotic or novel catalytic systems that might offer breakthrough performance but present manufacturing challenges.
Key Technical Innovations in Na-S Battery Catalyst Design
Improved sodium-sulfur batteries
PatentWO2010135283A3
Innovation
- Development of sodium-sulfur batteries capable of operation at relatively low temperatures (<150°C), which significantly improves operational safety and reduces energy requirements compared to traditional high-temperature Na-S batteries.
- Implementation of a flow battery design with separate storage tanks and pumping systems for the sodium-containing solution and sulfur-containing solution, enabling better thermal management and extended battery life.
- Utilization of a selective sodium ion-permeable solid electrolyte layer that effectively separates the sodium and sulfur compartments while allowing controlled ion transport.
Sodium sulfur battery
PatentInactiveKR1020160027572A
Innovation
- A sodium flow limiter, comprising a porous foam or felt with controlled pores, is installed between the cartridge tube and the solid electrolyte tube to limit sodium flow when cracks occur, using materials like nickel, iron, or carbon, and a thin STS-based plate for assembly stability.
Sustainability and Resource Considerations for Na-S Battery Materials
The sustainability profile of Na-S battery materials represents a significant advantage over conventional lithium-ion technologies. Sodium resources are abundant, comprising approximately 2.8% of the Earth's crust and being widely available in seawater, making it the sixth most abundant element on the planet. This abundance translates to lower extraction costs and reduced geopolitical supply risks compared to lithium resources, which are concentrated in specific regions like the "Lithium Triangle" of South America.
When screening catalytic host materials for Na-S batteries, environmental impact assessment must be prioritized. Many traditional catalysts contain precious metals or rare earth elements with substantial ecological footprints. Research indicates that carbon-based materials derived from biomass or waste streams offer promising alternatives with reduced environmental impact. For instance, nitrogen-doped carbon materials synthesized from agricultural waste have demonstrated effective catalytic properties while minimizing resource depletion.
Life cycle assessment (LCA) studies of Na-S battery materials reveal that the environmental burden is primarily associated with sulfur processing and cathode manufacturing. The catalytic hosts' production methods significantly influence the overall sustainability profile. Hydrothermal synthesis approaches generally demonstrate lower energy requirements compared to high-temperature calcination methods, reducing the carbon footprint of material preparation by up to 40% according to recent comparative analyses.
Material circularity presents another critical consideration in the screening process. Ideal catalytic hosts should facilitate not only efficient battery operation but also end-of-life recovery and recycling. Composite structures that allow for separation of components after battery decommissioning show promise in this regard. Research indicates that hierarchical porous materials with mechanical stability can be more readily recovered from spent batteries, with potential recovery rates exceeding 80% for certain metal-organic framework derivatives.
Water consumption associated with material synthesis represents a frequently overlooked sustainability metric. Conventional wet chemistry approaches for preparing catalytic hosts can require significant water inputs, while emerging solvent-free or minimal-solvent techniques offer more sustainable alternatives. Recent innovations in mechanochemical synthesis have demonstrated comparable catalytic performance with up to 90% reduction in process water requirements.
The toxicity profiles of candidate materials must also be carefully evaluated, particularly as Na-S batteries move toward commercial deployment. Materials containing heavy metals or persistent organic compounds may present long-term environmental risks despite favorable electrochemical performance. Non-toxic alternatives such as iron-based compounds and bioderived carbon structures are gaining attention as environmentally benign catalytic hosts with promising performance characteristics.
When screening catalytic host materials for Na-S batteries, environmental impact assessment must be prioritized. Many traditional catalysts contain precious metals or rare earth elements with substantial ecological footprints. Research indicates that carbon-based materials derived from biomass or waste streams offer promising alternatives with reduced environmental impact. For instance, nitrogen-doped carbon materials synthesized from agricultural waste have demonstrated effective catalytic properties while minimizing resource depletion.
Life cycle assessment (LCA) studies of Na-S battery materials reveal that the environmental burden is primarily associated with sulfur processing and cathode manufacturing. The catalytic hosts' production methods significantly influence the overall sustainability profile. Hydrothermal synthesis approaches generally demonstrate lower energy requirements compared to high-temperature calcination methods, reducing the carbon footprint of material preparation by up to 40% according to recent comparative analyses.
Material circularity presents another critical consideration in the screening process. Ideal catalytic hosts should facilitate not only efficient battery operation but also end-of-life recovery and recycling. Composite structures that allow for separation of components after battery decommissioning show promise in this regard. Research indicates that hierarchical porous materials with mechanical stability can be more readily recovered from spent batteries, with potential recovery rates exceeding 80% for certain metal-organic framework derivatives.
