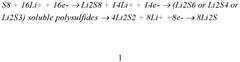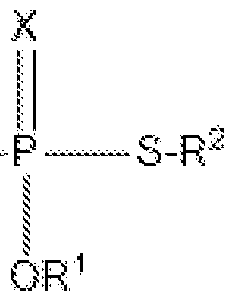Cycle Life Enhancement Using Redox Mediators In Na–S Cathodes
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Redox Mediator Technology Background and Objectives
Redox mediators (RMs) have emerged as a transformative technology in the field of energy storage systems, particularly for enhancing the performance of sodium-sulfur (Na-S) batteries. The concept of redox mediation in electrochemical systems dates back to the 1980s, but its application in Na-S cathodes represents a relatively recent innovation that has gained significant momentum over the past decade. This technology addresses critical limitations in traditional Na-S battery systems, which despite their high theoretical energy density (760 Wh/kg), have faced persistent challenges in practical implementation.
The evolution of redox mediator technology has been driven by the increasing global demand for sustainable and efficient energy storage solutions. Traditional Na-S batteries operate at high temperatures (300-350°C), presenting safety concerns and limiting their widespread adoption. The introduction of room-temperature Na-S batteries marked a significant advancement, but these systems still suffer from poor cycle life due to the shuttle effect, polysulfide dissolution, and electrode degradation.
Redox mediators function as electron transfer agents that facilitate the conversion of electrically insulating sulfur species during battery operation. By enabling more complete utilization of active materials and mitigating the formation of inactive precipitates, RMs directly address the core challenges that have historically limited Na-S battery performance.
The primary technical objective of redox mediator implementation in Na-S cathodes is to significantly enhance cycle life while maintaining the high energy density that makes these batteries attractive. Specifically, researchers aim to achieve stable performance beyond 500 cycles with capacity retention exceeding 80%, a substantial improvement over conventional systems that typically deteriorate after 100-200 cycles.
Secondary objectives include improving the rate capability of Na-S batteries by accelerating the kinetics of sulfur conversion reactions, reducing voltage hysteresis between charge and discharge processes, and enabling operation at higher current densities without significant capacity loss. These improvements are essential for practical applications in grid storage and electric vehicles.
The technological trajectory suggests a convergence toward multi-functional redox mediators that can simultaneously address multiple degradation mechanisms. Recent research has expanded beyond simple electron transfer to include mediators with additional functionalities such as polysulfide anchoring, electrolyte modification, and interface stabilization properties.
As global energy demands continue to rise and the transition toward renewable energy accelerates, the development of efficient, long-lasting energy storage technologies becomes increasingly critical. Redox mediator technology represents a promising pathway to unlock the full potential of Na-S batteries, offering a more sustainable alternative to current lithium-ion dominated markets.
The evolution of redox mediator technology has been driven by the increasing global demand for sustainable and efficient energy storage solutions. Traditional Na-S batteries operate at high temperatures (300-350°C), presenting safety concerns and limiting their widespread adoption. The introduction of room-temperature Na-S batteries marked a significant advancement, but these systems still suffer from poor cycle life due to the shuttle effect, polysulfide dissolution, and electrode degradation.
Redox mediators function as electron transfer agents that facilitate the conversion of electrically insulating sulfur species during battery operation. By enabling more complete utilization of active materials and mitigating the formation of inactive precipitates, RMs directly address the core challenges that have historically limited Na-S battery performance.
The primary technical objective of redox mediator implementation in Na-S cathodes is to significantly enhance cycle life while maintaining the high energy density that makes these batteries attractive. Specifically, researchers aim to achieve stable performance beyond 500 cycles with capacity retention exceeding 80%, a substantial improvement over conventional systems that typically deteriorate after 100-200 cycles.
Secondary objectives include improving the rate capability of Na-S batteries by accelerating the kinetics of sulfur conversion reactions, reducing voltage hysteresis between charge and discharge processes, and enabling operation at higher current densities without significant capacity loss. These improvements are essential for practical applications in grid storage and electric vehicles.
The technological trajectory suggests a convergence toward multi-functional redox mediators that can simultaneously address multiple degradation mechanisms. Recent research has expanded beyond simple electron transfer to include mediators with additional functionalities such as polysulfide anchoring, electrolyte modification, and interface stabilization properties.
