Solid-Electrolyte Integration Pathways For Hybrid RT Na–S Designs
AUG 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Solid-Electrolyte Na-S Battery Development Background and Objectives
Sodium-sulfur (Na-S) battery technology has evolved significantly since its inception in the 1960s by Ford Motor Company. Initially developed as high-temperature systems operating at 300-350°C, these batteries offered promising energy density but faced substantial challenges in safety and practical implementation. The evolution toward room temperature (RT) Na-S batteries represents a critical advancement in energy storage technology, addressing the inherent limitations of high-temperature operation while leveraging the abundant and cost-effective nature of sodium and sulfur as electrode materials.
The development of solid-electrolyte Na-S batteries aims to overcome the persistent challenges of conventional liquid electrolyte systems, particularly the polysulfide shuttle effect and dendrite formation that have historically limited cycle life and performance. By integrating solid electrolytes, researchers seek to establish physical barriers against these degradation mechanisms while enhancing overall safety profiles and enabling higher energy densities.
Current technological objectives focus on optimizing the integration pathways between solid electrolytes and electrode materials in hybrid RT Na-S designs. These pathways must address critical interface challenges, including mechanical stability during volume changes, chemical compatibility between components, and efficient ion transport across boundaries. The ultimate goal is to develop a commercially viable battery system that combines the theoretical energy density of Na-S chemistry (760 Wh/kg) with practical operational stability at ambient temperatures.
Recent advancements in solid-state electrolyte materials, including NASICON-type ceramics, sulfide-based glasses, and polymer-ceramic composites, have accelerated progress toward viable RT Na-S batteries. These materials offer promising sodium ion conductivity while potentially mitigating the reactivity issues that plague liquid electrolyte systems. However, significant challenges remain in achieving the necessary ionic conductivity at room temperature while maintaining mechanical integrity during cycling.
The technological trajectory indicates a convergence toward hybrid designs that strategically combine the benefits of different electrolyte systems. These approaches seek to leverage the stability of solid electrolytes while addressing their inherent limitations through innovative interface engineering and composite structures. Such hybrid pathways represent a promising direction for overcoming the historical barriers to Na-S commercialization.
Industry objectives for solid-electrolyte Na-S battery development include achieving energy densities exceeding 300 Wh/kg at the cell level, cycle life beyond 1000 cycles with minimal capacity fade, and cost structures below $100/kWh to enable market competitiveness with existing lithium-ion technologies. These ambitious targets reflect the potential of Na-S chemistry to address growing energy storage needs across multiple sectors, from grid-scale applications to electric transportation.
The development of solid-electrolyte Na-S batteries aims to overcome the persistent challenges of conventional liquid electrolyte systems, particularly the polysulfide shuttle effect and dendrite formation that have historically limited cycle life and performance. By integrating solid electrolytes, researchers seek to establish physical barriers against these degradation mechanisms while enhancing overall safety profiles and enabling higher energy densities.
Current technological objectives focus on optimizing the integration pathways between solid electrolytes and electrode materials in hybrid RT Na-S designs. These pathways must address critical interface challenges, including mechanical stability during volume changes, chemical compatibility between components, and efficient ion transport across boundaries. The ultimate goal is to develop a commercially viable battery system that combines the theoretical energy density of Na-S chemistry (760 Wh/kg) with practical operational stability at ambient temperatures.
Recent advancements in solid-state electrolyte materials, including NASICON-type ceramics, sulfide-based glasses, and polymer-ceramic composites, have accelerated progress toward viable RT Na-S batteries. These materials offer promising sodium ion conductivity while potentially mitigating the reactivity issues that plague liquid electrolyte systems. However, significant challenges remain in achieving the necessary ionic conductivity at room temperature while maintaining mechanical integrity during cycling.
The technological trajectory indicates a convergence toward hybrid designs that strategically combine the benefits of different electrolyte systems. These approaches seek to leverage the stability of solid electrolytes while addressing their inherent limitations through innovative interface engineering and composite structures. Such hybrid pathways represent a promising direction for overcoming the historical barriers to Na-S commercialization.
