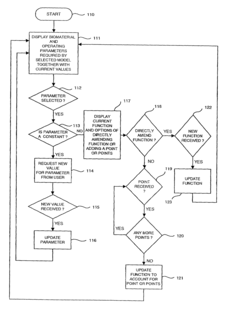
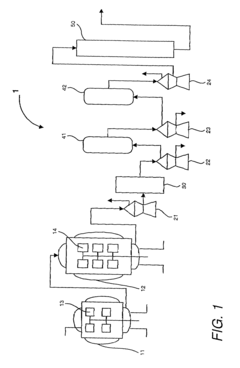
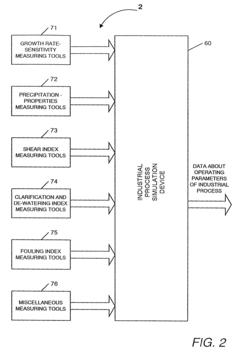
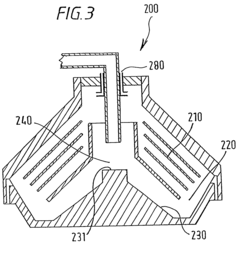
Safety Assessment Frameworks For Industrial Biomaterial Manufacturing
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomaterial Safety Assessment Background and Objectives
The industrial biomaterial manufacturing sector has witnessed significant evolution over the past decades, transitioning from traditional biological products to advanced biomaterials with diverse applications across healthcare, agriculture, and environmental remediation. This technological progression has introduced complex safety considerations that extend beyond conventional manufacturing paradigms, necessitating comprehensive assessment frameworks to ensure public health protection and environmental sustainability.
Historical safety incidents in biomaterial production, including contamination events in biopharmaceuticals and unexpected ecological impacts from engineered biomaterials, have highlighted the critical importance of robust safety protocols. These incidents have shaped regulatory approaches and industry practices, driving the development of increasingly sophisticated assessment methodologies tailored to the unique characteristics of biological manufacturing processes.
The convergence of biotechnology with other emerging fields such as nanotechnology and synthetic biology has further expanded the complexity of safety considerations. Novel biomaterials with unprecedented properties require evaluation frameworks that can adequately address their potential interactions with biological systems and ecosystems. Traditional risk assessment models often prove insufficient when applied to these innovative biomaterials, creating an urgent need for specialized approaches.
Current safety assessment practices in industrial biomaterial manufacturing exhibit considerable variation across different regions and sectors. While pharmaceutical biomaterials operate under stringent regulatory frameworks like Good Manufacturing Practices (GMP), other biomaterial applications may face regulatory gaps or inconsistent standards. This fragmentation presents challenges for manufacturers operating in global markets and potentially compromises safety assurance.
The primary objective of biomaterial safety assessment is to establish comprehensive, science-based frameworks that can effectively identify, evaluate, and mitigate risks throughout the entire lifecycle of industrial biomaterials. These frameworks must balance innovation enablement with rigorous safety standards, allowing technological advancement while protecting human health and environmental integrity.
Specific goals include developing standardized testing protocols that address the unique characteristics of different biomaterial categories, creating harmonized international standards to facilitate global trade while maintaining safety, and implementing adaptive regulatory approaches that can evolve alongside technological innovation. Additionally, there is a pressing need for assessment methodologies that can predict long-term safety implications beyond immediate toxicity concerns.
The evolution toward more predictive and comprehensive safety assessment frameworks represents a critical enabler for the sustainable growth of the industrial biomaterial sector, potentially unlocking significant economic and societal benefits while ensuring responsible innovation practices.
Historical safety incidents in biomaterial production, including contamination events in biopharmaceuticals and unexpected ecological impacts from engineered biomaterials, have highlighted the critical importance of robust safety protocols. These incidents have shaped regulatory approaches and industry practices, driving the development of increasingly sophisticated assessment methodologies tailored to the unique characteristics of biological manufacturing processes.
The convergence of biotechnology with other emerging fields such as nanotechnology and synthetic biology has further expanded the complexity of safety considerations. Novel biomaterials with unprecedented properties require evaluation frameworks that can adequately address their potential interactions with biological systems and ecosystems. Traditional risk assessment models often prove insufficient when applied to these innovative biomaterials, creating an urgent need for specialized approaches.