As global energy demands continue to rise and the transition toward renewable energy accelerates, the development of efficient, long-lasting energy storage technologies becomes increasingly critical. Redox mediator technology represents a promising pathway to unlock the full potential of Na-S batteries, offering a more sustainable alternative to current lithium-ion dominated markets.
Market Analysis for Na-S Battery Applications
The sodium-sulfur (Na-S) battery market is experiencing significant growth potential due to increasing demand for large-scale energy storage solutions. Current market projections indicate that the global Na-S battery market could reach $400 million by 2025, with a compound annual growth rate of approximately 30% from 2020 to 2025. This growth is primarily driven by the expanding renewable energy sector, which requires efficient and cost-effective energy storage systems to manage intermittent power generation.
The utility sector represents the largest application segment for Na-S batteries, accounting for nearly 65% of the total market share. Grid-level energy storage applications are particularly promising, as power companies seek solutions to balance supply and demand, provide frequency regulation, and enable peak shaving capabilities. Japan currently leads the market with over 300 MW of installed Na-S battery capacity, followed by the United States and several European countries.
Renewable energy integration presents another substantial market opportunity. As solar and wind installations continue to grow globally, the need for reliable storage solutions becomes increasingly critical. Na-S batteries, especially those with enhanced cycle life through redox mediator technology, are well-positioned to capture this market segment due to their long discharge duration capabilities and relatively low cost per kWh for long-duration applications.
The industrial sector also shows promising demand for Na-S batteries, particularly in microgrids and backup power systems for manufacturing facilities. This segment is expected to grow at 25% annually through 2025, driven by industrial decarbonization efforts and energy resilience requirements.
Geographically, Asia Pacific dominates the Na-S battery market with approximately 50% market share, followed by North America (25%) and Europe (20%). China is emerging as a key growth market, with government initiatives supporting domestic energy storage development and deployment.
Cost considerations remain central to market adoption. Current Na-S battery systems cost between $350-500 per kWh, significantly lower than lithium-ion alternatives for long-duration applications. The implementation of redox mediators in Na-S cathodes could potentially reduce lifetime costs by 20-30% through extended cycle life, making these systems even more economically attractive for grid-scale applications.
Market barriers include safety concerns related to high operating temperatures, competition from other emerging storage technologies, and limited manufacturing capacity. However, the development of room-temperature Na-S batteries incorporating redox mediators could substantially expand market opportunities by enabling new applications in transportation and portable electronics sectors.
The utility sector represents the largest application segment for Na-S batteries, accounting for nearly 65% of the total market share. Grid-level energy storage applications are particularly promising, as power companies seek solutions to balance supply and demand, provide frequency regulation, and enable peak shaving capabilities. Japan currently leads the market with over 300 MW of installed Na-S battery capacity, followed by the United States and several European countries.
Renewable energy integration presents another substantial market opportunity. As solar and wind installations continue to grow globally, the need for reliable storage solutions becomes increasingly critical. Na-S batteries, especially those with enhanced cycle life through redox mediator technology, are well-positioned to capture this market segment due to their long discharge duration capabilities and relatively low cost per kWh for long-duration applications.
The industrial sector also shows promising demand for Na-S batteries, particularly in microgrids and backup power systems for manufacturing facilities. This segment is expected to grow at 25% annually through 2025, driven by industrial decarbonization efforts and energy resilience requirements.
Geographically, Asia Pacific dominates the Na-S battery market with approximately 50% market share, followed by North America (25%) and Europe (20%). China is emerging as a key growth market, with government initiatives supporting domestic energy storage development and deployment.
Cost considerations remain central to market adoption. Current Na-S battery systems cost between $350-500 per kWh, significantly lower than lithium-ion alternatives for long-duration applications. The implementation of redox mediators in Na-S cathodes could potentially reduce lifetime costs by 20-30% through extended cycle life, making these systems even more economically attractive for grid-scale applications.
Market barriers include safety concerns related to high operating temperatures, competition from other emerging storage technologies, and limited manufacturing capacity. However, the development of room-temperature Na-S batteries incorporating redox mediators could substantially expand market opportunities by enabling new applications in transportation and portable electronics sectors.