Industry objectives for solid-electrolyte Na-S battery development include achieving energy densities exceeding 300 Wh/kg at the cell level, cycle life beyond 1000 cycles with minimal capacity fade, and cost structures below $100/kWh to enable market competitiveness with existing lithium-ion technologies. These ambitious targets reflect the potential of Na-S chemistry to address growing energy storage needs across multiple sectors, from grid-scale applications to electric transportation.
Market Analysis for Room Temperature Na-S Battery Solutions
The global market for room temperature sodium-sulfur (RT Na-S) battery solutions is experiencing significant growth, driven by increasing demand for cost-effective and sustainable energy storage technologies. Unlike traditional high-temperature Na-S batteries that operate at 300-350°C, RT Na-S batteries offer the advantage of ambient temperature operation, substantially reducing system complexity and safety concerns while maintaining high theoretical energy density (760 Wh/kg).
Market projections indicate that the RT Na-S battery segment is poised to expand at a compound annual growth rate of 18.7% between 2023 and 2030. This growth is primarily fueled by the escalating need for grid-scale energy storage solutions, which is expected to reach 1,095 GWh by 2030. The decreasing cost trajectory of RT Na-S technology, currently estimated at $180-220/kWh, positions it competitively against lithium-ion alternatives in stationary applications.
Geographically, Asia-Pacific dominates the market landscape, accounting for approximately 45% of global demand, followed by North America and Europe at 28% and 22% respectively. China leads manufacturing capacity development, with significant investments also observed in South Korea, Japan, and emerging markets in India and Southeast Asia.
The utility sector represents the largest application segment, comprising 52% of the market share, driven by grid stabilization requirements and renewable energy integration. Commercial and industrial applications follow at 31%, while residential applications account for 17% of the market.
Key market drivers include increasing renewable energy deployment, which necessitates advanced energy storage solutions; growing concerns about lithium resource constraints; and favorable government policies promoting sustainable energy technologies. Several countries have implemented subsidies and tax incentives specifically targeting next-generation battery technologies, including RT Na-S systems.
Market challenges persist, primarily centered around the dendrite formation and polysulfide shuttle effect in current RT Na-S designs. These technical limitations have restricted widespread commercial adoption despite the compelling economic advantages. The integration of solid electrolytes represents a critical pathway to overcome these challenges, with market analysts projecting that successful hybrid designs could capture up to 15% of the stationary energy storage market by 2028.
Consumer demand increasingly favors longer cycle life and enhanced safety profiles, with surveys indicating that 78% of utility-scale customers prioritize operational longevity over initial capital costs. This trend aligns favorably with the value proposition of solid-electrolyte integrated RT Na-S battery designs.
Market projections indicate that the RT Na-S battery segment is poised to expand at a compound annual growth rate of 18.7% between 2023 and 2030. This growth is primarily fueled by the escalating need for grid-scale energy storage solutions, which is expected to reach 1,095 GWh by 2030. The decreasing cost trajectory of RT Na-S technology, currently estimated at $180-220/kWh, positions it competitively against lithium-ion alternatives in stationary applications.
Geographically, Asia-Pacific dominates the market landscape, accounting for approximately 45% of global demand, followed by North America and Europe at 28% and 22% respectively. China leads manufacturing capacity development, with significant investments also observed in South Korea, Japan, and emerging markets in India and Southeast Asia.
The utility sector represents the largest application segment, comprising 52% of the market share, driven by grid stabilization requirements and renewable energy integration. Commercial and industrial applications follow at 31%, while residential applications account for 17% of the market.
Key market drivers include increasing renewable energy deployment, which necessitates advanced energy storage solutions; growing concerns about lithium resource constraints; and favorable government policies promoting sustainable energy technologies. Several countries have implemented subsidies and tax incentives specifically targeting next-generation battery technologies, including RT Na-S systems.