Current safety assessment practices in industrial biomaterial manufacturing exhibit considerable variation across different regions and sectors. While pharmaceutical biomaterials operate under stringent regulatory frameworks like Good Manufacturing Practices (GMP), other biomaterial applications may face regulatory gaps or inconsistent standards. This fragmentation presents challenges for manufacturers operating in global markets and potentially compromises safety assurance.
The primary objective of biomaterial safety assessment is to establish comprehensive, science-based frameworks that can effectively identify, evaluate, and mitigate risks throughout the entire lifecycle of industrial biomaterials. These frameworks must balance innovation enablement with rigorous safety standards, allowing technological advancement while protecting human health and environmental integrity.
Specific goals include developing standardized testing protocols that address the unique characteristics of different biomaterial categories, creating harmonized international standards to facilitate global trade while maintaining safety, and implementing adaptive regulatory approaches that can evolve alongside technological innovation. Additionally, there is a pressing need for assessment methodologies that can predict long-term safety implications beyond immediate toxicity concerns.
The evolution toward more predictive and comprehensive safety assessment frameworks represents a critical enabler for the sustainable growth of the industrial biomaterial sector, potentially unlocking significant economic and societal benefits while ensuring responsible innovation practices.
Market Demand Analysis for Industrial Biomaterial Products
The industrial biomaterials market has experienced significant growth in recent years, driven by increasing environmental concerns, regulatory pressures, and consumer demand for sustainable products. The global industrial biomaterials market was valued at approximately 107.5 billion USD in 2022 and is projected to reach 215.6 billion USD by 2030, growing at a CAGR of 9.1% during the forecast period.
Healthcare and medical applications represent the largest segment of the industrial biomaterials market, accounting for roughly 38% of the total market share. This dominance is attributed to the rising prevalence of chronic diseases, an aging global population, and increasing investments in healthcare infrastructure. Orthopedic applications, tissue engineering, and drug delivery systems are particularly driving demand in this sector.
The packaging industry has emerged as the fastest-growing application segment for industrial biomaterials, with a growth rate exceeding 12% annually. This surge is primarily due to stringent regulations against single-use plastics and growing consumer preference for environmentally friendly packaging solutions. Major food and beverage companies have committed to transitioning to biodegradable packaging materials, creating substantial market opportunities.
Automotive and transportation sectors are increasingly adopting biomaterials for lightweight components, interior parts, and insulation materials. This trend is driven by fuel efficiency requirements and emissions regulations, with biomaterials offering weight reduction of 10-30% compared to traditional materials while maintaining comparable performance characteristics.
Regional analysis indicates that North America currently leads the industrial biomaterials market with approximately 35% market share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the highest growth rate, driven by rapid industrialization, increasing healthcare expenditure, and government initiatives promoting sustainable manufacturing practices in countries like China, India, and Japan.
Consumer awareness and preference for sustainable products have significantly influenced market dynamics. According to recent surveys, 73% of global consumers are willing to pay a premium for products made with sustainable materials. This shift in consumer behavior has prompted manufacturers across various industries to incorporate biomaterials into their product development strategies.
Despite the promising growth trajectory, several challenges affect market demand, including high production costs, technical limitations in certain applications, and inconsistent regulatory frameworks across different regions. The cost premium for biomaterials compared to conventional materials remains a significant barrier, particularly in price-sensitive markets and applications where performance requirements are stringent.
Healthcare and medical applications represent the largest segment of the industrial biomaterials market, accounting for roughly 38% of the total market share. This dominance is attributed to the rising prevalence of chronic diseases, an aging global population, and increasing investments in healthcare infrastructure. Orthopedic applications, tissue engineering, and drug delivery systems are particularly driving demand in this sector.
The packaging industry has emerged as the fastest-growing application segment for industrial biomaterials, with a growth rate exceeding 12% annually. This surge is primarily due to stringent regulations against single-use plastics and growing consumer preference for environmentally friendly packaging solutions. Major food and beverage companies have committed to transitioning to biodegradable packaging materials, creating substantial market opportunities.
Automotive and transportation sectors are increasingly adopting biomaterials for lightweight components, interior parts, and insulation materials. This trend is driven by fuel efficiency requirements and emissions regulations, with biomaterials offering weight reduction of 10-30% compared to traditional materials while maintaining comparable performance characteristics.
