Electrolyte Design For High Coulombic Efficiency In RT Na–S Systems
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Na-S Battery Electrolyte Evolution and Objectives
Sodium-sulfur (Na-S) battery technology has evolved significantly since its inception in the 1960s by Ford Motor Company. Initially developed as high-temperature systems operating at 300-350°C, these batteries utilized molten sodium and sulfur electrodes separated by a solid beta-alumina ceramic electrolyte. While demonstrating impressive energy density (theoretical 760 Wh/kg), the high operating temperatures posed significant safety concerns and limited practical applications to stationary grid storage.
The paradigm shift toward room temperature Na-S batteries began in the early 2000s, driven by the need for safer, more versatile energy storage solutions. This transition necessitated fundamental redesign of electrolyte systems to accommodate the solid-state electrochemical reactions occurring at ambient temperatures. Early room temperature designs suffered from rapid capacity fading, poor cycle life, and low Coulombic efficiency, primarily due to the shuttle effect of soluble polysulfide intermediates.
Electrolyte development for room temperature Na-S batteries has progressed through several distinct phases. The first generation utilized conventional carbonate-based liquid electrolytes (e.g., NaClO₄ in EC/DMC), which proved inadequate due to side reactions with polysulfides. The second generation explored ether-based electrolytes (e.g., NaCF₃SO₃ in TEGDME), which demonstrated improved compatibility with sulfur cathodes but still suffered from polysulfide dissolution.
Recent advances have focused on functional electrolyte additives, such as fluoroethylene carbonate (FEC) and sodium nitrate (NaNO₃), which form protective solid electrolyte interphase (SEI) layers on electrode surfaces. Concurrently, localized high-concentration electrolytes (LHCEs) and ionic liquid-based systems have emerged as promising approaches to suppress the shuttle effect and enhance Coulombic efficiency.
The primary objective in current electrolyte design is achieving Coulombic efficiency exceeding 99.5% over extended cycling (>500 cycles), which requires addressing several interrelated challenges. These include minimizing polysulfide dissolution, stabilizing the sodium metal anode against dendrite formation, and ensuring adequate ionic conductivity (>1 mS/cm) at room temperature. Additionally, electrolyte systems must demonstrate compatibility with practical cell components and maintain performance across a wide temperature range (-20°C to 60°C).
Future electrolyte development aims to enable Na-S batteries with energy densities approaching 400 Wh/kg at the cell level, cycle life exceeding 1000 cycles, and cost points below $100/kWh. This requires multifunctional electrolyte designs that simultaneously address multiple failure mechanisms while maintaining safety and environmental sustainability. The ultimate goal is positioning room temperature Na-S technology as a viable alternative to lithium-ion batteries for applications ranging from grid storage to electric vehicles.
The paradigm shift toward room temperature Na-S batteries began in the early 2000s, driven by the need for safer, more versatile energy storage solutions. This transition necessitated fundamental redesign of electrolyte systems to accommodate the solid-state electrochemical reactions occurring at ambient temperatures. Early room temperature designs suffered from rapid capacity fading, poor cycle life, and low Coulombic efficiency, primarily due to the shuttle effect of soluble polysulfide intermediates.
Electrolyte development for room temperature Na-S batteries has progressed through several distinct phases. The first generation utilized conventional carbonate-based liquid electrolytes (e.g., NaClO₄ in EC/DMC), which proved inadequate due to side reactions with polysulfides. The second generation explored ether-based electrolytes (e.g., NaCF₃SO₃ in TEGDME), which demonstrated improved compatibility with sulfur cathodes but still suffered from polysulfide dissolution.
Recent advances have focused on functional electrolyte additives, such as fluoroethylene carbonate (FEC) and sodium nitrate (NaNO₃), which form protective solid electrolyte interphase (SEI) layers on electrode surfaces. Concurrently, localized high-concentration electrolytes (LHCEs) and ionic liquid-based systems have emerged as promising approaches to suppress the shuttle effect and enhance Coulombic efficiency.