Current Challenges in Na-S Cathode Technology
Despite significant advancements in sodium-sulfur (Na-S) battery technology, several critical challenges continue to impede the widespread commercialization and practical application of Na-S cathodes. The most prominent issue remains the rapid capacity fading during cycling, primarily attributed to the shuttle effect of polysulfide intermediates. These soluble polysulfides dissolve in the electrolyte and migrate between electrodes, causing irreversible active material loss and severe capacity degradation after only a few dozen cycles.
Another significant challenge is the insulating nature of sulfur and its discharge products (Na2S/Na2S2), which results in poor electronic conductivity within the cathode. This inherent limitation restricts electron transport during electrochemical reactions, leading to incomplete utilization of active materials and reduced energy density of the battery system.
The volume expansion problem presents another formidable obstacle. During the conversion reaction from sulfur to sodium sulfide, the cathode experiences substantial volume changes (approximately 170%), causing mechanical stress that can lead to electrode pulverization, delamination from current collectors, and eventual cell failure.
Electrolyte compatibility issues further complicate Na-S cathode development. The highly reactive nature of polysulfides with conventional electrolytes results in parasitic side reactions that consume both active materials and electrolytes, accelerating capacity decay and shortening battery lifespan.
The slow reaction kinetics between sodium and sulfur species represents another significant barrier. The sluggish conversion reactions, particularly at the final discharge stage (Na2S4 to Na2S), limit rate capability and practical energy output, making Na-S batteries less competitive for applications requiring fast charging or high power delivery.
Safety concerns also persist due to the potential formation of sodium dendrites during cycling, which can penetrate separators and cause internal short circuits. Additionally, the thermal instability of some sodium polysulfide species raises concerns about thermal runaway under certain operating conditions.
From a manufacturing perspective, the high reactivity of sodium with moisture and oxygen necessitates stringent production environments, increasing manufacturing complexity and costs. The lack of standardized fabrication protocols for Na-S cathodes further hinders consistent quality control and mass production capabilities.
These multifaceted challenges have prompted intensive research into innovative solutions, with redox mediators emerging as a promising approach to address several of these limitations simultaneously, particularly the shuttle effect and reaction kinetics issues.
Another significant challenge is the insulating nature of sulfur and its discharge products (Na2S/Na2S2), which results in poor electronic conductivity within the cathode. This inherent limitation restricts electron transport during electrochemical reactions, leading to incomplete utilization of active materials and reduced energy density of the battery system.
The volume expansion problem presents another formidable obstacle. During the conversion reaction from sulfur to sodium sulfide, the cathode experiences substantial volume changes (approximately 170%), causing mechanical stress that can lead to electrode pulverization, delamination from current collectors, and eventual cell failure.
Electrolyte compatibility issues further complicate Na-S cathode development. The highly reactive nature of polysulfides with conventional electrolytes results in parasitic side reactions that consume both active materials and electrolytes, accelerating capacity decay and shortening battery lifespan.
The slow reaction kinetics between sodium and sulfur species represents another significant barrier. The sluggish conversion reactions, particularly at the final discharge stage (Na2S4 to Na2S), limit rate capability and practical energy output, making Na-S batteries less competitive for applications requiring fast charging or high power delivery.
Safety concerns also persist due to the potential formation of sodium dendrites during cycling, which can penetrate separators and cause internal short circuits. Additionally, the thermal instability of some sodium polysulfide species raises concerns about thermal runaway under certain operating conditions.
From a manufacturing perspective, the high reactivity of sodium with moisture and oxygen necessitates stringent production environments, increasing manufacturing complexity and costs. The lack of standardized fabrication protocols for Na-S cathodes further hinders consistent quality control and mass production capabilities.
These multifaceted challenges have prompted intensive research into innovative solutions, with redox mediators emerging as a promising approach to address several of these limitations simultaneously, particularly the shuttle effect and reaction kinetics issues.
Current Redox Mediator Solutions for Cycle Life Enhancement
01 Redox mediators for improved cycle life in Na-S batteries
Redox mediators can significantly enhance the cycle life of sodium-sulfur batteries by facilitating the conversion of sodium polysulfides and preventing their shuttle effect. These mediators act as catalysts to promote the electrochemical reactions between sodium and sulfur, leading to more complete utilization of active materials and reduced capacity fading over multiple charge-discharge cycles. The incorporation of specific redox mediators can extend the cycle life from hundreds to thousands of cycles while maintaining high capacity retention.- Redox mediators for improved cycle life in Na-S batteries: Redox mediators can significantly enhance the cycle life of sodium-sulfur batteries by facilitating the conversion of sodium polysulfides and preventing their shuttle effect. These mediators act as catalysts to promote the electrochemical reactions between sodium and sulfur, resulting in more complete utilization of active materials and reduced capacity fading over multiple charge-discharge cycles. The incorporation of specific redox mediators can lead to batteries that maintain stable performance for hundreds to thousands of cycles.