Regional analysis indicates that North America currently leads the industrial biomaterials market with approximately 35% market share, followed by Europe (30%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to witness the highest growth rate, driven by rapid industrialization, increasing healthcare expenditure, and government initiatives promoting sustainable manufacturing practices in countries like China, India, and Japan.
Consumer awareness and preference for sustainable products have significantly influenced market dynamics. According to recent surveys, 73% of global consumers are willing to pay a premium for products made with sustainable materials. This shift in consumer behavior has prompted manufacturers across various industries to incorporate biomaterials into their product development strategies.
Despite the promising growth trajectory, several challenges affect market demand, including high production costs, technical limitations in certain applications, and inconsistent regulatory frameworks across different regions. The cost premium for biomaterials compared to conventional materials remains a significant barrier, particularly in price-sensitive markets and applications where performance requirements are stringent.
Current Safety Assessment Challenges in Biomaterial Manufacturing
The industrial biomaterial manufacturing sector faces significant safety assessment challenges that impede innovation and market entry. Current regulatory frameworks often struggle to keep pace with rapidly evolving biomaterial technologies, creating uncertainty for manufacturers and potentially delaying beneficial products from reaching consumers.
One primary challenge is the lack of standardized testing protocols specifically designed for novel biomaterials. Traditional safety assessment methods developed for conventional materials frequently fail to address the unique properties and behaviors of biomaterials, particularly those with living components or those designed to interact with biological systems. This gap necessitates case-by-case evaluations that are time-consuming, costly, and yield inconsistent results across different regulatory jurisdictions.
Risk characterization presents another substantial hurdle. Many biomaterials exhibit complex degradation profiles and can transform within biological environments, making long-term safety predictions difficult. Current assessment frameworks often inadequately account for these dynamic properties, potentially overlooking delayed adverse effects or failing to recognize beneficial adaptations that occur over time.
Cross-contamination risks and sterility assurance pose unique challenges in biomaterial manufacturing environments. Unlike traditional manufacturing, many biomaterial production processes involve biological components that can be susceptible to contamination at levels undetectable by standard quality control measures. Existing safety protocols may not sufficiently address these specialized concerns.
The global regulatory landscape for biomaterials remains fragmented, with significant variations in requirements between regions. This inconsistency creates compliance burdens for manufacturers operating internationally and may lead to regulatory arbitrage, where companies seek approval in jurisdictions with less stringent requirements, potentially compromising safety standards.
Emerging technologies such as 3D bioprinting, gene editing, and nanobiomaterials further complicate safety assessments. These innovations often combine multiple technological approaches, creating novel risk profiles that existing frameworks are ill-equipped to evaluate. Regulatory agencies frequently lack the specialized expertise needed to properly assess these cutting-edge developments.
Data gaps represent another critical challenge. Limited historical safety data exists for many novel biomaterials, and ethical considerations may restrict certain types of testing. This scarcity of evidence complicates risk-benefit analyses and may lead to overly cautious regulatory approaches that stifle innovation or, conversely, insufficient safeguards that could endanger public health.
Finally, the interdisciplinary nature of biomaterial development requires collaboration between experts from diverse fields including materials science, biology, medicine, and engineering. Current assessment frameworks often operate in disciplinary silos, failing to integrate these varied perspectives into comprehensive safety evaluations.
One primary challenge is the lack of standardized testing protocols specifically designed for novel biomaterials. Traditional safety assessment methods developed for conventional materials frequently fail to address the unique properties and behaviors of biomaterials, particularly those with living components or those designed to interact with biological systems. This gap necessitates case-by-case evaluations that are time-consuming, costly, and yield inconsistent results across different regulatory jurisdictions.
Risk characterization presents another substantial hurdle. Many biomaterials exhibit complex degradation profiles and can transform within biological environments, making long-term safety predictions difficult. Current assessment frameworks often inadequately account for these dynamic properties, potentially overlooking delayed adverse effects or failing to recognize beneficial adaptations that occur over time.
Cross-contamination risks and sterility assurance pose unique challenges in biomaterial manufacturing environments. Unlike traditional manufacturing, many biomaterial production processes involve biological components that can be susceptible to contamination at levels undetectable by standard quality control measures. Existing safety protocols may not sufficiently address these specialized concerns.
The global regulatory landscape for biomaterials remains fragmented, with significant variations in requirements between regions. This inconsistency creates compliance burdens for manufacturers operating internationally and may lead to regulatory arbitrage, where companies seek approval in jurisdictions with less stringent requirements, potentially compromising safety standards.