The primary objective in current electrolyte design is achieving Coulombic efficiency exceeding 99.5% over extended cycling (>500 cycles), which requires addressing several interrelated challenges. These include minimizing polysulfide dissolution, stabilizing the sodium metal anode against dendrite formation, and ensuring adequate ionic conductivity (>1 mS/cm) at room temperature. Additionally, electrolyte systems must demonstrate compatibility with practical cell components and maintain performance across a wide temperature range (-20°C to 60°C).
Future electrolyte development aims to enable Na-S batteries with energy densities approaching 400 Wh/kg at the cell level, cycle life exceeding 1000 cycles, and cost points below $100/kWh. This requires multifunctional electrolyte designs that simultaneously address multiple failure mechanisms while maintaining safety and environmental sustainability. The ultimate goal is positioning room temperature Na-S technology as a viable alternative to lithium-ion batteries for applications ranging from grid storage to electric vehicles.
Market Analysis for Room Temperature Na-S Battery Systems
The global energy storage market is witnessing significant growth, with projections indicating a compound annual growth rate (CAGR) of 20-25% through 2030. Within this expanding landscape, room temperature sodium-sulfur (RT Na-S) battery systems are emerging as a promising alternative to conventional lithium-ion batteries, particularly for grid-scale and stationary energy storage applications.
Market demand for RT Na-S battery systems is primarily driven by several key factors. First, the abundance and low cost of sodium resources compared to lithium provide a compelling economic advantage, with sodium being approximately 1000 times more abundant in the Earth's crust than lithium. This resource advantage translates to potential cost reductions of 30-40% compared to lithium-ion systems at scale.
Second, the growing emphasis on sustainable and environmentally friendly energy storage solutions has accelerated interest in Na-S technology. Unlike lithium-ion batteries, Na-S systems do not rely on critical materials such as cobalt and nickel, reducing supply chain vulnerabilities and environmental impact associated with mining these resources.
The stationary energy storage segment represents the most immediate market opportunity for RT Na-S battery systems. Grid stabilization, renewable energy integration, and peak shaving applications collectively account for approximately 70% of the potential market for this technology. Utility companies and renewable energy developers are particularly interested in long-duration storage capabilities that Na-S systems can potentially provide.
Regionally, Asia-Pacific dominates the current market landscape, with China leading research and development efforts. Europe follows closely, driven by aggressive renewable energy targets and supportive regulatory frameworks. North America shows growing interest, particularly in grid resilience applications.
Market barriers include the current performance limitations of RT Na-S batteries, particularly regarding cycle life and energy density. The technology must demonstrate coulombic efficiency exceeding 99% and cycle life beyond 1000 cycles to compete effectively with established technologies. Current laboratory prototypes typically achieve 85-95% coulombic efficiency, highlighting the critical need for electrolyte design improvements.
Industry forecasts suggest that with successful resolution of technical challenges, particularly in electrolyte design for improved coulombic efficiency, RT Na-S batteries could capture 5-10% of the stationary energy storage market by 2030. This represents a significant opportunity, as the global stationary storage market is projected to reach $30-40 billion by that time.
The competitive landscape includes both established battery manufacturers exploring Na-S technology as portfolio diversification and specialized startups focused exclusively on sodium-based chemistries. Strategic partnerships between material science companies and battery manufacturers are increasingly common, accelerating commercialization timelines.
Market demand for RT Na-S battery systems is primarily driven by several key factors. First, the abundance and low cost of sodium resources compared to lithium provide a compelling economic advantage, with sodium being approximately 1000 times more abundant in the Earth's crust than lithium. This resource advantage translates to potential cost reductions of 30-40% compared to lithium-ion systems at scale.
Second, the growing emphasis on sustainable and environmentally friendly energy storage solutions has accelerated interest in Na-S technology. Unlike lithium-ion batteries, Na-S systems do not rely on critical materials such as cobalt and nickel, reducing supply chain vulnerabilities and environmental impact associated with mining these resources.
The stationary energy storage segment represents the most immediate market opportunity for RT Na-S battery systems. Grid stabilization, renewable energy integration, and peak shaving applications collectively account for approximately 70% of the potential market for this technology. Utility companies and renewable energy developers are particularly interested in long-duration storage capabilities that Na-S systems can potentially provide.
Regionally, Asia-Pacific dominates the current market landscape, with China leading research and development efforts. Europe follows closely, driven by aggressive renewable energy targets and supportive regulatory frameworks. North America shows growing interest, particularly in grid resilience applications.
