Emerging Bioreactor Formats For Low-Cost High-Volume Production
SEP 2, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bioreactor Technology Evolution and Objectives
Bioreactor technology has undergone significant evolution since its inception in the early 20th century. Initially developed for wastewater treatment, bioreactors have transformed into sophisticated systems capable of supporting complex biological processes for pharmaceutical, food, and chemical industries. The 1970s marked a pivotal shift with the introduction of recombinant DNA technology, catalyzing the development of specialized bioreactors for protein production. This technological advancement established the foundation for modern biotechnology manufacturing.
The 1980s and 1990s witnessed the emergence of stirred-tank bioreactors as the industry standard, characterized by their scalability and robust mixing capabilities. However, these traditional formats presented limitations in terms of oxygen transfer, shear stress management, and operational costs at large scales. The early 2000s introduced wave-mixed bioreactors, representing a paradigm shift toward single-use technologies that significantly reduced contamination risks and cleaning validation requirements.
Recent technological trajectories have focused on intensifying bioprocesses through perfusion systems, which enable continuous cell culture with higher cell densities and productivity. This evolution reflects the industry's response to increasing demand for biologics and the economic pressures to reduce production costs while maintaining product quality. The integration of advanced monitoring and control systems has further enhanced bioreactor performance through real-time process optimization.
The primary objective of emerging bioreactor formats is to address the critical challenge of producing biological products at significantly lower costs while accommodating high-volume demands. This goal is particularly relevant for biosimilars, vaccines, and emerging cell and gene therapies, where cost-effectiveness directly impacts global accessibility. Technical objectives include developing systems with improved oxygen transfer efficiency, reduced shear stress, enhanced mixing capabilities, and simplified scale-up processes.
Another key objective is the development of modular and flexible manufacturing platforms that can rapidly adapt to changing production requirements. This flexibility is essential for responding to market fluctuations and emerging health crises, as demonstrated during the COVID-19 pandemic. Additionally, these new formats aim to minimize environmental impact through reduced resource consumption, waste generation, and energy requirements.
The convergence of these technological advancements and objectives is driving innovation toward bioreactor formats that combine the benefits of single-use technology with continuous processing capabilities. These hybrid systems represent the frontier of biomanufacturing, promising to revolutionize production economics while maintaining product quality and regulatory compliance. The ultimate goal remains clear: democratizing access to biological therapeutics through transformative production technologies that significantly reduce costs while enhancing manufacturing capacity.
The 1980s and 1990s witnessed the emergence of stirred-tank bioreactors as the industry standard, characterized by their scalability and robust mixing capabilities. However, these traditional formats presented limitations in terms of oxygen transfer, shear stress management, and operational costs at large scales. The early 2000s introduced wave-mixed bioreactors, representing a paradigm shift toward single-use technologies that significantly reduced contamination risks and cleaning validation requirements.
Recent technological trajectories have focused on intensifying bioprocesses through perfusion systems, which enable continuous cell culture with higher cell densities and productivity. This evolution reflects the industry's response to increasing demand for biologics and the economic pressures to reduce production costs while maintaining product quality. The integration of advanced monitoring and control systems has further enhanced bioreactor performance through real-time process optimization.
The primary objective of emerging bioreactor formats is to address the critical challenge of producing biological products at significantly lower costs while accommodating high-volume demands. This goal is particularly relevant for biosimilars, vaccines, and emerging cell and gene therapies, where cost-effectiveness directly impacts global accessibility. Technical objectives include developing systems with improved oxygen transfer efficiency, reduced shear stress, enhanced mixing capabilities, and simplified scale-up processes.
Another key objective is the development of modular and flexible manufacturing platforms that can rapidly adapt to changing production requirements. This flexibility is essential for responding to market fluctuations and emerging health crises, as demonstrated during the COVID-19 pandemic. Additionally, these new formats aim to minimize environmental impact through reduced resource consumption, waste generation, and energy requirements.
The convergence of these technological advancements and objectives is driving innovation toward bioreactor formats that combine the benefits of single-use technology with continuous processing capabilities. These hybrid systems represent the frontier of biomanufacturing, promising to revolutionize production economics while maintaining product quality and regulatory compliance. The ultimate goal remains clear: democratizing access to biological therapeutics through transformative production technologies that significantly reduce costs while enhancing manufacturing capacity.
Market Analysis for Low-Cost Biomanufacturing
The global biomanufacturing market is experiencing significant growth, driven by increasing demand for biopharmaceuticals, vaccines, and cell therapies. Current market valuations place the bioreactor segment at approximately $3.5 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 12-15% through 2030. This growth trajectory is particularly pronounced in the low-cost biomanufacturing sector, which is emerging as a critical focus area for both established pharmaceutical companies and biotech startups.