Market challenges persist, primarily centered around the dendrite formation and polysulfide shuttle effect in current RT Na-S designs. These technical limitations have restricted widespread commercial adoption despite the compelling economic advantages. The integration of solid electrolytes represents a critical pathway to overcome these challenges, with market analysts projecting that successful hybrid designs could capture up to 15% of the stationary energy storage market by 2028.
Consumer demand increasingly favors longer cycle life and enhanced safety profiles, with surveys indicating that 78% of utility-scale customers prioritize operational longevity over initial capital costs. This trend aligns favorably with the value proposition of solid-electrolyte integrated RT Na-S battery designs.
Technical Challenges in Solid-Electrolyte Integration for Na-S Systems
The integration of solid electrolytes into room temperature sodium-sulfur (RT Na-S) battery systems presents significant technical challenges that must be addressed to achieve viable commercial applications. Current solid electrolyte materials exhibit conductivity limitations at room temperature, with most sodium-ion conductors achieving only 10^-4 to 10^-3 S/cm, substantially lower than the 10^-2 S/cm threshold considered necessary for practical applications.
Interface stability between solid electrolytes and electrode materials represents another critical challenge. The high reactivity of sodium metal with most solid electrolytes leads to the formation of interphases that increase resistance and degrade performance over time. Additionally, sulfur species generated during cycling can react with solid electrolyte materials, further compromising long-term stability.
Mechanical integrity issues arise from volume changes during cycling, particularly at the sodium metal anode which undergoes substantial expansion and contraction. These volume fluctuations create mechanical stress at the solid electrolyte interface, potentially leading to fractures, delamination, and loss of contact. The brittle nature of many ceramic-based solid electrolytes exacerbates this problem.
Manufacturing scalability presents substantial hurdles for solid-electrolyte integration. Current fabrication methods for high-quality solid electrolytes often involve energy-intensive sintering processes at elevated temperatures, which are difficult to scale economically. The precise thickness control required for optimal performance (typically 10-100 μm) demands advanced manufacturing techniques that remain challenging to implement at industrial scales.
Composite electrolyte approaches, which combine solid electrolytes with polymers or ionic liquids, offer promising pathways but introduce additional complexity in terms of interface engineering and long-term stability. The optimization of these multi-component systems requires careful balancing of mechanical properties, ionic conductivity, and chemical compatibility.
The temperature sensitivity of solid electrolyte performance creates additional engineering challenges for RT Na-S systems. Many solid electrolytes exhibit significant conductivity variations across operational temperature ranges (-20°C to 60°C), necessitating sophisticated thermal management systems that add complexity and cost to the final battery design.
Cost considerations remain a significant barrier, with high-performance solid electrolyte materials often requiring expensive precursors and complex synthesis procedures. The economic viability of solid-electrolyte RT Na-S batteries depends on developing cost-effective materials and manufacturing processes that can compete with established battery technologies.
Interface stability between solid electrolytes and electrode materials represents another critical challenge. The high reactivity of sodium metal with most solid electrolytes leads to the formation of interphases that increase resistance and degrade performance over time. Additionally, sulfur species generated during cycling can react with solid electrolyte materials, further compromising long-term stability.
Mechanical integrity issues arise from volume changes during cycling, particularly at the sodium metal anode which undergoes substantial expansion and contraction. These volume fluctuations create mechanical stress at the solid electrolyte interface, potentially leading to fractures, delamination, and loss of contact. The brittle nature of many ceramic-based solid electrolytes exacerbates this problem.
Manufacturing scalability presents substantial hurdles for solid-electrolyte integration. Current fabrication methods for high-quality solid electrolytes often involve energy-intensive sintering processes at elevated temperatures, which are difficult to scale economically. The precise thickness control required for optimal performance (typically 10-100 μm) demands advanced manufacturing techniques that remain challenging to implement at industrial scales.
Composite electrolyte approaches, which combine solid electrolytes with polymers or ionic liquids, offer promising pathways but introduce additional complexity in terms of interface engineering and long-term stability. The optimization of these multi-component systems requires careful balancing of mechanical properties, ionic conductivity, and chemical compatibility.