Market barriers include the current performance limitations of RT Na-S batteries, particularly regarding cycle life and energy density. The technology must demonstrate coulombic efficiency exceeding 99% and cycle life beyond 1000 cycles to compete effectively with established technologies. Current laboratory prototypes typically achieve 85-95% coulombic efficiency, highlighting the critical need for electrolyte design improvements.
Industry forecasts suggest that with successful resolution of technical challenges, particularly in electrolyte design for improved coulombic efficiency, RT Na-S batteries could capture 5-10% of the stationary energy storage market by 2030. This represents a significant opportunity, as the global stationary storage market is projected to reach $30-40 billion by that time.
The competitive landscape includes both established battery manufacturers exploring Na-S technology as portfolio diversification and specialized startups focused exclusively on sodium-based chemistries. Strategic partnerships between material science companies and battery manufacturers are increasingly common, accelerating commercialization timelines.
Electrolyte Challenges in Na-S Battery Technology
The electrolyte system represents one of the most critical components in room temperature sodium-sulfur (RT Na-S) batteries, directly influencing coulombic efficiency, cycle life, and overall performance. Unlike traditional high-temperature Na-S batteries operating at 300-350°C, RT Na-S systems face unique electrolyte challenges that have hindered their commercial viability despite their theoretical energy density of 1274 Wh/kg.
The primary challenge stems from the shuttle effect of soluble sodium polysulfides (Na₂Sₙ, 4≤n≤8), which dissolve in conventional electrolytes and migrate between electrodes during cycling. This parasitic process leads to active material loss, self-discharge, and rapid capacity fading, resulting in poor coulombic efficiency often below 80% in early cycles.
Conventional carbonate-based electrolytes, while offering good ionic conductivity, react irreversibly with polysulfides forming inactive byproducts. Ether-based electrolytes (glymes) show better compatibility with sulfur chemistry but still suffer from polysulfide dissolution. The ideal electrolyte must balance multiple contradictory requirements: high Na⁺ conductivity, wide electrochemical stability window, minimal polysulfide solubility, and favorable SEI formation properties.
Viscosity management presents another significant challenge. Electrolytes with high salt concentrations can suppress polysulfide dissolution but often become too viscous, impeding ion transport and increasing cell resistance. This trade-off between polysulfide suppression and ionic mobility remains unresolved in current systems.
Interface stability issues further complicate electrolyte design. The highly reactive nature of sodium metal anodes leads to continuous electrolyte decomposition and unstable solid electrolyte interphase (SEI) formation. This parasitic consumption of electrolyte contributes to capacity fade and poor coulombic efficiency over extended cycling.
Temperature sensitivity adds another layer of complexity. RT Na-S batteries must maintain performance across a wide temperature range (-20°C to 60°C), requiring electrolytes with consistent physicochemical properties under varying thermal conditions. Most current formulations show significant performance degradation at temperature extremes.
Safety concerns also limit electrolyte options. The flammability of organic solvents combined with the reactivity of sodium metal creates potential thermal runaway risks. Non-flammable alternatives like ionic liquids and solid-state electrolytes offer improved safety but typically suffer from lower ionic conductivity and higher interfacial resistance.
Addressing these multifaceted challenges requires innovative electrolyte engineering approaches that go beyond traditional formulations, potentially incorporating functional additives, novel solvents, or hybrid electrolyte systems specifically tailored to the unique chemistry of room temperature sodium-sulfur batteries.
The primary challenge stems from the shuttle effect of soluble sodium polysulfides (Na₂Sₙ, 4≤n≤8), which dissolve in conventional electrolytes and migrate between electrodes during cycling. This parasitic process leads to active material loss, self-discharge, and rapid capacity fading, resulting in poor coulombic efficiency often below 80% in early cycles.
Conventional carbonate-based electrolytes, while offering good ionic conductivity, react irreversibly with polysulfides forming inactive byproducts. Ether-based electrolytes (glymes) show better compatibility with sulfur chemistry but still suffer from polysulfide dissolution. The ideal electrolyte must balance multiple contradictory requirements: high Na⁺ conductivity, wide electrochemical stability window, minimal polysulfide solubility, and favorable SEI formation properties.