The demand for low-cost biomanufacturing solutions is being fueled by several market factors. Healthcare systems worldwide are under increasing pressure to reduce costs while maintaining quality, creating a strong market pull for more economical production methods. Additionally, the rise of biosimilars and the expiration of key biologic patents have intensified competition, compelling manufacturers to optimize production economics.
Geographically, North America currently dominates the biomanufacturing market with approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 25%. However, the most rapid growth is occurring in emerging markets, particularly in countries like China, India, and Brazil, where annual growth rates exceed 18%. These regions are increasingly becoming manufacturing hubs due to lower operational costs and supportive government policies.
By application segment, monoclonal antibody production represents the largest market share at 35%, followed by vaccines (25%), recombinant proteins (20%), and cell therapies (15%). The remaining 5% encompasses various niche applications. The cell therapy segment, though currently smaller, is demonstrating the fastest growth rate at nearly 20% annually, driven by advancements in regenerative medicine and personalized treatments.
End-user analysis reveals that pharmaceutical and biotechnology companies account for 65% of the market, contract manufacturing organizations (CMOs) for 25%, and academic and research institutions for 10%. The CMO segment is growing particularly rapidly as more companies outsource biomanufacturing to reduce capital expenditure and leverage specialized expertise.
Key market drivers for low-cost biomanufacturing include increasing prevalence of chronic diseases, growing demand for personalized medicine, pressure to reduce healthcare costs, and expanding applications of biologics in various therapeutic areas. Conversely, market restraints include stringent regulatory requirements, high initial investment costs, and technical challenges in scaling production while maintaining product quality.
The market opportunity for emerging bioreactor formats is substantial, with particular potential in single-use systems, continuous processing technologies, and miniaturized bioreactor platforms that enable more efficient and cost-effective production processes.
The demand for low-cost biomanufacturing solutions is being fueled by several market factors. Healthcare systems worldwide are under increasing pressure to reduce costs while maintaining quality, creating a strong market pull for more economical production methods. Additionally, the rise of biosimilars and the expiration of key biologic patents have intensified competition, compelling manufacturers to optimize production economics.
Geographically, North America currently dominates the biomanufacturing market with approximately 40% market share, followed by Europe at 30% and Asia-Pacific at 25%. However, the most rapid growth is occurring in emerging markets, particularly in countries like China, India, and Brazil, where annual growth rates exceed 18%. These regions are increasingly becoming manufacturing hubs due to lower operational costs and supportive government policies.
By application segment, monoclonal antibody production represents the largest market share at 35%, followed by vaccines (25%), recombinant proteins (20%), and cell therapies (15%). The remaining 5% encompasses various niche applications. The cell therapy segment, though currently smaller, is demonstrating the fastest growth rate at nearly 20% annually, driven by advancements in regenerative medicine and personalized treatments.
End-user analysis reveals that pharmaceutical and biotechnology companies account for 65% of the market, contract manufacturing organizations (CMOs) for 25%, and academic and research institutions for 10%. The CMO segment is growing particularly rapidly as more companies outsource biomanufacturing to reduce capital expenditure and leverage specialized expertise.
Key market drivers for low-cost biomanufacturing include increasing prevalence of chronic diseases, growing demand for personalized medicine, pressure to reduce healthcare costs, and expanding applications of biologics in various therapeutic areas. Conversely, market restraints include stringent regulatory requirements, high initial investment costs, and technical challenges in scaling production while maintaining product quality.
The market opportunity for emerging bioreactor formats is substantial, with particular potential in single-use systems, continuous processing technologies, and miniaturized bioreactor platforms that enable more efficient and cost-effective production processes.
Current Challenges in High-Volume Bioreactor Systems
Despite significant advancements in bioreactor technology, high-volume biomanufacturing faces several persistent challenges that limit production efficiency and cost-effectiveness. Traditional stirred-tank bioreactors, while well-established, struggle with scalability issues when production volumes exceed 20,000 liters. At larger scales, maintaining homogeneous conditions becomes increasingly difficult, resulting in gradient formation of nutrients, dissolved oxygen, and pH that negatively impact cell growth and product quality.
Oxygen transfer limitations represent a critical bottleneck in high-volume systems. As production scale increases, the surface area-to-volume ratio decreases substantially, making efficient oxygen delivery to cells problematic. Current sparging systems often create excessive shear stress that can damage sensitive cell lines, particularly mammalian cells used for complex protein production.