Emerging technologies such as 3D bioprinting, gene editing, and nanobiomaterials further complicate safety assessments. These innovations often combine multiple technological approaches, creating novel risk profiles that existing frameworks are ill-equipped to evaluate. Regulatory agencies frequently lack the specialized expertise needed to properly assess these cutting-edge developments.
Data gaps represent another critical challenge. Limited historical safety data exists for many novel biomaterials, and ethical considerations may restrict certain types of testing. This scarcity of evidence complicates risk-benefit analyses and may lead to overly cautious regulatory approaches that stifle innovation or, conversely, insufficient safeguards that could endanger public health.
Finally, the interdisciplinary nature of biomaterial development requires collaboration between experts from diverse fields including materials science, biology, medicine, and engineering. Current assessment frameworks often operate in disciplinary silos, failing to integrate these varied perspectives into comprehensive safety evaluations.
Existing Safety Assessment Methodologies and Protocols
01 Risk assessment methodologies for safety frameworks
Various methodologies are employed to assess risks within safety frameworks, including systematic approaches to identify, analyze, and mitigate potential hazards. These methodologies incorporate data analysis techniques, predictive modeling, and scenario-based assessments to evaluate safety risks across different operational contexts. By implementing structured risk assessment processes, organizations can proactively address safety concerns and establish appropriate control measures to prevent incidents.- Risk assessment methodologies for safety frameworks: Various methodologies are employed to assess risks within safety frameworks, including quantitative and qualitative approaches. These methodologies help identify potential hazards, evaluate their likelihood and impact, and prioritize mitigation strategies. Advanced algorithms and data analysis techniques enable more accurate risk predictions and assessments, supporting decision-making processes in safety management systems.
- Automated safety monitoring and compliance systems: Automated systems for continuous monitoring of safety parameters and ensuring compliance with regulatory requirements are increasingly being implemented. These systems utilize sensors, IoT devices, and real-time data collection to detect anomalies, trigger alerts, and maintain comprehensive safety records. They can automatically generate compliance reports and documentation, reducing manual effort while improving accuracy and consistency in safety management.
- AI and machine learning in safety prediction and prevention: Artificial intelligence and machine learning technologies are being integrated into safety frameworks to predict potential safety incidents before they occur. These technologies analyze patterns from historical data, identify leading indicators of safety risks, and suggest preventive measures. The predictive capabilities enable proactive safety management rather than reactive responses, significantly reducing the likelihood of accidents and improving overall safety outcomes.
- Integrated safety management platforms: Comprehensive platforms that integrate various aspects of safety management into a unified system are being developed. These platforms combine risk assessment, incident reporting, compliance tracking, training management, and performance analytics in a single interface. The integration enables better coordination between different safety functions, improves information sharing, and provides holistic visibility into the organization's safety status and performance metrics.
- Human factors and behavioral safety approaches: Safety frameworks are increasingly incorporating human factors and behavioral aspects to address the root causes of safety incidents. These approaches focus on understanding human behavior, decision-making processes, and organizational culture that influence safety outcomes. By addressing psychological and social factors, organizations can develop more effective safety interventions, improve safety communication, and foster a positive safety culture that encourages reporting and continuous improvement.