Viscosity management presents another significant challenge. Electrolytes with high salt concentrations can suppress polysulfide dissolution but often become too viscous, impeding ion transport and increasing cell resistance. This trade-off between polysulfide suppression and ionic mobility remains unresolved in current systems.
Interface stability issues further complicate electrolyte design. The highly reactive nature of sodium metal anodes leads to continuous electrolyte decomposition and unstable solid electrolyte interphase (SEI) formation. This parasitic consumption of electrolyte contributes to capacity fade and poor coulombic efficiency over extended cycling.
Temperature sensitivity adds another layer of complexity. RT Na-S batteries must maintain performance across a wide temperature range (-20°C to 60°C), requiring electrolytes with consistent physicochemical properties under varying thermal conditions. Most current formulations show significant performance degradation at temperature extremes.
Safety concerns also limit electrolyte options. The flammability of organic solvents combined with the reactivity of sodium metal creates potential thermal runaway risks. Non-flammable alternatives like ionic liquids and solid-state electrolytes offer improved safety but typically suffer from lower ionic conductivity and higher interfacial resistance.
Addressing these multifaceted challenges requires innovative electrolyte engineering approaches that go beyond traditional formulations, potentially incorporating functional additives, novel solvents, or hybrid electrolyte systems specifically tailored to the unique chemistry of room temperature sodium-sulfur batteries.
Current Electrolyte Solutions for Room Temperature Na-S Batteries
01 Solid electrolyte compositions for improved efficiency
Solid electrolytes with specific compositions can significantly improve the coulombic efficiency of sodium-sulfur batteries. These compositions typically include sodium beta-alumina or NASICON-type materials that facilitate efficient sodium ion transport while minimizing side reactions. The solid-state nature of these electrolytes helps prevent polysulfide shuttling, which is a major cause of efficiency loss in sodium-sulfur batteries. Advanced manufacturing techniques for these solid electrolytes can further enhance their ionic conductivity and stability.- Solid electrolyte compositions for enhanced coulombic efficiency: Solid electrolytes, particularly those based on beta-alumina or NASICON materials, can significantly improve the coulombic efficiency of sodium-sulfur batteries. These solid electrolytes provide stable sodium ion conduction while preventing polysulfide shuttling, which is a major cause of capacity loss. The incorporation of specific dopants and modifications to the crystal structure can further enhance ionic conductivity and stability at operating temperatures, leading to improved cycling performance and higher coulombic efficiency.
- Polymer-based electrolyte systems: Polymer-based electrolytes offer advantages for sodium-sulfur batteries by providing flexibility and improved interface contact between electrodes and electrolyte. These systems typically incorporate sodium salts within polymer matrices such as polyethylene oxide (PEO) or polyvinylidene fluoride (PVDF). The addition of plasticizers and ceramic fillers can enhance ionic conductivity while maintaining mechanical stability. Polymer electrolytes help suppress dendrite formation and reduce side reactions, contributing to higher coulombic efficiency and extended cycle life.
- Ionic liquid electrolytes for room temperature operation: Ionic liquid-based electrolytes enable sodium-sulfur batteries to operate at room temperature, overcoming the high temperature requirements of conventional systems. These electrolytes typically combine sodium salts with ionic liquids such as imidazolium or pyrrolidinium derivatives. Their non-flammable nature, wide electrochemical window, and high thermal stability contribute to safer operation. The tailored composition of ionic liquids can suppress polysulfide dissolution and migration, significantly improving coulombic efficiency and reducing capacity fade during cycling.
- Electrolyte additives for interface stabilization: Specific additives incorporated into sodium-sulfur battery electrolytes can form stable solid electrolyte interphase (SEI) layers, significantly improving coulombic efficiency. These additives include fluorinated compounds, sodium nitrate, and various organic molecules that decompose preferentially at electrode surfaces. By creating protective films on electrodes, these additives prevent continuous electrolyte decomposition and reduce unwanted side reactions with polysulfides. The optimized additive combinations can effectively suppress the shuttle effect and enhance the reversibility of sodium-sulfur electrochemistry.