Heat transfer inefficiency emerges as another significant challenge. Large-volume bioreactors generate substantial metabolic heat that must be removed to maintain optimal growth conditions. Conventional cooling jacket designs become less effective at industrial scales, requiring additional cooling systems that increase complexity and capital costs.
Monitoring and control systems face limitations in large-scale operations. While small bioreactors can be equipped with multiple sensors, larger systems often have fewer measurement points relative to their volume. This creates "blind spots" where process deviations may go undetected, potentially compromising batch consistency and product quality.
Contamination risk increases proportionally with bioreactor size due to longer processing times and more complex operations. Current sterilization methods for large-scale systems are energy-intensive and time-consuming, contributing significantly to operational costs and environmental impact.
Economic constraints further complicate high-volume production. Traditional stainless steel bioreactors require substantial capital investment, with costs ranging from $500-1,000 per liter of working volume. This creates significant financial barriers for smaller companies and limits manufacturing flexibility in responding to market demands.
Regulatory compliance presents additional challenges for high-volume systems. Validation of large-scale processes is complex and time-consuming, with regulatory agencies requiring extensive documentation of process consistency and product quality. Any modifications to established processes necessitate revalidation, further increasing costs and time-to-market.
Water and energy consumption in conventional high-volume bioreactors is substantial, with estimates suggesting that water usage can exceed 100 liters per gram of product in some bioprocesses. This resource intensity contradicts growing sustainability imperatives and increases production costs in regions with high utility prices.
Oxygen transfer limitations represent a critical bottleneck in high-volume systems. As production scale increases, the surface area-to-volume ratio decreases substantially, making efficient oxygen delivery to cells problematic. Current sparging systems often create excessive shear stress that can damage sensitive cell lines, particularly mammalian cells used for complex protein production.
Heat transfer inefficiency emerges as another significant challenge. Large-volume bioreactors generate substantial metabolic heat that must be removed to maintain optimal growth conditions. Conventional cooling jacket designs become less effective at industrial scales, requiring additional cooling systems that increase complexity and capital costs.
Monitoring and control systems face limitations in large-scale operations. While small bioreactors can be equipped with multiple sensors, larger systems often have fewer measurement points relative to their volume. This creates "blind spots" where process deviations may go undetected, potentially compromising batch consistency and product quality.
Contamination risk increases proportionally with bioreactor size due to longer processing times and more complex operations. Current sterilization methods for large-scale systems are energy-intensive and time-consuming, contributing significantly to operational costs and environmental impact.
Economic constraints further complicate high-volume production. Traditional stainless steel bioreactors require substantial capital investment, with costs ranging from $500-1,000 per liter of working volume. This creates significant financial barriers for smaller companies and limits manufacturing flexibility in responding to market demands.
Regulatory compliance presents additional challenges for high-volume systems. Validation of large-scale processes is complex and time-consuming, with regulatory agencies requiring extensive documentation of process consistency and product quality. Any modifications to established processes necessitate revalidation, further increasing costs and time-to-market.
Water and energy consumption in conventional high-volume bioreactors is substantial, with estimates suggesting that water usage can exceed 100 liters per gram of product in some bioprocesses. This resource intensity contradicts growing sustainability imperatives and increases production costs in regions with high utility prices.
Contemporary Low-Cost Bioreactor Solutions
01 Continuous flow bioreactor systems
Continuous flow bioreactor systems offer advantages for low-cost high-volume production by allowing constant nutrient supply and waste removal. These systems maintain optimal growth conditions for extended periods, increasing productivity while reducing labor costs. The continuous nature of the process eliminates downtime between batches, making it ideal for industrial-scale production of biologics, enzymes, and other bioactive compounds.- Continuous flow bioreactor systems: Continuous flow bioreactor systems offer advantages for low-cost high-volume production by allowing constant nutrient supply and waste removal. These systems maintain optimal growth conditions for extended periods, increasing productivity and reducing downtime between batches. The continuous operation reduces labor costs and improves space utilization, making them ideal for industrial-scale production of biologics, enzymes, and other bioactive compounds.
- Modular and scalable bioreactor designs: Modular bioreactor designs enable flexible scaling of production capacity while maintaining cost efficiency. These systems consist of standardized units that can be connected or operated in parallel to increase production volume as needed. The modular approach reduces initial capital investment by allowing gradual expansion and facilitates maintenance since individual modules can be serviced without shutting down the entire production line. This design philosophy is particularly valuable for facilities that need to adapt to changing market demands.