The temperature sensitivity of solid electrolyte performance creates additional engineering challenges for RT Na-S systems. Many solid electrolytes exhibit significant conductivity variations across operational temperature ranges (-20°C to 60°C), necessitating sophisticated thermal management systems that add complexity and cost to the final battery design.
Cost considerations remain a significant barrier, with high-performance solid electrolyte materials often requiring expensive precursors and complex synthesis procedures. The economic viability of solid-electrolyte RT Na-S batteries depends on developing cost-effective materials and manufacturing processes that can compete with established battery technologies.
Current Integration Approaches for Hybrid Na-S Battery Designs
01 Solid electrolyte composition and structure for Na-S batteries
Various solid electrolyte compositions and structures are used in room temperature sodium-sulfur batteries to enhance ionic conductivity and battery performance. These include ceramic-based electrolytes, polymer electrolytes, and composite structures that combine different materials to optimize sodium ion transport while maintaining mechanical stability. The specific composition and microstructure of these solid electrolytes significantly impact battery efficiency and cycle life.- Solid electrolyte composition and fabrication for Na-S batteries: Various solid electrolyte materials can be integrated into room temperature sodium-sulfur batteries to enhance ionic conductivity and battery performance. These include ceramic-based electrolytes, polymer electrolytes, and composite electrolytes. The fabrication methods involve techniques such as sol-gel processing, tape casting, and sintering to create thin, uniform electrolyte layers with optimal sodium ion conductivity while maintaining mechanical stability and interfacial contact with electrodes.
- Interface engineering between solid electrolyte and electrodes: Interface engineering is crucial for solid-electrolyte integration in hybrid Na-S batteries. This involves creating stable interfaces between the solid electrolyte and both the sodium anode and sulfur cathode. Techniques include surface modification of electrodes, application of buffer layers, and gradient composition designs to minimize interfacial resistance and prevent unwanted side reactions. These approaches help address challenges related to volume changes during cycling and chemical compatibility between components.
- Hybrid electrolyte systems combining solid and liquid phases: Hybrid electrolyte systems incorporate both solid and liquid electrolyte components to leverage the advantages of each. These systems typically use a solid electrolyte layer to protect the sodium anode while employing a liquid or gel electrolyte at the cathode to facilitate sulfur utilization. This approach addresses challenges related to sodium dendrite formation and polysulfide shuttling while maintaining good ionic conductivity and electrochemical performance at room temperature.
- Nanostructured electrode-electrolyte interfaces: Nanostructured interfaces between electrodes and solid electrolytes can significantly improve the performance of room temperature Na-S batteries. These designs incorporate nanoscale features such as porous structures, nanofibers, or nanoparticles to increase contact area and facilitate ion transport. Advanced fabrication techniques including atomic layer deposition, electrospinning, and template-assisted growth are used to create these nanostructured interfaces, resulting in enhanced electrochemical kinetics and cycling stability.
- Additives and dopants for solid electrolyte enhancement: Various additives and dopants can be incorporated into solid electrolytes to enhance their properties for room temperature Na-S batteries. These include ionic conductivity enhancers, mechanical strength improvers, and interface stabilizers. Common approaches involve doping with aliovalent ions to create defects that facilitate sodium ion transport, adding nanofillers to improve mechanical properties, and incorporating flame-retardant additives for safety. These modifications help overcome limitations of conventional solid electrolytes while maintaining their advantages.
