Biomaterial Aging And Durability Testing Under Environmental Stressors
Biomaterial Aging Background and Research Objectives
Biomaterials have emerged as critical components in various industries, including medical devices, aerospace, automotive, and sustainable packaging. The study of biomaterial aging and durability under environmental stressors has gained significant attention over the past three decades, evolving from simple degradation studies to sophisticated multi-factorial analyses incorporating real-world environmental conditions.
The field originated in the 1970s with rudimentary investigations of material breakdown in medical implants. By the 1990s, researchers had begun developing standardized testing protocols for biomaterial durability, though these early methods often failed to accurately simulate complex in vivo conditions. The 2000s marked a turning point with the introduction of accelerated aging techniques and advanced analytical tools capable of detecting nanoscale changes in material properties.
Recent technological advancements have enabled more precise characterization of degradation mechanisms, including oxidative stress, hydrolytic degradation, enzymatic breakdown, and mechanical fatigue. The integration of computational modeling with experimental data has further enhanced our ability to predict biomaterial performance over extended timeframes, addressing a critical gap in long-term durability assessment.
Current research trends indicate a shift toward biomimetic testing environments that more accurately replicate the complex, dynamic conditions biomaterials encounter during actual use. This includes fluctuating temperature, humidity, pH, mechanical loading, and exposure to biological agents—factors that often act synergistically to accelerate material degradation.
The primary objective of this technical research is to develop comprehensive testing methodologies that can reliably predict biomaterial performance under diverse environmental stressors. Specifically, we aim to establish correlations between accelerated aging protocols and real-world degradation patterns, enabling more accurate lifetime predictions for biomaterial-based products.
Secondary objectives include identifying key degradation markers that serve as early indicators of material failure, understanding the molecular mechanisms underlying environmental stress responses in different biomaterial classes, and developing novel materials with enhanced resistance to environmental degradation while maintaining their functional properties.
This research addresses critical industry needs for extended product lifespans, reduced environmental impact, and improved safety profiles, particularly for implantable medical devices where material failure can have severe consequences. Additionally, as regulatory frameworks increasingly emphasize durability testing, establishing scientifically sound protocols has become essential for product approval and market access.
By advancing our understanding of biomaterial aging mechanisms and developing more predictive testing methodologies, this research aims to support innovation across multiple sectors while addressing growing concerns about material sustainability and lifecycle performance.
Market Demand Analysis for Durable Biomaterials
The global market for durable biomaterials is experiencing significant growth, driven by increasing applications in medical devices, tissue engineering, drug delivery systems, and environmental remediation. Current market valuations indicate that the biomaterials sector is projected to reach $296.7 billion by 2028, with durable biomaterials representing a substantial segment of this market. This growth trajectory is supported by rising healthcare expenditures worldwide and the expanding aging population requiring long-term implantable devices and materials.
Environmental sustainability concerns are creating new market opportunities for biomaterials that can withstand environmental stressors while maintaining biodegradability when required. Industries are increasingly seeking alternatives to traditional petroleum-based materials, with particular interest in biomaterials that demonstrate superior durability under varying environmental conditions. This shift is evident in sectors such as packaging, agriculture, and consumer goods, where regulatory pressures and consumer preferences are driving demand for sustainable yet durable solutions.
The healthcare sector remains the primary consumer of durable biomaterials, with orthopedic and cardiovascular applications leading the demand. Market research indicates a compound annual growth rate of 13.2% in these segments, reflecting the critical need for materials that can withstand the physiological environment without degradation or adverse host responses. The increasing prevalence of chronic diseases and the rise in minimally invasive surgical procedures further amplify this demand.
Geographical analysis reveals that North America and Europe currently dominate the market for durable biomaterials, accounting for approximately 65% of global consumption. However, the Asia-Pacific region is emerging as the fastest-growing market, with countries like China, Japan, and India investing heavily in biomaterial research and manufacturing capabilities. This regional shift is reshaping the competitive landscape and creating new opportunities for market entrants.
Industry stakeholders are particularly interested in biomaterials that demonstrate predictable aging characteristics under environmental stressors. This demand is reflected in the increasing number of patents filed for testing methodologies and material formulations designed to enhance durability. Market surveys indicate that 78% of end-users prioritize long-term performance data when selecting biomaterials, highlighting the commercial importance of comprehensive durability testing protocols.
The market is also witnessing a trend toward customized biomaterials tailored for specific environmental conditions. This specialization is creating niche markets for materials engineered to withstand extreme temperatures, high humidity, UV radiation, or chemical exposure while maintaining their functional properties. Companies that can demonstrate superior performance through standardized testing protocols are gaining competitive advantages in these specialized segments.