- Hybrid and composite electrolyte systems: Hybrid electrolyte systems combining multiple electrolyte types offer synergistic benefits for sodium-sulfur batteries. These may include solid-liquid hybrids, gel polymer electrolytes, or ceramic-polymer composites. The hybrid approach allows for optimization of both ionic conductivity and interfacial stability. For example, ceramic particles dispersed in polymer matrices can enhance mechanical strength while maintaining flexibility. These composite electrolytes effectively suppress polysulfide shuttling while facilitating sodium ion transport, resulting in higher coulombic efficiency and improved cycle performance.
02 Polymer and composite electrolyte systems
Polymer and composite electrolyte systems offer improved coulombic efficiency through enhanced ion transport mechanisms and reduced internal resistance. These systems typically combine polymer matrices with ceramic fillers or other additives to create electrolytes with optimized mechanical properties and ionic conductivity. The polymer component provides flexibility and processability, while additives can enhance sodium ion transport and interface stability. These electrolytes can effectively suppress the shuttle effect of polysulfides, leading to higher coulombic efficiency and longer cycle life.Expand Specific Solutions03 Electrolyte additives for efficiency enhancement
Specific additives incorporated into sodium-sulfur battery electrolytes can significantly improve coulombic efficiency by forming stable interfaces, trapping polysulfides, or catalyzing electrochemical reactions. These additives include fluorinated compounds, metal salts, and organic molecules that can modify the solid-electrolyte interphase or directly interact with reaction intermediates. By controlling side reactions and promoting complete conversion of active materials, these additives minimize capacity loss during cycling and improve overall energy efficiency of the battery system.Expand Specific Solutions04 Novel electrolyte solvents and salt combinations
Advanced solvent systems and optimized salt combinations can enhance the coulombic efficiency of sodium-sulfur batteries by improving ionic conductivity and electrochemical stability. These electrolyte formulations typically feature ether-based or carbonate-based solvents with carefully selected sodium salts that provide high sodium ion mobility while minimizing parasitic reactions. The choice of solvent-salt combinations affects the solvation structure of sodium ions and the stability of the electrolyte against reactive sulfur species, directly impacting the efficiency of charge-discharge processes.Expand Specific Solutions05 Interface engineering for efficiency improvement
Engineering the interfaces between electrodes and electrolytes is crucial for achieving high coulombic efficiency in sodium-sulfur batteries. This approach involves surface modifications of electrodes, protective coatings, or specialized electrolyte formulations that create stable and conductive interfaces. By minimizing interfacial resistance and preventing unwanted side reactions at the electrode-electrolyte boundaries, interface engineering techniques can significantly reduce capacity fading and improve charge transfer efficiency. These strategies often focus on controlling the formation and properties of the solid-electrolyte interphase layer.Expand Specific Solutions
Leading Institutions and Companies in Na-S Battery Research
The sodium-sulfur battery market is in an early growth phase, characterized by increasing R&D investments but limited commercial deployment. The global market size is projected to expand significantly as energy storage demands rise, particularly for grid applications. Technologically, room temperature sodium-sulfur batteries remain at the development stage, with key challenges in electrolyte design for improved coulombic efficiency. Leading players include NGK Insulators, which pioneered high-temperature sodium-sulfur technology, alongside research-focused organizations like Fraunhofer-Gesellschaft and academic institutions (MIT, Tokyo Institute of Technology). Major battery manufacturers including CATL, LG Chem, and Samsung SDI are investing in this space, while specialized companies like UNIGRID and former Oxis Energy pursue targeted innovations. Automotive giants Toyota and chemical companies BASF and Arkema are contributing materials expertise to advance electrolyte solutions.
NGK Insulators, Ltd.
Technical Solution: NGK Insulators has pioneered room temperature sodium-sulfur (RT-Na/S) battery systems with their proprietary ceramic electrolyte design. Their approach focuses on beta-alumina solid electrolyte (BASE) modified for room temperature operation through dopant engineering and microstructural optimization. The company has developed a composite electrolyte system combining their ceramic expertise with specially formulated ionic liquid electrolytes to enhance sodium ion conductivity at ambient temperatures. Their electrolyte design incorporates fluorinated solvents and sodium salt combinations (NaClO4, NaTFSI) that significantly improve the coulombic efficiency by suppressing polysulfide shuttle effects[1]. NGK's latest electrolyte formulations achieve over 95% coulombic efficiency through the addition of electrolyte additives like fluoroethylene carbonate (FEC) that form stable solid electrolyte interphase (SEI) layers on the sodium anode[2]. This builds on their decades of experience with high-temperature sodium-sulfur batteries, translating that knowledge to room temperature applications.