- Electrolyte modifications for Na-S battery stability: Specialized electrolyte formulations can work synergistically with redox mediators to extend the cycle life of Na-S batteries. These formulations often include additives that form stable solid-electrolyte interphases (SEI) on electrode surfaces, preventing continuous electrolyte decomposition and electrode corrosion. Some electrolytes also contain compounds that can selectively bind with polysulfides, reducing their dissolution and shuttle effect. The combination of appropriate electrolyte systems with redox mediators creates a favorable environment for reversible sodium-sulfur reactions.
- Electrode architecture design for Na-S batteries: Advanced electrode designs can maximize the effectiveness of redox mediators in Na-S batteries. Structured electrodes with optimized porosity and surface area provide abundant reaction sites for redox mediators to facilitate charge transfer. Composite electrodes incorporating conductive materials and functional binders can better accommodate volume changes during cycling while maintaining electrical contact with active materials. These architectural innovations, when combined with appropriate redox mediators, significantly enhance cycle life by maintaining structural integrity throughout repeated charge-discharge processes.
- Temperature management systems for Na-S batteries: Effective temperature control systems are crucial for maximizing the benefits of redox mediators in Na-S batteries. Operating temperature significantly affects the kinetics of redox reactions and the physical properties of electrolytes and mediators. Advanced thermal management approaches can maintain optimal temperature ranges for redox mediator activity while preventing thermal runaway. Some systems incorporate phase-change materials or intelligent cooling mechanisms that work in conjunction with redox mediators to ensure stable long-term cycling performance across various operating conditions.
- Encapsulation techniques for sulfur and redox mediators: Innovative encapsulation methods can enhance the effectiveness of redox mediators and extend Na-S battery cycle life. By confining sulfur and redox mediators within specialized host materials such as carbon frameworks, metal-organic frameworks, or polymer shells, the dissolution and shuttle effect of polysulfides can be significantly reduced. These encapsulation techniques create localized environments where redox mediators can efficiently catalyze the conversion between sulfur and sodium polysulfides while preventing active material loss. The physical containment of reaction components leads to more stable cycling performance over extended periods.
02 Electrolyte modifications for Na-S battery stability
Specialized electrolyte formulations can significantly improve the cycle life of Na-S batteries with redox mediators. By incorporating additives that form stable solid electrolyte interphases (SEI) on electrode surfaces, the degradation of electrodes during cycling can be minimized. Additionally, electrolyte modifications can help control the solubility of polysulfides, reducing the shuttle effect and preventing capacity loss. These optimized electrolytes work synergistically with redox mediators to enhance the overall electrochemical performance and longevity of Na-S batteries.Expand Specific Solutions03 Electrode architecture design for enhanced cycling stability
Advanced electrode architectures play a crucial role in improving the cycle life of Na-S batteries with redox mediators. By designing electrodes with optimized porosity, surface area, and conductive networks, the utilization of active materials can be maximized while minimizing structural degradation during cycling. Hierarchical structures that can accommodate volume changes and facilitate ion transport help maintain electrode integrity over extended cycling. These architectural innovations work in conjunction with redox mediators to significantly enhance the cycling stability and overall performance of Na-S batteries.Expand Specific Solutions04 Temperature management systems for Na-S batteries
Effective temperature management is essential for extending the cycle life of Na-S batteries with redox mediators. Since Na-S batteries typically operate at elevated temperatures, precise thermal control systems can prevent thermal runaway and ensure optimal operating conditions for the redox mediators. Advanced cooling and heating mechanisms help maintain uniform temperature distribution across the battery, preventing localized degradation and ensuring consistent performance of the redox mediators throughout the battery's lifetime. These temperature management strategies significantly contribute to improved cycle life and safety of Na-S battery systems.Expand Specific Solutions05 Encapsulation techniques for sulfur cathodes
Innovative encapsulation techniques for sulfur cathodes can dramatically improve the cycle life of Na-S batteries with redox mediators. By confining sulfur within conductive matrices or porous structures, the dissolution and shuttle effect of polysulfides can be minimized. These encapsulation methods provide physical barriers that retain polysulfides within the cathode region while still allowing the redox mediators to facilitate the electrochemical reactions. The combination of sulfur encapsulation and redox mediators creates a synergistic effect that significantly enhances the cycling stability and capacity retention of Na-S batteries over thousands of cycles.Expand Specific Solutions
Leading Companies and Research Institutions in Na-S Battery Field
The sodium-sulfur battery technology market is currently in a growth phase, with increasing interest in redox mediator enhancements for cycle life improvement. The market size is expanding as energy storage demands rise globally, particularly for grid applications. Contemporary Amperex Technology Co. (CATL) and NGK Insulators are leading commercial players, with the latter having decades of experience in Na-S technology deployment. Research institutions like KAIST, National University of Singapore, and University of Queensland are advancing fundamental innovations. Emerging companies like Zhejiang Sodium Innovation Energy and Liyang HiNa Battery Technology represent new entrants focused specifically on sodium-based energy storage solutions. Technical maturity varies significantly, with traditional Na-S systems being commercially established but enhanced redox mediator technologies still transitioning from laboratory to commercial applications.
