From Lab To Pilot: Tech Transfer Considerations In Biomaterials Manufacturing
Biomaterials Manufacturing Evolution and Objectives
Biomaterials manufacturing has undergone a remarkable evolution over the past several decades, transitioning from rudimentary processing techniques to sophisticated, precision-controlled manufacturing systems. The field emerged in the 1960s with the development of the first synthetic biomaterials primarily focused on inert substances that could replace damaged tissues. These early biomaterials were characterized by their mechanical properties rather than biological functionality, with limited interaction with surrounding tissues.
The 1980s marked a significant shift toward bioactive materials designed to elicit specific biological responses. This period saw the emergence of biodegradable polymers and bioactive ceramics, expanding the application scope beyond simple tissue replacement to tissue regeneration. Manufacturing processes during this era remained largely batch-oriented with limited scalability and reproducibility.
By the early 2000s, biomaterials manufacturing entered a new phase with the integration of nanotechnology and advanced fabrication techniques. The ability to control material properties at the nanoscale opened new possibilities for mimicking natural tissue structures and functions. Concurrently, the rise of additive manufacturing technologies enabled the production of complex, patient-specific implants with unprecedented precision.
Today's biomaterials manufacturing landscape is characterized by convergent technologies incorporating principles from materials science, biology, engineering, and computational modeling. Smart biomaterials responsive to biological stimuli, biomimetic materials that replicate natural tissue architecture, and hybrid materials combining synthetic and biological components represent the cutting edge of the field. Advanced biofabrication techniques such as 3D bioprinting have revolutionized the production of tissue-engineered constructs with spatially organized cells and biomaterials.
The primary objective in biomaterials manufacturing is to bridge the significant gap between laboratory-scale production and industrial-scale manufacturing while maintaining product quality, consistency, and biological functionality. This challenge is particularly acute given the inherent complexity and variability of biological systems. Specific goals include developing robust, reproducible manufacturing processes that can accommodate the sensitive nature of biomaterials while meeting regulatory requirements.
Additional objectives include reducing production costs to improve accessibility, enhancing shelf life and stability of biomaterial products, and establishing standardized quality control metrics specific to biomaterials. The field also aims to develop sustainable manufacturing practices that minimize environmental impact while maximizing resource efficiency. Ultimately, the overarching goal is to accelerate the translation of innovative biomaterial technologies from research laboratories to clinical applications, improving patient outcomes across a spectrum of medical conditions.
Market Analysis for Biomaterials Applications
The global biomaterials market demonstrates robust growth, valued at approximately $106.5 billion in 2022 and projected to reach $283.1 billion by 2030, with a compound annual growth rate (CAGR) of 13.2%. This expansion is primarily driven by increasing applications in medical devices, tissue engineering, drug delivery systems, and regenerative medicine.
Healthcare applications dominate the biomaterials landscape, accounting for over 60% of market share. Orthopedic and cardiovascular applications represent the largest segments, with dental and wound healing applications showing the fastest growth rates. The aging global population and rising prevalence of chronic diseases continue to fuel demand for biomaterial-based medical solutions.
Regionally, North America leads the market with approximately 38% share, followed by Europe at 30% and Asia-Pacific at 25%. However, emerging economies in Asia-Pacific, particularly China and India, are experiencing the most rapid growth rates due to improving healthcare infrastructure, increasing medical tourism, and growing government investments in healthcare technology.
The biomaterials market segmentation reveals interesting trends across material types. Metallic biomaterials (titanium alloys, stainless steel) currently hold the largest market share at 35%, followed by polymeric biomaterials at 28%, ceramics at 20%, and natural biomaterials at 17%. However, natural and biodegradable biomaterials are experiencing the fastest growth due to sustainability concerns and improved biocompatibility profiles.
End-user analysis indicates hospitals and clinics remain the primary consumers of biomaterial products, accounting for 45% of market demand. Research institutions represent 25%, while pharmaceutical and biotechnology companies comprise 20%. The remaining 10% is distributed among cosmetic surgery centers and other specialized facilities.
Key market drivers include technological advancements in material science, increasing prevalence of chronic diseases, growing demand for minimally invasive surgical procedures, and rising investments in regenerative medicine research. The shift toward personalized medicine and 3D bioprinting technologies is creating new market opportunities for customized biomaterial solutions.
Market challenges persist, including high development and manufacturing costs, stringent regulatory approval processes, and technical challenges in scaling laboratory innovations to commercial production. The average time from laboratory discovery to market entry for new biomaterials remains between 8-12 years, significantly impacting return on investment calculations for manufacturers and investors.