02 Automated safety monitoring and compliance systems
Advanced automated systems are developed to continuously monitor safety parameters and ensure compliance with established safety standards. These systems utilize sensors, real-time data collection, and automated alerts to detect deviations from safety thresholds. By integrating automated monitoring with compliance verification mechanisms, organizations can maintain consistent adherence to safety protocols and quickly respond to potential safety issues before they escalate into incidents.Expand Specific Solutions03 AI and machine learning applications in safety assessment
Artificial intelligence and machine learning technologies are increasingly applied to enhance safety assessment frameworks. These technologies enable pattern recognition in safety data, predictive analysis of potential incidents, and automated identification of safety anomalies. By leveraging AI algorithms, safety systems can continuously learn from operational data, improving their ability to identify emerging risks and recommend appropriate preventive measures.Expand Specific Solutions04 Integrated safety management systems
Comprehensive safety frameworks incorporate integrated management systems that connect various safety components across an organization. These systems coordinate safety policies, procedures, training, and incident reporting within a unified framework. By implementing integrated approaches, organizations can ensure consistent safety practices, facilitate information sharing between departments, and create a holistic view of safety performance across operations.Expand Specific Solutions05 Safety certification and validation processes
Structured certification and validation processes are essential components of safety assessment frameworks. These processes establish standardized methods for verifying that safety systems meet required specifications and performance criteria. Through rigorous testing, documentation, and third-party verification, safety certification ensures that implemented safety measures are effective and compliant with relevant standards and regulations.Expand Specific Solutions
Leading Organizations in Industrial Biomaterial Safety Standards
The industrial biomaterial manufacturing safety assessment landscape is currently in a growth phase, with increasing market demand driven by healthcare and environmental applications. The market is expected to reach significant scale as regulatory frameworks evolve and adoption increases across industries. Technologically, the field shows varying maturity levels, with academic institutions like MIT, University of California, and University College London leading fundamental research, while companies such as Janssen Biotech, Straumann Holding, and SiO2 Medical Products are advancing commercial applications. Research organizations including CNRS and Battelle Memorial Institute bridge the gap between theoretical frameworks and practical implementation. The ecosystem demonstrates a collaborative approach between academia, industry, and research institutions, with specialized players like DiFusion and SupraPolix developing niche biomaterial technologies with enhanced safety profiles.
The Regents of the University of California
Technical Solution: The University of California system has developed a multidisciplinary safety assessment framework for industrial biomaterial manufacturing that emphasizes sustainability and circular economy principles. Their approach integrates traditional toxicological assessments with advanced ecological impact evaluations, creating a holistic view of biomaterial safety. The framework employs a multi-tiered testing strategy that begins with in silico predictive modeling, followed by high-throughput in vitro screening, and culminates with targeted in vivo studies when necessary. UC researchers have pioneered the use of organ-on-chip technologies to create more physiologically relevant testing environments for biomaterial safety assessment, potentially reducing animal testing requirements. Their system incorporates specialized protocols for evaluating biomaterial degradation products and their potential environmental persistence. The framework also features a comprehensive risk communication component that translates complex safety data into actionable information for stakeholders including manufacturers, regulators, and the public. Additionally, UC has developed specific guidance for emerging biomaterials such as those derived from synthetic biology approaches, addressing unique safety considerations for genetically engineered production systems.
Strengths: Excellent integration of environmental and human health considerations; strong scientific foundation with continuous updates based on emerging research; adaptable to diverse biomaterial types. Weaknesses: Complex implementation requiring significant expertise across multiple disciplines; framework still evolving for certain novel biomaterial categories; may exceed regulatory requirements in some jurisdictions.
Battelle Memorial Institute
Technical Solution: Battelle has established a robust industrial safety assessment framework for biomaterial manufacturing that emphasizes practical implementation in production environments. Their approach features a comprehensive hazard identification system that categorizes potential risks according to severity and likelihood, enabling prioritized mitigation strategies. The framework incorporates specialized exposure assessment methodologies tailored to different biomaterial manufacturing processes, including fermentation, cell culture, and synthetic production routes. Battelle's system includes detailed protocols for evaluating process containment effectiveness, particularly important for genetically modified organisms used in biomaterial production. Their methodology features a strong focus on worker safety, with specialized monitoring approaches for potential biological and chemical hazards in manufacturing environments. The framework also incorporates emergency response protocols specific to biomaterial manufacturing incidents, including containment breaches and accidental exposures. Additionally, Battelle has developed specialized risk assessment tools for scale-up activities, addressing how safety profiles may change as processes move from laboratory to industrial production scales. Their approach also includes comprehensive documentation systems that support regulatory compliance across multiple jurisdictions.
Strengths: Highly practical implementation focus; excellent integration with industrial hygiene practices; comprehensive coverage of both routine operations and emergency scenarios. Weaknesses: Less emphasis on fundamental research aspects of biomaterial safety; framework primarily focused on production environment rather than end-product safety; requires significant resources for full implementation.
Critical Safety Testing Technologies and Validation Methods
Method and apparatus for producing a biomaterial product
PatentInactiveUS7099721B2
Innovation
- A method involving scale-down experiments using specialized devices to mimic industrial processes, allowing for the determination of biomaterial indices and simulation of industrial bioprocesses, thereby optimizing operating conditions without the need for large-scale machinery or expensive analysis techniques.