- Single-use bioreactor technology: Single-use bioreactor systems utilize disposable components to eliminate cleaning and sterilization requirements, significantly reducing operational costs and turnaround time. These systems minimize cross-contamination risks and water consumption while increasing production flexibility. The reduced need for validation and cleaning verification processes makes them particularly suitable for contract manufacturing organizations and facilities producing multiple products. Despite higher consumable costs, the overall economic benefits make them attractive for high-volume production.
- Advanced monitoring and control systems: Integration of advanced monitoring and control systems in bioreactors optimizes production efficiency and reduces costs. These systems employ sensors and automated feedback mechanisms to maintain ideal growth conditions, including temperature, pH, dissolved oxygen, and nutrient levels. Real-time data analysis enables predictive maintenance and early detection of process deviations, minimizing batch failures. The automation reduces labor requirements and improves consistency across production runs, essential for high-volume manufacturing operations.
- Alternative energy-efficient bioreactor designs: Energy-efficient bioreactor designs incorporate innovative approaches to reduce operational costs in high-volume production. These include improved mixing technologies that maintain homogeneity with lower power input, heat exchange systems that recover and reuse thermal energy, and alternative agitation methods that reduce shear stress on cells while ensuring adequate mixing. Some designs utilize gravity-based or air-lift principles to reduce energy consumption. These innovations significantly lower production costs while maintaining or improving productivity.
02 Modular and scalable bioreactor designs
Modular bioreactor designs provide flexibility and scalability for high-volume production while keeping costs low. These systems can be expanded incrementally as production demands increase, avoiding large upfront capital investments. The standardized components allow for easier maintenance, reduced downtime, and simplified validation processes. Modular designs also enable parallel processing, increasing overall throughput while maintaining consistent product quality.Expand Specific Solutions03 Single-use bioreactor technology
Single-use bioreactor technology eliminates the need for cleaning, sterilization, and validation between production runs, significantly reducing operational costs and turnaround time. These disposable systems minimize cross-contamination risks and water consumption while increasing production flexibility. The reduced infrastructure requirements make them particularly suitable for facilities with space constraints or those seeking to quickly scale up production with minimal capital investment.Expand Specific Solutions04 Advanced monitoring and control systems
Advanced monitoring and control systems optimize bioreactor performance through real-time data collection and automated adjustments. These systems maintain optimal growth conditions by continuously monitoring parameters such as pH, temperature, dissolved oxygen, and nutrient levels. The integration of artificial intelligence and machine learning algorithms enables predictive maintenance and process optimization, reducing waste and improving yield while minimizing human intervention requirements.Expand Specific Solutions05 Novel agitation and aeration methods
Innovative agitation and aeration methods improve mass transfer efficiency while reducing energy consumption in large-scale bioreactors. These technologies ensure homogeneous distribution of nutrients and oxygen throughout the culture medium, preventing gradient formation that can limit productivity. Low-shear mixing systems protect sensitive cell cultures while maintaining efficient gas exchange, particularly important for mammalian cell cultures used in biopharmaceutical production.Expand Specific Solutions
Leading Companies in Bioreactor Innovation
The bioreactor market for low-cost high-volume production is currently in a growth phase, with increasing demand driven by biopharmaceutical manufacturing expansion. The global market is estimated to reach $5-6 billion by 2025, growing at 8-10% annually. Technology maturity varies across formats, with traditional stirred-tank systems being well-established while newer single-use and continuous processing technologies are rapidly evolving. Key players demonstrate diverse approaches: Applikon Biotechnology and ABEC lead in scalable systems; EMD Millipore and Cytiva (Global Life Sciences Solutions) dominate single-use technologies; academic institutions like MIT, Zhejiang University, and Karlsruhe Institute of Technology drive innovation in novel formats; while pharmaceutical companies like Sanofi and Roche Diagnostics implement advanced manufacturing platforms to reduce production costs.
Applikon Biotechnology BV
Technical Solution: Applikon Biotechnology has developed the AppliFlex ST single-use bioreactor system that combines traditional stirred-tank design principles with modern single-use technology. Their bioreactors feature proprietary impeller configurations that achieve high oxygen transfer rates (kLa values >30 h-1) while maintaining low shear stress environments suitable for sensitive cell cultures[1]. The company has pioneered advanced sensor integration through their AppliFlex sensor technology, which enables reliable real-time monitoring of critical parameters in disposable formats with performance comparable to traditional reusable sensors. Applikon's systems incorporate innovative gas management strategies including microsparger technology and controlled headspace aeration that optimize gas transfer efficiency while minimizing foam formation and cell damage[2]. Their bioreactors support perfusion processes through integration with various cell retention devices, enabling high-density cultures with cell concentrations exceeding 100 million cells/mL in continuous operation modes. The company has also developed scalable control strategies that ensure consistent performance from bench-scale development (250mL) through pilot (50L) to production scale (1000L)[3].