Consumer trends indicate growing preference for biomaterials with enhanced functionality, such as antimicrobial properties, controlled drug release capabilities, and improved integration with biological tissues. Additionally, sustainability concerns are driving demand for eco-friendly biomaterials with reduced environmental impact throughout their lifecycle.
Current Challenges in Biomaterials Scale-up
The transition from laboratory-scale production to pilot manufacturing represents a critical bottleneck in biomaterials development. Despite promising research outcomes, many biomaterial innovations fail to reach commercial viability due to significant scale-up challenges. Current manufacturing processes often struggle with maintaining consistent quality parameters when production volumes increase, resulting in materials with altered physical properties, compromised biocompatibility, or reduced functionality.
A primary challenge lies in process parameter translation, where laboratory conditions cannot be directly replicated at larger scales. Variables such as mixing dynamics, temperature gradients, and reaction kinetics behave differently in industrial equipment compared to laboratory settings. For instance, heat transfer efficiency decreases with increasing batch size, potentially affecting crosslinking density in hydrogels or crystallization patterns in biopolymers.
Regulatory compliance presents another substantial hurdle. Biomaterials intended for medical applications must adhere to stringent quality standards throughout the manufacturing process. Current Good Manufacturing Practice (cGMP) requirements demand comprehensive documentation, validation protocols, and quality control measures that are often underdeveloped during laboratory research phases. The establishment of robust quality management systems represents a significant investment that many startups and research institutions are unprepared to undertake.
Material characterization methodologies frequently prove inadequate during scale-up. Analytical techniques suitable for small-batch testing may become impractical or insufficiently sensitive when applied to industrial production. This creates difficulties in establishing appropriate quality control metrics and ensuring batch-to-batch consistency, particularly for complex biomaterials with multiple functional components or hierarchical structures.
Supply chain vulnerabilities further complicate scale-up efforts. Many advanced biomaterials rely on specialized raw materials with limited suppliers, creating potential bottlenecks in production. Variations in starting material quality can propagate through manufacturing processes, resulting in unpredictable final product characteristics. Additionally, the shelf-life limitations of biological components necessitate careful logistics planning that laboratory protocols rarely address.
Cost-effectiveness remains a persistent challenge, with many laboratory-developed biomaterials utilizing expensive production methods or components that become economically prohibitive at scale. Current manufacturing approaches often fail to incorporate design-for-manufacturing principles early in development, resulting in processes that are difficult to optimize for industrial efficiency. Without significant process engineering innovations, many promising biomaterials remain commercially nonviable despite their technical merits.
Equipment design and availability represent another significant constraint. Specialized bioreactors, purification systems, and processing equipment for novel biomaterials often do not exist as off-the-shelf solutions, requiring custom engineering that introduces additional technical risks and capital requirements to the scale-up process.
Current Scale-up Methodologies and Approaches
01 Advanced manufacturing processes for biomaterials
Advanced manufacturing techniques are employed to produce biomaterials with specific properties and functionalities. These processes include precision molding, additive manufacturing, and specialized fabrication methods that enable the creation of complex biomaterial structures. These manufacturing processes are critical for ensuring the quality, consistency, and performance of biomaterials used in medical applications, tissue engineering, and regenerative medicine.- Additive Manufacturing of Biomaterials: Additive manufacturing technologies are increasingly used for biomaterial production, enabling precise control over structure and properties. These techniques allow for the creation of customized implants, scaffolds, and medical devices with complex geometries. The technology transfer in this area involves specialized equipment, process parameters, and quality control methods to ensure biocompatibility and mechanical integrity of the final products.
- Biomaterial Surface Modification Techniques: Surface modification of biomaterials enhances their functionality, biocompatibility, and integration with biological tissues. These techniques include plasma treatment, chemical etching, coating applications, and nanopatterning. Technology transfer in this domain focuses on optimizing surface properties for specific medical applications while maintaining the bulk material characteristics and ensuring reproducibility in manufacturing processes.
- Biopolymer Processing and Scale-up: The manufacturing of biopolymers for medical applications requires specialized processing techniques and scale-up strategies. This includes extraction, purification, and modification of natural polymers or synthesis of novel biomaterials. Technology transfer in this field addresses challenges in maintaining consistent material properties during scale-up, regulatory compliance, and integration of sustainable manufacturing practices.
- Quality Control Systems for Biomaterials Production: Effective quality control systems are essential for biomaterials manufacturing to ensure product safety, efficacy, and regulatory compliance. These systems incorporate advanced analytical techniques, in-process monitoring, and validation protocols. Technology transfer in this area involves implementing robust testing methodologies, establishing acceptance criteria, and developing documentation systems that meet international standards for medical-grade materials.