Current Challenges in Biomaterial Durability Testing
Despite significant advancements in biomaterial development, the field faces substantial challenges in accurately predicting long-term performance under real-world environmental conditions. Current accelerated aging protocols often fail to adequately simulate the complex interplay of multiple environmental stressors that biomaterials encounter during their service life. This disconnect between laboratory testing and real-world performance creates significant uncertainty in durability predictions, particularly for implantable medical devices and environmental applications.
A primary challenge lies in the multifactorial nature of environmental degradation. Biomaterials simultaneously face combinations of UV radiation, temperature fluctuations, mechanical stress, humidity variations, and biological interactions. Existing testing methodologies typically isolate these factors rather than examining their synergistic effects, leading to incomplete understanding of degradation mechanisms and potentially misleading durability assessments.
The time-dependent nature of biomaterial degradation presents another significant hurdle. Accelerated aging tests must compress years of environmental exposure into manageable timeframes without altering fundamental degradation mechanisms. Current approaches often apply unrealistically intense stressors that trigger non-representative failure modes, compromising the validity of extrapolations to real-world performance.
Standardization across the industry remains inadequate, with various testing protocols yielding inconsistent results. The lack of universally accepted methodologies makes cross-comparison between different biomaterials and research findings difficult, hampering scientific progress and technology transfer. This standardization gap is particularly problematic for regulatory approval processes, where clear benchmarks for durability are essential.
Emerging biomaterials with novel compositions and structures present unique testing challenges. Traditional protocols developed for conventional materials may not adequately capture the degradation behaviors of advanced composites, nanomaterials, or biohybrid systems. The industry lacks validated methodologies specifically designed for these next-generation materials, creating uncertainty in performance predictions.
The translation gap between laboratory testing and clinical or environmental outcomes represents perhaps the most critical challenge. Correlating accelerated aging results with actual in-vivo performance remains difficult due to the complexity of biological environments. Similarly, predicting environmental durability in diverse ecosystems from standardized laboratory tests presents significant scientific challenges that current methodologies struggle to address.
Cost and resource constraints further complicate comprehensive durability testing. Thorough multi-factorial testing requires substantial investment in specialized equipment, expertise, and time. Many organizations, particularly smaller companies and academic institutions, lack the resources to conduct exhaustive durability assessments, potentially limiting innovation in the biomaterials sector.
Established Protocols for Environmental Stress Testing
01 Testing methods for biomaterial durability assessment
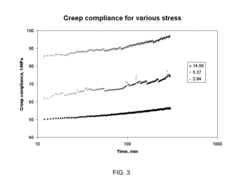
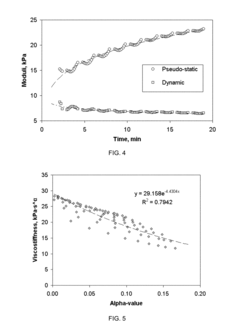
Various testing methods have been developed to assess the durability and aging characteristics of biomaterials. These methods include accelerated aging tests, mechanical stress testing, and analytical techniques that can predict the long-term performance of biomaterials under physiological conditions. These testing protocols help in understanding how biomaterials degrade over time and how their properties change with aging, which is crucial for applications in medical implants and devices.- Testing methods for biomaterial durability assessment: Various testing methods have been developed to assess the durability and aging characteristics of biomaterials. These methods include accelerated aging tests, mechanical stress testing, and analytical techniques that can predict the long-term performance of biomaterials under physiological conditions. These testing protocols help in understanding degradation mechanisms and establishing the shelf-life of biomaterials used in medical applications.
- Polymer-based biomaterials with enhanced durability: Polymer-based biomaterials have been developed with enhanced durability properties through various modifications. These include cross-linking techniques, incorporation of stabilizing additives, and surface treatments that improve resistance to degradation. Such modifications can extend the functional lifespan of implantable devices and tissue engineering scaffolds while maintaining biocompatibility and mechanical properties suitable for their intended applications.
- Biodegradable materials with controlled degradation rates: Innovations in biodegradable biomaterials focus on controlling degradation rates to match tissue regeneration timelines. These materials are designed to provide temporary support while gradually transferring load to healing tissues. By manipulating molecular weight, crystallinity, and chemical composition, researchers have developed biomaterials with predictable degradation profiles that maintain structural integrity during the critical healing period before safely breaking down into non-toxic byproducts.