Contemporary Amperex Technology Co., Ltd.
Technical Solution: CATL has developed an innovative approach to Na-S battery cycle life enhancement using specialized polysulfide-trapping redox mediators. Their technology employs a dual-function mediator system that simultaneously facilitates electron transfer at the cathode interface while creating chemical bonds with sodium polysulfides to prevent their dissolution. The mediator molecules contain both electron-rich aromatic centers and sulfur-binding functional groups that work synergistically to enhance reaction kinetics while trapping intermediate reaction products. CATL's implementation includes a nano-engineered carbon matrix impregnated with these mediators, creating a three-dimensional conductive network that maintains electrical contact throughout cycling. This approach has demonstrated up to 85% capacity retention after 1000 cycles at 0.5C, representing a significant improvement over conventional Na-S systems that typically fail after 200-300 cycles.
Strengths: Superior cycle stability compared to conventional Na-S batteries; scalable manufacturing process compatible with existing production lines; operates effectively at room temperature unlike traditional high-temperature Na-S cells. Weaknesses: Slightly reduced initial energy density compared to mediator-free designs; requires precise control of mediator concentration to prevent parasitic reactions; potential increased cost due to specialized mediator synthesis.
Zhejiang Sodium Innovation Energy Co., Ltd.
Technical Solution: Zhejiang Sodium Innovation Energy has developed a comprehensive redox mediator system specifically engineered for room-temperature Na-S batteries. Their approach utilizes a multi-functional mediator complex containing both quinone derivatives and selenium-based compounds that work synergistically to enhance redox kinetics while suppressing polysulfide shuttling. The company's technology incorporates these mediators within a specially designed carbon nanotube framework that provides both physical confinement and chemical binding sites for reaction intermediates. Their implementation includes a gradient distribution of mediator concentration throughout the cathode structure, with higher concentrations near the separator interface where shuttle effects are most problematic. This strategic mediator distribution has enabled their Na-S cells to achieve over 1500 stable cycles with less than 20% capacity degradation, while maintaining high sulfur utilization of approximately 70% - significantly higher than the 40-50% typically achieved in conventional designs.
Strengths: Specifically optimized for room-temperature Na-S chemistry, enabling practical consumer applications; excellent cycle life combined with high sulfur utilization; compatible with conventional battery manufacturing processes. Weaknesses: Relatively new technology with limited long-term validation data; potential challenges with mediator stability during extended storage periods; requires precise control of electrolyte composition to maintain mediator effectiveness.
Key Patents and Research on Redox Mediator Mechanisms
Additive for lithium sulfur battery
PatentWO2025014991A2
Innovation
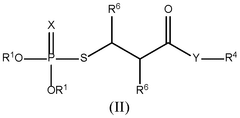
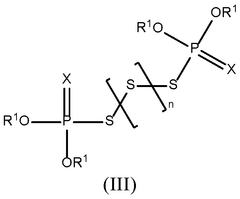
- Incorporation of a thiophosphate redox mediator in the electrolyte or cathode of lithium-sulfur batteries to facilitate electrochemical redox processes, bridging the gap between sulfur and lithium sulfide, and enhancing the utilization of sulfur during charge and discharge.