- Biomaterial Manufacturing Automation and Industry 4.0: Integration of automation, robotics, and Industry 4.0 technologies is transforming biomaterials manufacturing. These advancements include AI-driven process optimization, IoT-enabled equipment monitoring, and digital twins for production simulation. Technology transfer in this domain focuses on implementing smart manufacturing systems that improve efficiency, reduce contamination risks, and enhance traceability throughout the biomaterial production lifecycle.
02 Technology transfer frameworks for biomaterial innovations
Structured frameworks and methodologies facilitate the transfer of biomaterial technologies from research laboratories to commercial production. These frameworks include protocols for knowledge sharing, intellectual property management, and collaborative development between academic institutions and industry partners. Effective technology transfer ensures that innovative biomaterial solutions can be scaled up and commercialized efficiently.Expand Specific Solutions03 Quality control systems for biomaterial manufacturing
Specialized quality control systems are implemented to ensure the consistency, safety, and efficacy of manufactured biomaterials. These systems include testing protocols, validation procedures, and monitoring technologies that verify the properties and performance of biomaterials throughout the manufacturing process. Quality control is essential for meeting regulatory requirements and ensuring that biomaterials perform as intended in their applications.Expand Specific Solutions04 Scalable production methods for biomaterial commercialization
Scalable production methods enable the transition of biomaterial innovations from laboratory-scale to commercial-scale manufacturing. These methods address challenges related to process efficiency, cost-effectiveness, and consistency when producing biomaterials in larger quantities. Scalable production is crucial for making advanced biomaterials economically viable and widely available for various applications.Expand Specific Solutions05 Regulatory compliance strategies for biomaterial technology transfer
Comprehensive strategies are developed to navigate the regulatory landscape during the transfer of biomaterial manufacturing technologies. These strategies include documentation practices, validation protocols, and compliance frameworks that address the requirements of regulatory agencies. Effective regulatory compliance ensures that biomaterial products can be approved for market entry and meet safety and efficacy standards.Expand Specific Solutions
Leading Organizations in Biomaterials Manufacturing
The biomaterials manufacturing technology transfer landscape is currently in a growth phase, with an estimated market size of $150-200 billion by 2025. The industry is transitioning from early-stage development to commercial scaling, with varying degrees of technical maturity. Leading academic institutions like University College London and University of Zurich are driving fundamental research, while established companies including Cytiva, 3M, and DuPont are advancing commercial applications. Pharmaceutical giants such as Genentech and Dr. Reddy's are integrating biomaterials into therapeutic pipelines. The ecosystem also features specialized players like Advanced Solutions Life Sciences and Contraline developing niche applications. Key challenges in technology transfer include standardization, regulatory compliance, and scaling production while maintaining quality—requiring cross-sector collaboration between academia and industry.
University College London
Genentech, Inc.
Critical Patents and Innovations in Biomaterials Production
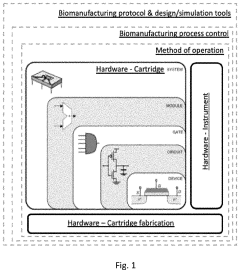
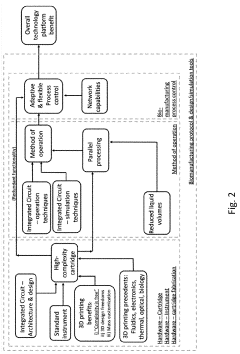
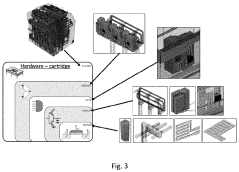
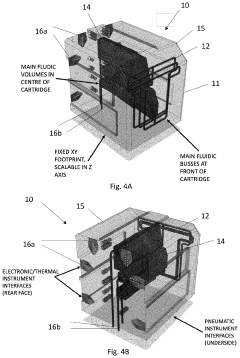
- A method using scale-down devices and experiments to determine biomaterial indices and input parameters for computer simulations of industrial bioprocesses, allowing for the prediction of industrial-scale behavior without large-scale machinery, and reducing the risk of substantial process changes during pilot trials.
- The development of modular reactor systems with standardized, compact, and easy-to-use hardware that allows for the agile design and manufacturing of various bioproducts using additive manufacturing, enabling on-demand production and implementation of complex biomanufacturing protocols closer to the point of need through modular reactor devices with interchangeable components and a base station for control.