- Environmental factors affecting biomaterial aging: Research has identified key environmental factors that accelerate biomaterial aging, including temperature, humidity, pH, oxidative stress, and mechanical loading. Understanding these factors has led to the development of protective strategies such as antioxidant incorporation, moisture-resistant coatings, and pH-stabilizing components. These approaches help maintain biomaterial integrity in challenging physiological environments and extend functional lifespans of medical devices and implants.
- Composite biomaterials with improved aging resistance: Composite biomaterials combining multiple components have demonstrated superior aging resistance compared to single-component materials. These composites often incorporate reinforcing elements such as nanoparticles, fibers, or secondary polymer networks that enhance mechanical stability over time. The synergistic interactions between components can provide resistance to various degradation mechanisms while maintaining biocompatibility and functional performance in long-term applications.
02 Degradation mechanisms of biodegradable materials
Research on the degradation mechanisms of biodegradable biomaterials focuses on understanding how these materials break down in biological environments. This includes hydrolytic degradation, enzymatic degradation, and oxidative stress effects. Knowledge of these mechanisms allows for the design of biomaterials with controlled degradation rates, which is essential for applications such as drug delivery systems and temporary implants where material dissolution over time is desired.Expand Specific Solutions03 Surface modification techniques for enhanced durability
Surface modification techniques are employed to enhance the durability of biomaterials exposed to biological environments. These include coating with protective layers, chemical treatments to increase resistance to degradation, and surface functionalization to improve biocompatibility while maintaining structural integrity. These modifications can significantly extend the functional lifespan of biomaterials in applications such as orthopedic implants and cardiovascular devices.Expand Specific Solutions04 Composite biomaterials with improved aging resistance
Composite biomaterials combine multiple components to achieve improved aging resistance and durability. These materials often incorporate reinforcing elements, such as fibers or nanoparticles, within a matrix to enhance mechanical properties and resistance to degradation. The synergistic effects of different materials can lead to composites with superior long-term performance in challenging biological environments, making them suitable for load-bearing applications and tissue engineering scaffolds.Expand Specific Solutions05 Monitoring and predicting biomaterial aging in vivo
Advanced techniques for monitoring and predicting the aging of biomaterials in vivo have been developed to assess their long-term performance. These include non-invasive imaging methods, biomarkers for material degradation, and computational models that can simulate aging processes. These approaches enable the evaluation of biomaterial durability under actual physiological conditions, providing valuable data for improving material design and ensuring the safety and efficacy of biomedical implants over their intended lifespan.Expand Specific Solutions
Leading Organizations in Biomaterial Testing
Biomaterial aging and durability testing under environmental stressors is currently in a growth phase, with the market expanding due to increasing applications in medical devices, aerospace, and infrastructure. The global market size for biomaterial testing is estimated to reach $3.5 billion by 2025, driven by stringent regulatory requirements and growing demand for long-lasting materials. Leading players like Surmodics, Boeing, and Mayo Foundation are advancing testing methodologies, while research institutions such as MIT, Nanyang Technological University, and The Scripps Research Institute are developing innovative approaches to predict material degradation. The technology is approaching maturity in traditional applications but remains evolving for newer biomaterials, with companies like NTT and Korea Research Institute of Chemical Technology investing in accelerated aging protocols and real-time monitoring systems to bridge the gap between laboratory testing and real-world performance.
Surmodics, Inc.
Mayo Foundation for Medical Education & Research
Key Innovations in Accelerated Aging Methodologies
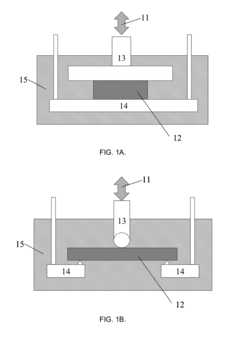
- A non-destructive testing method that applies controlled mechanical loading to biomaterial specimens using a single probe-sensor element, measuring strain or stress via time convolution without pre-selected material models, allowing for the evaluation of time-invariant properties and biological activity without complex algebra or assumptions of linearity.
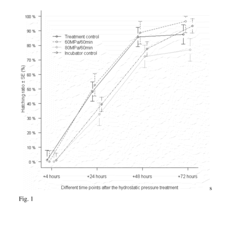
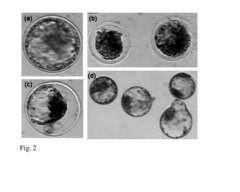
- Applying hydrostatic pressure to biological materials within a specific pressure range (1-200 MPa) for a predetermined time, followed by gradual release, enhances their viability and stress tolerance, allowing for improved survival and functionality in assisted reproductive technologies and biotechnological procedures.