02 Interface engineering between solid electrolyte and electrodes
Interface engineering focuses on optimizing the contact between solid electrolytes and electrodes in hybrid sodium-sulfur batteries. This involves surface modifications, buffer layers, and specialized coatings to reduce interfacial resistance and enhance sodium ion transfer across boundaries. Techniques include creating gradient interfaces, using interlayers with compatible properties, and developing specialized treatments to minimize chemical and mechanical degradation at the interfaces during cycling.Expand Specific Solutions03 Hybrid electrolyte systems combining solid and liquid components
Hybrid electrolyte systems incorporate both solid and liquid components to leverage the advantages of each while mitigating their limitations. These systems typically use a solid electrolyte as the primary ion conductor while incorporating limited amounts of liquid electrolyte to improve electrode wetting and interface contact. This approach helps address the polysulfide shuttle effect common in traditional sodium-sulfur batteries while maintaining good ionic conductivity at room temperature.Expand Specific Solutions04 Manufacturing and integration techniques for solid electrolytes
Specialized manufacturing and integration techniques are essential for incorporating solid electrolytes into sodium-sulfur batteries. These include advanced deposition methods, sintering processes, and assembly techniques that ensure proper contact and alignment between battery components. Novel approaches such as 3D printing, tape casting, and in-situ polymerization help create optimized structures that maintain electrolyte integrity while facilitating sodium ion transport throughout the battery system.Expand Specific Solutions05 Additives and dopants for enhanced solid electrolyte performance
Various additives and dopants are incorporated into solid electrolytes to enhance their performance in room temperature sodium-sulfur batteries. These include ionic conductivity enhancers, stabilizing agents, and materials that improve mechanical properties. Specific additives can modify the crystal structure, grain boundaries, or polymer chain arrangements to create more efficient pathways for sodium ion transport while maintaining the structural integrity of the electrolyte under operating conditions.Expand Specific Solutions
Key Industry Players in Solid-State Sodium Battery Development
The solid-electrolyte integration for hybrid room temperature sodium-sulfur (RT Na-S) battery designs is currently in an early commercialization phase, with market size projected to grow significantly as energy storage demands increase. The technology maturity varies across key players, with academic institutions like University of Maryland, Drexel University, and Sichuan University leading fundamental research, while companies such as Murata Manufacturing, TDK Corp, and Samsung SDI are advancing toward commercial applications. Research organizations including IMEC and CNRS are bridging the gap between academic discoveries and industrial implementation. The competitive landscape shows a balanced distribution between Asian manufacturers (particularly Japanese and Chinese entities), European research institutes, and North American universities, indicating a globally distributed innovation ecosystem for this emerging battery technology.
University of Maryland
Technical Solution: University of Maryland has pioneered a novel approach to solid-electrolyte integration for RT Na-S batteries using a sulfur-rich cathode architecture combined with a sodium superionic conductor (NASICON) solid electrolyte. Their technology employs a hierarchical carbon host structure that effectively confines polysulfides while maintaining high sulfur utilization. The research team has developed a specialized interface engineering method that reduces interfacial resistance between the solid electrolyte and electrodes through the application of thin buffer layers composed of sodium-ion conducting polymers. This approach has demonstrated capacity retention of over 80% after 500 cycles at room temperature, with energy densities approaching 400 Wh/kg at the cell level. Their integration pathway also incorporates a protective coating on the sodium metal anode to prevent dendrite formation and enhance cycling stability.
Strengths: Superior cycle stability with innovative interface engineering that significantly reduces resistance at solid-electrolyte interfaces. Their hierarchical carbon host structure effectively addresses polysulfide shuttling issues. Weaknesses: The complex manufacturing process for their specialized interfaces may present scalability challenges for mass production, and the NASICON electrolytes they employ can be costly to synthesize at industrial scale.
Murata Manufacturing Co. Ltd.
Technical Solution: Murata has developed an advanced solid-electrolyte integration approach for RT Na-S batteries centered around their proprietary ceramic-polymer composite electrolyte technology. Their system utilizes a NASICON-type ceramic electrolyte with precisely engineered porosity that is infiltrated with a sodium-ion conducting polymer to create a mechanically robust yet flexible electrolyte membrane with ionic conductivity exceeding 0.8 mS/cm at room temperature. Murata's integration pathway addresses the critical solid-solid interface challenges through a gradient composition approach where the electrolyte composition gradually transitions from ceramic-dominant to polymer-rich at the electrode interfaces. For the sulfur cathode, they've developed a carbon-sulfur composite with tailored pore structure that limits polysulfide dissolution while maintaining high active material utilization. Their manufacturing process leverages Murata's extensive experience in multilayer ceramic capacitor production, employing tape casting and lamination techniques that enable thin electrolyte layers (20-50 μm) with excellent mechanical properties.