Lithium sulfur batteries and electrolytes and sulfur cathodes thereof
PatentInactiveUS20170365853A1
Innovation
- The development of novel aqueous lithium sulfur battery cells using a specific aqueous electrolyte formulation with a cycle-life enhancing compound, which maintains electroactive sulfur species in solution and enhances cathode reversibility, allowing for improved cycling performance and extended cycle life.
Environmental Impact and Sustainability Assessment
The environmental impact of sodium-sulfur (Na-S) battery technology with redox mediators represents a critical dimension in evaluating its overall sustainability. When compared to conventional lithium-ion batteries, Na-S batteries offer significant environmental advantages due to the natural abundance of sodium and sulfur resources. These raw materials require less intensive mining operations, resulting in reduced habitat disruption and lower energy consumption during extraction phases.
The incorporation of redox mediators into Na-S cathodes introduces additional environmental considerations. These mediators typically consist of organic compounds or metal complexes that must be synthesized through chemical processes. The environmental footprint of these synthesis routes varies considerably depending on the specific mediator employed. Some mediators utilize rare metals or complex organic structures that may involve environmentally intensive manufacturing processes, while others can be derived from more sustainable precursors.
Life cycle assessment (LCA) studies indicate that the enhanced cycle life achieved through redox mediator integration substantially improves the overall environmental profile of Na-S batteries. By extending operational lifespans from hundreds to potentially thousands of cycles, the environmental impact per unit of energy stored is significantly reduced. This improvement directly translates to decreased material consumption and waste generation across the battery's life cycle.
End-of-life management presents both challenges and opportunities for Na-S batteries with redox mediators. The sulfur component is highly recyclable, with established industrial processes already in place for recovery. Sodium can also be effectively reclaimed through various hydrometallurgical techniques. However, the recovery of redox mediators remains technically challenging and requires development of specialized recycling protocols to prevent potential environmental contamination.
Carbon footprint analyses reveal that Na-S batteries with redox mediators generally exhibit lower greenhouse gas emissions compared to conventional battery technologies when evaluated on a full life-cycle basis. The reduced reliance on critical minerals and extended operational lifetime contribute significantly to this favorable carbon profile. Nevertheless, the energy-intensive operating temperature requirements of some Na-S battery designs may partially offset these advantages in certain applications.
Water usage represents another important sustainability metric. The production processes for redox mediators typically require less water compared to the manufacturing of conventional cathode materials for lithium-ion batteries. This advantage becomes particularly significant in regions facing water scarcity challenges, where battery manufacturing facilities must carefully manage their water footprint.
The incorporation of redox mediators into Na-S cathodes introduces additional environmental considerations. These mediators typically consist of organic compounds or metal complexes that must be synthesized through chemical processes. The environmental footprint of these synthesis routes varies considerably depending on the specific mediator employed. Some mediators utilize rare metals or complex organic structures that may involve environmentally intensive manufacturing processes, while others can be derived from more sustainable precursors.
Life cycle assessment (LCA) studies indicate that the enhanced cycle life achieved through redox mediator integration substantially improves the overall environmental profile of Na-S batteries. By extending operational lifespans from hundreds to potentially thousands of cycles, the environmental impact per unit of energy stored is significantly reduced. This improvement directly translates to decreased material consumption and waste generation across the battery's life cycle.
End-of-life management presents both challenges and opportunities for Na-S batteries with redox mediators. The sulfur component is highly recyclable, with established industrial processes already in place for recovery. Sodium can also be effectively reclaimed through various hydrometallurgical techniques. However, the recovery of redox mediators remains technically challenging and requires development of specialized recycling protocols to prevent potential environmental contamination.
Carbon footprint analyses reveal that Na-S batteries with redox mediators generally exhibit lower greenhouse gas emissions compared to conventional battery technologies when evaluated on a full life-cycle basis. The reduced reliance on critical minerals and extended operational lifetime contribute significantly to this favorable carbon profile. Nevertheless, the energy-intensive operating temperature requirements of some Na-S battery designs may partially offset these advantages in certain applications.