A method and apparatus for producing a biomaterial product
PatentInactiveEP1297389B8
Innovation
- A method using scale-down devices and experiments to determine biomaterial indices and input parameters for computer simulations of industrial bioprocesses, allowing for the prediction of industrial-scale behavior without large-scale machinery, and reducing the risk of substantial process changes during pilot trials.
Regulatory Compliance and Global Harmonization Efforts
The regulatory landscape for industrial biomaterial manufacturing is characterized by complex and often fragmented frameworks across different regions. In the United States, the FDA has established tiered regulatory pathways based on risk classification, with biomaterials falling under various centers including CBER, CDRH, and CDER depending on their intended use and mechanism of action. The European Union operates under the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which introduced more stringent requirements for clinical evidence and post-market surveillance of biomaterials.
Asia-Pacific regions present varying approaches, with Japan's PMDA implementing the Sakigake designation system to expedite innovative biomaterials, while China's NMPA has recently reformed its regulatory framework to align more closely with international standards while maintaining distinct local requirements. These regional differences create significant compliance challenges for global manufacturers.
Global harmonization efforts are gaining momentum through initiatives like the International Medical Device Regulators Forum (IMDRF), which has developed the Medical Device Single Audit Program (MDSAP) to standardize quality management system requirements across multiple jurisdictions. The ISO 13485 standard for quality management systems has become a cornerstone for international compliance, though implementation specifics still vary by region.
Regulatory convergence is also evident in the adoption of Common Submission Dossier Templates (CSDT) and the development of internationally recognized biocompatibility testing standards under ISO 10993 series. These efforts reduce redundant testing requirements and facilitate multi-market access for biomaterial manufacturers.
Emerging challenges include the regulation of combination products that incorporate both biological and synthetic components, which often fall between traditional regulatory categories. Advanced therapy medicinal products (ATMPs) and tissue-engineered products present novel regulatory questions regarding long-term safety monitoring and risk assessment methodologies.
Industry stakeholders are increasingly participating in public-private partnerships to develop consensus standards and best practices. The Reagan-Udall Foundation in the US and the Innovative Medicines Initiative in Europe exemplify collaborative approaches to addressing regulatory science gaps in biomaterial safety assessment.
Looking forward, regulatory frameworks are evolving toward more adaptive approaches that accommodate rapid technological innovation while maintaining safety standards. Real-world evidence and post-market surveillance data are gaining importance in regulatory decision-making, complementing traditional pre-market testing requirements and creating more comprehensive safety assessment frameworks for industrial biomaterial manufacturing.
Asia-Pacific regions present varying approaches, with Japan's PMDA implementing the Sakigake designation system to expedite innovative biomaterials, while China's NMPA has recently reformed its regulatory framework to align more closely with international standards while maintaining distinct local requirements. These regional differences create significant compliance challenges for global manufacturers.
Global harmonization efforts are gaining momentum through initiatives like the International Medical Device Regulators Forum (IMDRF), which has developed the Medical Device Single Audit Program (MDSAP) to standardize quality management system requirements across multiple jurisdictions. The ISO 13485 standard for quality management systems has become a cornerstone for international compliance, though implementation specifics still vary by region.
Regulatory convergence is also evident in the adoption of Common Submission Dossier Templates (CSDT) and the development of internationally recognized biocompatibility testing standards under ISO 10993 series. These efforts reduce redundant testing requirements and facilitate multi-market access for biomaterial manufacturers.
Emerging challenges include the regulation of combination products that incorporate both biological and synthetic components, which often fall between traditional regulatory categories. Advanced therapy medicinal products (ATMPs) and tissue-engineered products present novel regulatory questions regarding long-term safety monitoring and risk assessment methodologies.
Industry stakeholders are increasingly participating in public-private partnerships to develop consensus standards and best practices. The Reagan-Udall Foundation in the US and the Innovative Medicines Initiative in Europe exemplify collaborative approaches to addressing regulatory science gaps in biomaterial safety assessment.
Looking forward, regulatory frameworks are evolving toward more adaptive approaches that accommodate rapid technological innovation while maintaining safety standards. Real-world evidence and post-market surveillance data are gaining importance in regulatory decision-making, complementing traditional pre-market testing requirements and creating more comprehensive safety assessment frameworks for industrial biomaterial manufacturing.