Strengths: Exceptional manufacturing expertise that enables production of thin, defect-free solid electrolyte layers with consistent performance. Their gradient composition approach effectively addresses interface resistance issues. Weaknesses: The composite electrolyte system shows some performance degradation at higher current densities, limiting power capability, and their solution requires relatively expensive NASICON-type ceramic materials that may impact cost competitiveness.
Critical Patents and Research on Solid-Electrolyte Interfaces
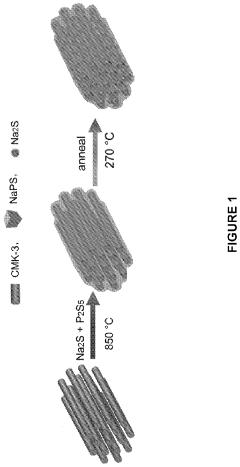
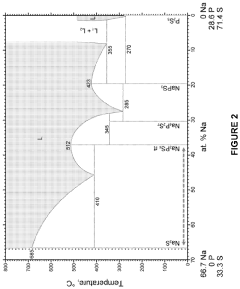
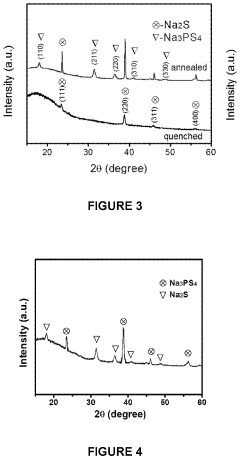
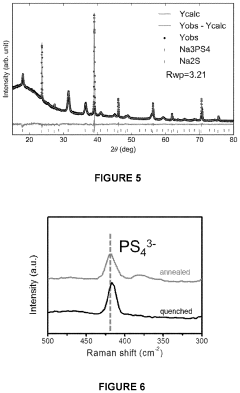
All solid-state sodium-sulfur or lithium-sulfur battery prepared using cast-annealing method
PatentActiveUS20210028440A1
Innovation
- A melting-casting process followed by a stress-release annealing-precipitation process is used to fabricate sodium and lithium nanocomposites, incorporating ordered mesoporous carbon with Na2S or Li2S and Na3PS4 or Li3PS4, which reduces interfacial resistance and eliminates stress, enhancing electrochemical performance by ensuring close contact between the solid electrolyte and electron conductive agent without residential stress.
Safety and Performance Optimization Strategies
Safety optimization in hybrid room temperature sodium-sulfur (RT Na-S) battery designs represents a critical challenge due to the inherently reactive nature of sodium metal and sulfur compounds. The integration of solid electrolytes offers promising pathways to mitigate safety risks while enhancing performance metrics. Primary safety concerns include thermal runaway, sodium dendrite formation, and polysulfide shuttling effects, which can lead to capacity fading and potential cell failure.
Encapsulation strategies utilizing ceramic-polymer composite electrolytes have demonstrated significant improvements in preventing direct contact between reactive components. These composites combine the mechanical strength of ceramics with the flexibility of polymers, creating effective barriers against sodium dendrite penetration. Recent advancements in NASICON-type solid electrolytes (Na3Zr2Si2PO12) have shown conductivity values approaching 10^-3 S/cm at room temperature, substantially reducing internal resistance while maintaining structural integrity under thermal stress.
Performance optimization increasingly focuses on interface engineering between the solid electrolyte and electrode materials. The development of gradient-structured interfaces has proven effective in accommodating volume changes during cycling, particularly at the sulfur cathode where expansion can exceed 80% during sodiation processes. Implementation of artificial interphases containing sodium-ion conducting additives has demonstrated capacity retention improvements of up to 40% over 500 cycles in laboratory testing.