Regulatory Compliance Framework for Biomaterials
The regulatory landscape for biomaterials manufacturing represents a complex and evolving framework that manufacturers must navigate throughout the technology transfer process. At the international level, organizations such as the International Organization for Standardization (ISO) have established standards like ISO 13485 for medical devices and ISO 10993 series specifically addressing biocompatibility evaluation of medical devices. These standards provide foundational requirements that influence regulatory approaches worldwide.
In the United States, the Food and Drug Administration (FDA) oversees biomaterials through various regulatory pathways depending on their classification and intended use. The Center for Devices and Radiological Health (CDRH) typically handles biomaterial-based medical devices, while the Center for Biologics Evaluation and Research (CBER) regulates biological components. The 510(k) clearance, Premarket Approval (PMA), and De Novo classification represent the primary pathways for bringing biomaterial products to market.
European regulations have undergone significant transformation with the implementation of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which impose more stringent requirements for clinical evidence, post-market surveillance, and technical documentation. The CE marking process now demands more comprehensive data on biomaterials' safety and performance characteristics.
Good Manufacturing Practices (GMPs) constitute a critical component of the regulatory framework, ensuring consistent production quality and safety. For biomaterials, these practices extend beyond standard manufacturing protocols to include specialized considerations for biocompatibility, sterility, and biological safety. The FDA's Quality System Regulation (21 CFR Part 820) and the EU's GMP guidelines provide specific requirements that manufacturers must implement.
During technology transfer from laboratory to pilot production, regulatory considerations must be integrated early in the process. This includes developing a regulatory strategy that identifies applicable standards, required testing protocols, and documentation needs. Risk management frameworks such as ISO 14971 should be implemented to systematically identify and mitigate potential hazards associated with biomaterial manufacturing and use.
Environmental regulations also impact biomaterials manufacturing, particularly regarding waste management, emissions control, and sustainable sourcing practices. Compliance with regulations such as the Environmental Protection Agency (EPA) guidelines in the US or the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) in Europe may be necessary depending on the materials and processes involved.
As emerging technologies like 3D bioprinting and nanomaterials continue to advance, regulatory frameworks are evolving to address novel challenges. Manufacturers engaged in technology transfer must maintain vigilance regarding regulatory developments and consider engaging with authorities through pre-submission consultations to navigate uncertainties in the regulatory pathway.
Quality Control Systems for Consistent Production
Quality control systems represent a critical cornerstone in the successful transition of biomaterials manufacturing from laboratory to pilot scale. The inherent complexity and biological variability of biomaterials necessitate robust quality control frameworks that can ensure consistent production outcomes while maintaining compliance with regulatory standards. These systems must be designed to address the unique challenges posed by biomaterials, including batch-to-batch variability, stability concerns, and biocompatibility requirements.
Implementing a comprehensive quality control system begins with the establishment of clear specifications and acceptance criteria for raw materials, intermediates, and final products. These specifications should be based on critical quality attributes (CQAs) that directly impact the safety, efficacy, and functionality of the biomaterial. For instance, in collagen-based scaffolds, parameters such as fiber diameter, porosity, mechanical strength, and degradation rate must be consistently monitored and controlled.
Process analytical technology (PAT) plays an instrumental role in modern biomaterials manufacturing quality systems. Real-time monitoring techniques such as spectroscopy, chromatography, and microscopy enable continuous assessment of process parameters and material properties during production. This approach facilitates immediate detection of deviations and allows for timely corrective actions, minimizing waste and ensuring consistent quality.
Statistical process control (SPC) methodologies provide a quantitative framework for monitoring process stability and capability. By establishing control charts for key parameters, manufacturers can distinguish between common cause variations (inherent to the process) and special cause variations (requiring intervention). Implementation of SPC in biomaterials production has demonstrated significant improvements in consistency, particularly in processes involving biological components with natural variability.
Documentation systems constitute another essential element of quality control infrastructure. Comprehensive batch records, standard operating procedures (SOPs), and testing protocols ensure traceability and reproducibility throughout the manufacturing process. Digital solutions such as laboratory information management systems (LIMS) and electronic batch records enhance data integrity and facilitate compliance with regulatory requirements.
Validation strategies must be tailored to the specific challenges of biomaterials manufacturing. This includes process validation to demonstrate that the manufacturing process consistently produces material meeting predetermined specifications, and analytical method validation to ensure that testing procedures provide accurate and reliable results. For biomaterials with living components, such as cell-seeded scaffolds, specialized validation approaches addressing cell viability and functionality are necessary.
Training programs for personnel represent a frequently overlooked yet crucial component of quality control systems. Operators must possess thorough understanding of both the technical aspects of production and the quality implications of process deviations. Regular competency assessments and continuous education ensure that human factors do not compromise product quality.