Environmental Impact Assessment of Biomaterial Production
The environmental impact assessment of biomaterial production represents a critical component in evaluating the sustainability of industrial biomaterial manufacturing processes. Traditional manufacturing methods often generate significant environmental footprints through resource consumption, waste generation, and emissions. In contrast, biomaterial production offers potential advantages but also presents unique environmental challenges that require comprehensive assessment.
Life cycle assessment (LCA) methodologies have emerged as the primary framework for quantifying environmental impacts across the entire biomaterial production chain. These assessments typically examine impacts from raw material extraction through processing, use, and end-of-life disposal. Recent advancements in LCA approaches have incorporated specialized metrics for biomaterial-specific concerns, including land use change, biodiversity impacts, and water footprint analysis.
Carbon footprint analysis reveals that biomaterial production generally demonstrates lower greenhouse gas emissions compared to petroleum-based alternatives, with reductions ranging from 25% to 80% depending on feedstock selection and processing methods. However, these advantages can be offset by land use requirements, particularly when agricultural feedstocks compete with food production or drive deforestation.
Water consumption represents another significant environmental consideration, with biomaterial production processes often requiring substantial water inputs. Advanced water recycling systems and closed-loop manufacturing approaches have demonstrated potential to reduce freshwater requirements by up to 60% in optimized facilities. Wastewater management presents additional challenges, as biological processing can generate effluents with high biochemical oxygen demand and potential biological contaminants.
Biodiversity impacts must be carefully assessed, particularly when biomaterial production involves large-scale cultivation of specific organisms or genetic modification. Monitoring frameworks that track ecosystem changes in production regions have become increasingly sophisticated, incorporating satellite imagery analysis and environmental DNA sampling to detect subtle ecological shifts.
Waste stream management in biomaterial production presents both challenges and opportunities. While some processes generate biological waste requiring specialized treatment, many biomaterial manufacturing systems can incorporate circular economy principles, with waste products from one process serving as feedstock for another. Emerging technologies for valorizing production residues have demonstrated potential to transform waste streams into valuable co-products, significantly improving overall environmental performance.
Regulatory frameworks for environmental impact assessment continue to evolve, with increasing emphasis on standardized reporting metrics and third-party verification. The development of industry-specific environmental performance benchmarks has facilitated meaningful comparisons between different biomaterial production pathways and conventional alternatives, driving continuous improvement across the sector.
Life cycle assessment (LCA) methodologies have emerged as the primary framework for quantifying environmental impacts across the entire biomaterial production chain. These assessments typically examine impacts from raw material extraction through processing, use, and end-of-life disposal. Recent advancements in LCA approaches have incorporated specialized metrics for biomaterial-specific concerns, including land use change, biodiversity impacts, and water footprint analysis.
Carbon footprint analysis reveals that biomaterial production generally demonstrates lower greenhouse gas emissions compared to petroleum-based alternatives, with reductions ranging from 25% to 80% depending on feedstock selection and processing methods. However, these advantages can be offset by land use requirements, particularly when agricultural feedstocks compete with food production or drive deforestation.
Water consumption represents another significant environmental consideration, with biomaterial production processes often requiring substantial water inputs. Advanced water recycling systems and closed-loop manufacturing approaches have demonstrated potential to reduce freshwater requirements by up to 60% in optimized facilities. Wastewater management presents additional challenges, as biological processing can generate effluents with high biochemical oxygen demand and potential biological contaminants.
Biodiversity impacts must be carefully assessed, particularly when biomaterial production involves large-scale cultivation of specific organisms or genetic modification. Monitoring frameworks that track ecosystem changes in production regions have become increasingly sophisticated, incorporating satellite imagery analysis and environmental DNA sampling to detect subtle ecological shifts.
Waste stream management in biomaterial production presents both challenges and opportunities. While some processes generate biological waste requiring specialized treatment, many biomaterial manufacturing systems can incorporate circular economy principles, with waste products from one process serving as feedstock for another. Emerging technologies for valorizing production residues have demonstrated potential to transform waste streams into valuable co-products, significantly improving overall environmental performance.
Regulatory frameworks for environmental impact assessment continue to evolve, with increasing emphasis on standardized reporting metrics and third-party verification. The development of industry-specific environmental performance benchmarks has facilitated meaningful comparisons between different biomaterial production pathways and conventional alternatives, driving continuous improvement across the sector.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!