Thermal management systems specifically designed for hybrid RT Na-S configurations represent another critical optimization pathway. Integration of phase-change materials within cell architecture provides passive thermal regulation, preventing localized hotspots that could trigger thermal runaway. Advanced battery management systems incorporating predictive algorithms can detect early signs of thermal anomalies, enabling preemptive intervention before safety thresholds are breached.
Electrolyte additives such as fluorinated compounds and ionic liquids have shown promise in forming stable solid-electrolyte interphase (SEI) layers on sodium metal surfaces, significantly reducing parasitic reactions. These additives function by promoting the formation of NaF-rich protective films that exhibit superior ionic conductivity while suppressing dendrite nucleation sites. Optimization studies indicate that concentrations between 2-5 wt% of these additives yield optimal performance without compromising overall ionic conductivity.
Recent innovations in cell design architecture incorporate mechanical pressure regulation systems that maintain optimal contact between solid electrolytes and electrodes throughout cycling. These pressure-modulated designs have demonstrated up to 30% improvement in rate capability by ensuring consistent interfacial contact, critical for maintaining performance during high-current operations. The implementation of these strategies collectively addresses the fundamental challenges in hybrid RT Na-S systems, paving the way for safer, higher-performing energy storage solutions.
Encapsulation strategies utilizing ceramic-polymer composite electrolytes have demonstrated significant improvements in preventing direct contact between reactive components. These composites combine the mechanical strength of ceramics with the flexibility of polymers, creating effective barriers against sodium dendrite penetration. Recent advancements in NASICON-type solid electrolytes (Na3Zr2Si2PO12) have shown conductivity values approaching 10^-3 S/cm at room temperature, substantially reducing internal resistance while maintaining structural integrity under thermal stress.
Performance optimization increasingly focuses on interface engineering between the solid electrolyte and electrode materials. The development of gradient-structured interfaces has proven effective in accommodating volume changes during cycling, particularly at the sulfur cathode where expansion can exceed 80% during sodiation processes. Implementation of artificial interphases containing sodium-ion conducting additives has demonstrated capacity retention improvements of up to 40% over 500 cycles in laboratory testing.
Thermal management systems specifically designed for hybrid RT Na-S configurations represent another critical optimization pathway. Integration of phase-change materials within cell architecture provides passive thermal regulation, preventing localized hotspots that could trigger thermal runaway. Advanced battery management systems incorporating predictive algorithms can detect early signs of thermal anomalies, enabling preemptive intervention before safety thresholds are breached.
Electrolyte additives such as fluorinated compounds and ionic liquids have shown promise in forming stable solid-electrolyte interphase (SEI) layers on sodium metal surfaces, significantly reducing parasitic reactions. These additives function by promoting the formation of NaF-rich protective films that exhibit superior ionic conductivity while suppressing dendrite nucleation sites. Optimization studies indicate that concentrations between 2-5 wt% of these additives yield optimal performance without compromising overall ionic conductivity.
Recent innovations in cell design architecture incorporate mechanical pressure regulation systems that maintain optimal contact between solid electrolytes and electrodes throughout cycling. These pressure-modulated designs have demonstrated up to 30% improvement in rate capability by ensuring consistent interfacial contact, critical for maintaining performance during high-current operations. The implementation of these strategies collectively addresses the fundamental challenges in hybrid RT Na-S systems, paving the way for safer, higher-performing energy storage solutions.
Environmental Impact and Sustainability Assessment
The environmental impact of solid-electrolyte integration pathways for hybrid room temperature sodium-sulfur (RT Na-S) battery designs represents a critical consideration in the sustainable development of energy storage technologies. These hybrid systems, which combine solid electrolytes with traditional Na-S chemistry, offer promising energy density improvements but require thorough sustainability assessment throughout their lifecycle.