Water consumption associated with material synthesis represents a frequently overlooked sustainability metric. Conventional wet chemistry approaches for preparing catalytic hosts can require significant water inputs, while emerging solvent-free or minimal-solvent techniques offer more sustainable alternatives. Recent innovations in mechanochemical synthesis have demonstrated comparable catalytic performance with up to 90% reduction in process water requirements.
The toxicity profiles of candidate materials must also be carefully evaluated, particularly as Na-S batteries move toward commercial deployment. Materials containing heavy metals or persistent organic compounds may present long-term environmental risks despite favorable electrochemical performance. Non-toxic alternatives such as iron-based compounds and bioderived carbon structures are gaining attention as environmentally benign catalytic hosts with promising performance characteristics.
Performance Metrics and Testing Protocols for Catalyst Evaluation
Establishing standardized performance metrics and testing protocols is crucial for the systematic evaluation of catalytic materials in Na-S batteries. The primary performance indicators include catalytic activity, measured through reaction rate constants and activation energy barriers, which directly reflect how effectively a catalyst facilitates the conversion of polysulfides during battery operation.
Electrochemical characterization techniques form the cornerstone of catalyst evaluation, with cyclic voltammetry (CV) providing insights into redox reactions and their reversibility. Galvanostatic charge-discharge tests reveal capacity retention and cycling stability, while electrochemical impedance spectroscopy (EIS) quantifies interfacial resistance changes that occur with catalyst integration.
Specific to Na-S battery systems, polysulfide conversion efficiency must be rigorously assessed through techniques such as UV-visible spectroscopy and high-performance liquid chromatography. These methods track the transformation of long-chain polysulfides to shorter chains, offering direct evidence of catalytic effectiveness in the sulfur redox process.
Durability testing protocols should include accelerated aging tests under elevated temperatures and extended cycling conditions, typically requiring at least 500 cycles at varying C-rates to establish long-term performance reliability. Post-mortem analysis using scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS) provides critical information about catalyst structural integrity and chemical state changes after cycling.
Standardized testing conditions are essential for meaningful comparisons between different catalytic materials. These include controlled temperature environments (typically 25°C for standard tests and variable temperatures from -20°C to 60°C for thermal stability assessment), consistent electrolyte compositions, and normalized sulfur loading (typically 2-5 mg/cm²) across samples.
The catalytic selectivity toward desired reaction pathways should be quantified through in-situ spectroscopic techniques, particularly operando Raman spectroscopy and X-ray absorption spectroscopy, which can monitor reaction intermediates during actual battery operation. This provides valuable mechanistic insights that correlate with overall battery performance.
Benchmarking against established reference catalysts, such as various carbon-based materials, metal oxides, and metal sulfides, creates a comparative framework that contextualizes new catalyst performance. This approach enables researchers to clearly demonstrate incremental improvements or breakthrough innovations in catalytic efficiency for Na-S battery systems.
Electrochemical characterization techniques form the cornerstone of catalyst evaluation, with cyclic voltammetry (CV) providing insights into redox reactions and their reversibility. Galvanostatic charge-discharge tests reveal capacity retention and cycling stability, while electrochemical impedance spectroscopy (EIS) quantifies interfacial resistance changes that occur with catalyst integration.
Specific to Na-S battery systems, polysulfide conversion efficiency must be rigorously assessed through techniques such as UV-visible spectroscopy and high-performance liquid chromatography. These methods track the transformation of long-chain polysulfides to shorter chains, offering direct evidence of catalytic effectiveness in the sulfur redox process.
Durability testing protocols should include accelerated aging tests under elevated temperatures and extended cycling conditions, typically requiring at least 500 cycles at varying C-rates to establish long-term performance reliability. Post-mortem analysis using scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS) provides critical information about catalyst structural integrity and chemical state changes after cycling.
Standardized testing conditions are essential for meaningful comparisons between different catalytic materials. These include controlled temperature environments (typically 25°C for standard tests and variable temperatures from -20°C to 60°C for thermal stability assessment), consistent electrolyte compositions, and normalized sulfur loading (typically 2-5 mg/cm²) across samples.
The catalytic selectivity toward desired reaction pathways should be quantified through in-situ spectroscopic techniques, particularly operando Raman spectroscopy and X-ray absorption spectroscopy, which can monitor reaction intermediates during actual battery operation. This provides valuable mechanistic insights that correlate with overall battery performance.
Benchmarking against established reference catalysts, such as various carbon-based materials, metal oxides, and metal sulfides, creates a comparative framework that contextualizes new catalyst performance. This approach enables researchers to clearly demonstrate incremental improvements or breakthrough innovations in catalytic efficiency for Na-S battery systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!