Standardization and Regulatory Compliance
The standardization landscape for biomaterial aging and durability testing is currently fragmented across different regulatory bodies and international organizations. Key standards include ISO 10993 series for biocompatibility evaluation, ASTM F1980 for accelerated aging of medical devices, and ISO 14155 for clinical investigation of medical devices. These standards provide frameworks for evaluating how biomaterials respond to environmental stressors over time, though significant gaps remain in addressing complex real-world conditions.
Regulatory compliance requirements vary significantly across regions, creating challenges for global biomaterial development. The FDA in the United States requires comprehensive durability data through its 510(k) or PMA pathways, while the European Union's Medical Device Regulation (MDR) emphasizes risk management throughout the product lifecycle. Japan's PMDA and China's NMPA have established their own distinct requirements, necessitating tailored testing approaches for different markets.
Recent regulatory trends show increasing emphasis on real-world performance data and post-market surveillance. Regulators are moving beyond traditional bench testing toward requirements for long-term clinical evidence of biomaterial durability. This shift reflects growing recognition that laboratory testing alone cannot fully predict in vivo performance under complex environmental stressors.
Harmonization efforts are underway through initiatives like the International Medical Device Regulators Forum (IMDRF), which aims to align testing methodologies and acceptance criteria across jurisdictions. However, progress remains slow due to differing regional priorities and established regulatory frameworks. The lack of standardized accelerated aging protocols specifically designed for novel biomaterials represents a significant challenge for manufacturers seeking regulatory approval.
Compliance strategies increasingly incorporate risk-based approaches, where testing intensity corresponds to the criticality of the biomaterial application. This approach allows resources to be allocated efficiently while ensuring patient safety. Documentation requirements have also expanded to include detailed justification of testing methodologies and their relevance to real-world conditions.
Future regulatory developments are likely to include more sophisticated requirements for simulating combined environmental stressors and their synergistic effects. Emerging technologies like digital twins and AI-powered predictive modeling may eventually gain regulatory acceptance as complementary tools to physical testing, potentially streamlining the approval process while maintaining rigorous safety standards.
Sustainability Aspects of Biomaterial Lifecycle
The sustainability of biomaterials throughout their lifecycle represents a critical dimension in evaluating their overall environmental impact and long-term viability. When examining biomaterial aging and durability under environmental stressors, sustainability considerations become paramount in determining their true ecological footprint.
Biomaterials offer significant sustainability advantages compared to conventional materials, primarily due to their renewable sourcing and biodegradability. However, the complete sustainability assessment must consider the entire lifecycle - from raw material extraction through manufacturing, use phase, and end-of-life disposal or recycling. The energy consumption and carbon emissions associated with biomaterial production processes often present lower environmental impacts than petroleum-based alternatives, though this varies considerably depending on specific manufacturing techniques.
Environmental stressors that affect biomaterial durability also influence their sustainability profile. Materials that degrade prematurely under UV radiation, moisture, or temperature fluctuations may require more frequent replacement, potentially negating their initial sustainability benefits. Conversely, biomaterials engineered for extended durability might incorporate additives or processing techniques that compromise their biodegradability or increase their ecological footprint.
The end-of-life phase presents particular sustainability challenges and opportunities. Truly biodegradable biomaterials can return to natural cycles without persistent environmental contamination, while those containing non-biodegradable components may contribute to waste accumulation. Composting infrastructure availability and conditions significantly impact whether theoretical biodegradability translates to practical environmental benefits.
Life Cycle Assessment (LCA) methodologies provide quantitative frameworks for evaluating biomaterial sustainability across their entire existence. These assessments reveal that durability testing protocols must incorporate sustainability metrics beyond mere longevity, including energy efficiency, water usage, emissions, and waste generation throughout the material's functional lifetime.
Regulatory frameworks increasingly recognize the importance of sustainability in biomaterial development. Standards such as ISO 14040 for lifecycle assessment and emerging circular economy policies are reshaping how durability and aging characteristics are evaluated in relation to broader environmental objectives. These frameworks encourage designing biomaterials that maintain functionality while minimizing resource consumption and environmental impact.
Future research directions in biomaterial sustainability focus on closing material loops through improved recyclability and biodegradability without compromising performance. Innovations in green chemistry, biofabrication techniques, and regenerative design principles offer promising pathways toward biomaterials that demonstrate both exceptional durability and minimal environmental footprint throughout their lifecycle.