Primary raw material extraction for solid electrolytes presents significant environmental challenges. The mining processes for sodium, sulfur, and various ceramic or polymer electrolyte components generate substantial carbon emissions and can lead to habitat disruption. Particularly concerning is the extraction of rare or strategic elements sometimes incorporated into advanced solid electrolytes, which may involve energy-intensive processes and create localized environmental degradation.
Manufacturing processes for solid-electrolyte integration in hybrid RT Na-S batteries currently demonstrate higher energy consumption compared to conventional liquid electrolyte systems. The high-temperature sintering often required for ceramic electrolytes contributes significantly to the carbon footprint of these technologies. However, emerging room-temperature processing techniques show promise for reducing these environmental impacts while maintaining performance characteristics.
Lifecycle analysis reveals that the extended cycle life potentially offered by solid-electrolyte integration may offset initial manufacturing impacts. Studies indicate that hybrid RT Na-S designs with properly integrated solid electrolytes can achieve 2-3 times longer operational lifespans than conventional designs, thereby reducing waste generation and resource consumption over time. This longevity factor must be weighted appropriately in comprehensive sustainability assessments.
Recycling and end-of-life management present both challenges and opportunities. The complex integration of solid electrolytes with sodium and sulfur components complicates separation processes, potentially reducing recyclability. However, the stability of solid electrolytes may facilitate safer recycling operations by minimizing reactive material exposure. Development of specialized recycling protocols for these hybrid systems remains an active research area with significant environmental implications.
Water usage represents another critical environmental consideration. Traditional Na-S battery manufacturing processes can be water-intensive, but certain solid-electrolyte integration pathways offer opportunities for reduced water consumption through dry processing techniques. This advantage becomes particularly significant in water-stressed regions where battery manufacturing may occur.
Comparative analysis with lithium-ion technologies demonstrates potential sustainability advantages for hybrid RT Na-S designs. The greater abundance of sodium resources compared to lithium suggests reduced extraction impacts, while the elimination of toxic organic electrolytes in solid-state designs addresses significant environmental and safety concerns associated with conventional battery technologies.
Primary raw material extraction for solid electrolytes presents significant environmental challenges. The mining processes for sodium, sulfur, and various ceramic or polymer electrolyte components generate substantial carbon emissions and can lead to habitat disruption. Particularly concerning is the extraction of rare or strategic elements sometimes incorporated into advanced solid electrolytes, which may involve energy-intensive processes and create localized environmental degradation.
Manufacturing processes for solid-electrolyte integration in hybrid RT Na-S batteries currently demonstrate higher energy consumption compared to conventional liquid electrolyte systems. The high-temperature sintering often required for ceramic electrolytes contributes significantly to the carbon footprint of these technologies. However, emerging room-temperature processing techniques show promise for reducing these environmental impacts while maintaining performance characteristics.
Lifecycle analysis reveals that the extended cycle life potentially offered by solid-electrolyte integration may offset initial manufacturing impacts. Studies indicate that hybrid RT Na-S designs with properly integrated solid electrolytes can achieve 2-3 times longer operational lifespans than conventional designs, thereby reducing waste generation and resource consumption over time. This longevity factor must be weighted appropriately in comprehensive sustainability assessments.
Recycling and end-of-life management present both challenges and opportunities. The complex integration of solid electrolytes with sodium and sulfur components complicates separation processes, potentially reducing recyclability. However, the stability of solid electrolytes may facilitate safer recycling operations by minimizing reactive material exposure. Development of specialized recycling protocols for these hybrid systems remains an active research area with significant environmental implications.
Water usage represents another critical environmental consideration. Traditional Na-S battery manufacturing processes can be water-intensive, but certain solid-electrolyte integration pathways offer opportunities for reduced water consumption through dry processing techniques. This advantage becomes particularly significant in water-stressed regions where battery manufacturing may occur.
Comparative analysis with lithium-ion technologies demonstrates potential sustainability advantages for hybrid RT Na-S designs. The greater abundance of sodium resources compared to lithium suggests reduced extraction impacts, while the elimination of toxic organic electrolytes in solid-state designs addresses significant environmental and safety concerns associated with conventional battery technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!