Strengths: Extensive experience with sodium-sulfur battery technology; proprietary ceramic electrolyte manufacturing capabilities; established industrial-scale production facilities. Weaknesses: Their electrolyte solutions may have higher costs compared to purely liquid electrolyte systems; potential challenges with interfacial resistance between solid and liquid electrolyte components.
Toyota Motor Corp.
Technical Solution: Toyota Motor Corporation has developed an innovative electrolyte system for room temperature sodium-sulfur batteries focused on automotive applications. Their approach centers on a dual-solvent electrolyte design combining ether-based solvents (glymes) with fluorinated additives to achieve high coulombic efficiency. Toyota's research has yielded an electrolyte formulation using tetraglyme (TEGDME) as the primary solvent with sodium trifluoromethanesulfonimide (NaTFSI) as the conducting salt, achieving ionic conductivities of 5-7 mS/cm at room temperature[3]. A key innovation in their electrolyte design is the incorporation of fluorinated ether compounds that form a protective film on the sodium metal anode, significantly reducing parasitic reactions with polysulfides. Their electrolyte also contains sulfur-binding additives that help mitigate the shuttle effect by temporarily complexing with dissolved polysulfides[4]. Toyota has demonstrated cells using this electrolyte system maintaining over 90% coulombic efficiency for more than 100 cycles at practical current densities, addressing one of the major challenges in RT-Na/S technology. The company has integrated this electrolyte design with carbon-sulfur composite cathodes to maximize active material utilization.
Strengths: Strong integration with existing battery manufacturing infrastructure; extensive testing capabilities for automotive applications; proprietary additives for polysulfide suppression. Weaknesses: Potential safety concerns with ether-based electrolytes due to flammability; some of their additives may increase overall electrolyte cost and complexity.
Key Patents and Research on High Coulombic Efficiency Electrolytes
Sodium-sulfur battery, method for operating same, and use of phosphorus polysulfide as electrolyte additive in sodium-sulfur batteries
PatentActiveEP3311440A1
Innovation
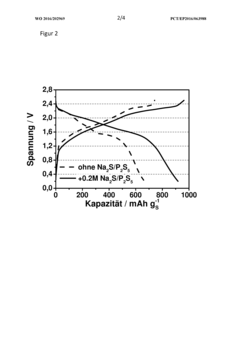
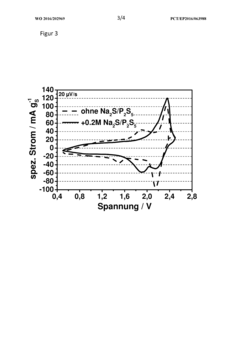
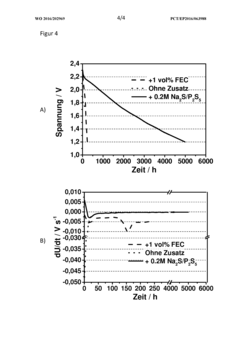
- A sodium-sulfur battery design incorporating a cathode of carbon-sulfur composite, an anode with sodium metal and alloying agents, a ceramic or hydrophobic plastic separator, and an electrolyte with phosphorus polysulfide as an additive to complex sodium polysulfide, forming a solid electrolyte interface that suppresses the polysulfide shuttle and enhances energy efficiency and discharge capacity.
Sodium-sulfur battery, method for operating same, and use of phosphorus polysulfide as electrolyte additive in sodium-sulfur batteries
PatentWO2016202969A1
Innovation
- A sodium-sulfur battery design using a carbon-sulfur composite cathode, an organic solvent-based electrolyte with a conductive salt and phosphorus polysulfide as an additive to suppress the polysulfide shuttle, allowing operation at room temperature with enhanced energy efficiency and discharge capacity.