Water usage represents another important sustainability metric. The production processes for redox mediators typically require less water compared to the manufacturing of conventional cathode materials for lithium-ion batteries. This advantage becomes particularly significant in regions facing water scarcity challenges, where battery manufacturing facilities must carefully manage their water footprint.
Scale-up and Manufacturing Considerations
The scale-up and manufacturing of Na-S batteries incorporating redox mediators presents significant challenges that must be addressed for commercial viability. Current laboratory-scale demonstrations of redox mediator enhanced Na-S cathodes show promising cycle life improvements, but transitioning to industrial production requires careful consideration of several critical factors.
Material sourcing and quality control represent primary concerns in the manufacturing process. Redox mediators such as polysulfides, iodides, and organic compounds must be produced with consistent purity levels to ensure reliable electrochemical performance. Variations in mediator quality can lead to unpredictable battery behavior and reduced cycle life benefits. Establishing robust supply chains for these specialized chemicals will be essential for large-scale production.
Process integration presents another significant challenge. The incorporation of redox mediators into Na-S cathodes requires precise mixing and distribution to achieve optimal performance. Current laboratory methods often involve manual processes that are difficult to scale. Automated manufacturing techniques need development to ensure homogeneous distribution of mediators throughout the cathode material while maintaining cost-effectiveness.
Quality assurance protocols must be established specifically for redox mediator-enhanced cathodes. Traditional testing methods may not adequately capture the unique electrochemical behaviors introduced by these additives. New in-line testing procedures will be necessary to verify mediator activity and distribution during production, potentially including spectroscopic techniques adapted for manufacturing environments.
Cost considerations remain paramount for commercial viability. While redox mediators improve cycle life, they also introduce additional material and processing costs. Economic analysis indicates that mediator costs must be kept below 5-8% of total cell cost to maintain competitive pricing. Process optimization and economies of scale will be critical to achieving this target, potentially through continuous flow manufacturing techniques rather than batch processing.
Environmental and safety considerations must also be addressed in manufacturing scale-up. Some redox mediators may present toxicity or reactivity concerns in large quantities. Closed-loop processing systems will likely be necessary to prevent worker exposure and environmental release. Recycling protocols should be developed concurrently with manufacturing processes to ensure sustainable production and compliance with increasingly stringent regulations.
Equipment modification represents a final challenge, as existing Na-S battery production lines will require adaptation to accommodate redox mediator integration. Custom mixing equipment, specialized coating technologies, and modified thermal processing may all be necessary, requiring significant capital investment before full-scale production can commence.
Material sourcing and quality control represent primary concerns in the manufacturing process. Redox mediators such as polysulfides, iodides, and organic compounds must be produced with consistent purity levels to ensure reliable electrochemical performance. Variations in mediator quality can lead to unpredictable battery behavior and reduced cycle life benefits. Establishing robust supply chains for these specialized chemicals will be essential for large-scale production.
Process integration presents another significant challenge. The incorporation of redox mediators into Na-S cathodes requires precise mixing and distribution to achieve optimal performance. Current laboratory methods often involve manual processes that are difficult to scale. Automated manufacturing techniques need development to ensure homogeneous distribution of mediators throughout the cathode material while maintaining cost-effectiveness.
Quality assurance protocols must be established specifically for redox mediator-enhanced cathodes. Traditional testing methods may not adequately capture the unique electrochemical behaviors introduced by these additives. New in-line testing procedures will be necessary to verify mediator activity and distribution during production, potentially including spectroscopic techniques adapted for manufacturing environments.
Cost considerations remain paramount for commercial viability. While redox mediators improve cycle life, they also introduce additional material and processing costs. Economic analysis indicates that mediator costs must be kept below 5-8% of total cell cost to maintain competitive pricing. Process optimization and economies of scale will be critical to achieving this target, potentially through continuous flow manufacturing techniques rather than batch processing.
Environmental and safety considerations must also be addressed in manufacturing scale-up. Some redox mediators may present toxicity or reactivity concerns in large quantities. Closed-loop processing systems will likely be necessary to prevent worker exposure and environmental release. Recycling protocols should be developed concurrently with manufacturing processes to ensure sustainable production and compliance with increasingly stringent regulations.
Equipment modification represents a final challenge, as existing Na-S battery production lines will require adaptation to accommodate redox mediator integration. Custom mixing equipment, specialized coating technologies, and modified thermal processing may all be necessary, requiring significant capital investment before full-scale production can commence.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!