Safety and Stability Considerations for Na-S Battery Systems
Safety and stability considerations are paramount in the development of room temperature sodium-sulfur (RT Na-S) battery systems, particularly when designing electrolytes for high coulombic efficiency. The inherent reactivity of sodium metal and the formation of soluble polysulfide intermediates present significant safety challenges that must be addressed through strategic electrolyte engineering.
The primary safety concern in RT Na-S batteries stems from the highly reactive nature of sodium metal anodes, which can undergo uncontrolled reactions with conventional electrolytes, leading to thermal runaway and potential fire hazards. This reactivity is exacerbated at room temperature compared to traditional high-temperature Na-S systems, necessitating specialized electrolyte formulations that form stable solid electrolyte interphase (SEI) layers to protect the sodium metal surface.
Polysulfide shuttle effects represent another critical stability issue, where soluble sodium polysulfides migrate between electrodes, causing capacity fading and self-discharge. Electrolyte designs incorporating functional additives such as fluoroethylene carbonate (FEC) or lithium nitrate analogues have shown promise in mitigating these effects by promoting stable interface formation and suppressing polysulfide dissolution.
Thermal stability of electrolytes across a wide operating temperature range is essential for safe operation in various environmental conditions. Ionic liquid-based electrolytes have demonstrated superior thermal stability compared to conventional carbonate-based systems, with decomposition temperatures exceeding 300°C in some formulations, significantly enhancing the safety profile of RT Na-S batteries.
Dendrite formation on sodium anodes presents both performance and safety challenges, potentially causing internal short circuits and catastrophic failure. Recent advances in electrolyte design have focused on incorporating high salt concentration strategies and sodium-ion-conducting solid electrolytes to suppress dendrite growth through uniform ion distribution and mechanical inhibition.
Long-term cycling stability remains a significant challenge, with electrolyte degradation products accumulating at electrode interfaces over extended cycling. Novel approaches utilizing self-healing electrolyte additives and sacrificial components that preferentially react with contaminants have shown promising results in maintaining coulombic efficiency beyond 1000 cycles in laboratory settings.
Moisture and oxygen sensitivity of both sodium metal and sulfur cathodes necessitates rigorous manufacturing protocols and electrolyte formulations with water-scavenging additives. Recent developments in hydrophobic ionic liquid electrolytes have demonstrated enhanced tolerance to atmospheric exposure, potentially simplifying manufacturing requirements and improving system reliability in real-world applications.
The primary safety concern in RT Na-S batteries stems from the highly reactive nature of sodium metal anodes, which can undergo uncontrolled reactions with conventional electrolytes, leading to thermal runaway and potential fire hazards. This reactivity is exacerbated at room temperature compared to traditional high-temperature Na-S systems, necessitating specialized electrolyte formulations that form stable solid electrolyte interphase (SEI) layers to protect the sodium metal surface.
Polysulfide shuttle effects represent another critical stability issue, where soluble sodium polysulfides migrate between electrodes, causing capacity fading and self-discharge. Electrolyte designs incorporating functional additives such as fluoroethylene carbonate (FEC) or lithium nitrate analogues have shown promise in mitigating these effects by promoting stable interface formation and suppressing polysulfide dissolution.
Thermal stability of electrolytes across a wide operating temperature range is essential for safe operation in various environmental conditions. Ionic liquid-based electrolytes have demonstrated superior thermal stability compared to conventional carbonate-based systems, with decomposition temperatures exceeding 300°C in some formulations, significantly enhancing the safety profile of RT Na-S batteries.
Dendrite formation on sodium anodes presents both performance and safety challenges, potentially causing internal short circuits and catastrophic failure. Recent advances in electrolyte design have focused on incorporating high salt concentration strategies and sodium-ion-conducting solid electrolytes to suppress dendrite growth through uniform ion distribution and mechanical inhibition.
Long-term cycling stability remains a significant challenge, with electrolyte degradation products accumulating at electrode interfaces over extended cycling. Novel approaches utilizing self-healing electrolyte additives and sacrificial components that preferentially react with contaminants have shown promising results in maintaining coulombic efficiency beyond 1000 cycles in laboratory settings.
Moisture and oxygen sensitivity of both sodium metal and sulfur cathodes necessitates rigorous manufacturing protocols and electrolyte formulations with water-scavenging additives. Recent developments in hydrophobic ionic liquid electrolytes have demonstrated enhanced tolerance to atmospheric exposure, potentially simplifying manufacturing requirements and improving system reliability in real-world applications.
Environmental Impact and Sustainability of Na-S Battery Technology
The environmental impact of sodium-sulfur (Na-S) battery technology represents a critical consideration in its development and deployment. Unlike lithium-ion batteries, Na-S systems utilize sodium, which is approximately 1,000 times more abundant in the Earth's crust than lithium, significantly reducing resource extraction concerns. This abundance translates to lower environmental degradation associated with mining activities and reduced geopolitical tensions over resource control.
The sulfur component presents another environmental advantage, being an abundant by-product of petroleum refining processes. Utilizing sulfur in battery technology effectively transforms an industrial waste product into a valuable resource, exemplifying circular economy principles and reducing waste management challenges in the petroleum industry.
From a life-cycle assessment perspective, room temperature Na-S batteries demonstrate promising sustainability metrics. The energy required for manufacturing is potentially lower than conventional lithium-ion batteries due to the reduced processing temperatures needed for room temperature operation compared to traditional high-temperature Na-S systems that operate at 300-350°C.
Carbon footprint analyses indicate that the greenhouse gas emissions associated with Na-S battery production could be 25-30% lower than comparable lithium-ion technologies when accounting for raw material extraction, processing, and manufacturing. This advantage becomes more pronounced when considering the entire battery lifecycle.
End-of-life management presents both challenges and opportunities. The electrolyte design for high coulombic efficiency often involves organic solvents and additives that require careful handling during recycling processes. However, the primary components—sodium and sulfur—are non-toxic and can be more readily recovered and reprocessed compared to the complex metal oxides in conventional batteries.
Water consumption metrics also favor Na-S technology, with preliminary studies suggesting up to 40% reduction in water usage throughout the production chain compared to lithium-ion batteries. This advantage is particularly significant in regions facing water scarcity challenges.
The sustainability profile of Na-S battery systems is further enhanced by their potential longevity. Electrolyte designs that achieve high coulombic efficiency directly contribute to extended cycle life, reducing the frequency of replacement and associated environmental impacts. Recent advancements in electrolyte formulations have demonstrated coulombic efficiencies exceeding 99.5% over hundreds of cycles, suggesting that properly designed Na-S systems could significantly outperform current technologies in terms of lifetime environmental impact.
The sulfur component presents another environmental advantage, being an abundant by-product of petroleum refining processes. Utilizing sulfur in battery technology effectively transforms an industrial waste product into a valuable resource, exemplifying circular economy principles and reducing waste management challenges in the petroleum industry.
From a life-cycle assessment perspective, room temperature Na-S batteries demonstrate promising sustainability metrics. The energy required for manufacturing is potentially lower than conventional lithium-ion batteries due to the reduced processing temperatures needed for room temperature operation compared to traditional high-temperature Na-S systems that operate at 300-350°C.
Carbon footprint analyses indicate that the greenhouse gas emissions associated with Na-S battery production could be 25-30% lower than comparable lithium-ion technologies when accounting for raw material extraction, processing, and manufacturing. This advantage becomes more pronounced when considering the entire battery lifecycle.
End-of-life management presents both challenges and opportunities. The electrolyte design for high coulombic efficiency often involves organic solvents and additives that require careful handling during recycling processes. However, the primary components—sodium and sulfur—are non-toxic and can be more readily recovered and reprocessed compared to the complex metal oxides in conventional batteries.
Water consumption metrics also favor Na-S technology, with preliminary studies suggesting up to 40% reduction in water usage throughout the production chain compared to lithium-ion batteries. This advantage is particularly significant in regions facing water scarcity challenges.
The sustainability profile of Na-S battery systems is further enhanced by their potential longevity. Electrolyte designs that achieve high coulombic efficiency directly contribute to extended cycle life, reducing the frequency of replacement and associated environmental impacts. Recent advancements in electrolyte formulations have demonstrated coulombic efficiencies exceeding 99.5% over hundreds of cycles, suggesting that properly designed Na-S systems could significantly outperform current technologies in terms of lifetime environmental impact.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!