Strengths: Excellent scalability with consistent performance across scales; robust design with industry-leading reliability; comprehensive data management and process control capabilities; extensive validation documentation. Weaknesses: Higher initial investment compared to some competitors; more complex setup procedures for certain applications; limited maximum scale compared to some newer single-use technologies.
EMD Millipore Corp.
Technical Solution: EMD Millipore has pioneered the Mobius® bioreactor platform, which integrates single-use technology with advanced process control capabilities for flexible biomanufacturing. Their systems feature the Flexsafe® film technology, specifically engineered to minimize leachables and extractables while providing consistent cell growth performance across multiple cell lines[1]. The company has developed innovative impeller and sparger designs that enhance oxygen transfer rates while minimizing shear stress, critical for sensitive cell cultures. EMD Millipore's bioreactors incorporate perfusion-capable systems that enable high-density cell cultures and extended production runs, significantly increasing volumetric productivity compared to traditional batch processes[2]. Their integrated sensing technology allows for real-time monitoring of critical parameters including dissolved oxygen, pH, and metabolite concentrations, enabling advanced process control strategies that optimize production conditions throughout the culture duration[3].
Strengths: Comprehensive validation package reducing implementation time; seamless scalability from development to production; robust supply chain for consumables ensuring manufacturing continuity. Weaknesses: Higher initial investment compared to some competitors; proprietary consumables creating potential vendor lock-in; limited maximum scale compared to traditional stainless steel options.
Key Patents in Cost-Effective Bioreactor Technology
Low cost simple design bioreactor
PatentInactiveIN201621029249A
Innovation
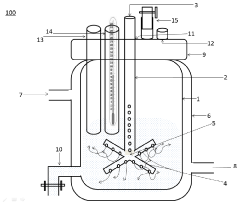
- A bioreactor design without mechanical agitation devices or baffles, utilizing a hollow aeration device with perforated arms for gas sparging to provide uniform mixing and aeration, reducing shear forces and foaming while maintaining yield, and featuring an adjustable aeration tube for optimal gas transfer.
Bioreactor systems
PatentWO2025096530A1
Innovation
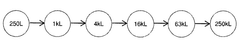
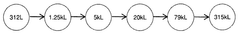
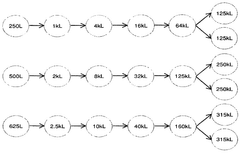
- The development of large-scale bioreactor systems with vessels having internal reaction chambers capable of holding at least 125,000 to 315,000 liters and maintaining viable cell densities of over 50 million cells per milliliter, along with advanced heat transfer systems and agitation mechanisms.
Sustainability Aspects of Modern Bioreactor Systems
The sustainability of modern bioreactor systems has become a critical consideration in the development of emerging bioreactor formats for low-cost, high-volume production. As industries strive to meet increasing demand while minimizing environmental impact, bioreactor design and operation must incorporate sustainable practices throughout the entire production lifecycle.
Energy efficiency represents one of the most significant sustainability aspects of modern bioreactor systems. Traditional bioreactors often require substantial energy inputs for mixing, aeration, temperature control, and downstream processing. Newer designs incorporate energy recovery systems, improved insulation, and more efficient mixing technologies that can reduce energy consumption by up to 40% compared to conventional systems.
Water usage optimization has emerged as another key sustainability factor. Modern bioreactor formats implement closed-loop water recycling systems that can recover and purify process water, reducing freshwater requirements by 60-80%. Single-use bioreactors, while generating plastic waste, actually demonstrate superior water efficiency profiles compared to traditional stainless steel systems that require extensive cleaning and sterilization between batches.
Raw material efficiency extends beyond water to include culture media components and supplements. Advanced formulations utilizing plant-based hydrolysates and chemically defined media reduce reliance on animal-derived components while improving batch-to-batch consistency. Waste valorization strategies are increasingly integrated into bioreactor operations, converting spent biomass into valuable byproducts or bioenergy feedstocks.
Carbon footprint reduction strategies have become standard in next-generation bioreactor design. Life cycle assessment (LCA) studies indicate that implementing renewable energy sources for bioreactor operations can reduce greenhouse gas emissions by 30-70% compared to fossil fuel-powered systems. Additionally, process intensification through continuous manufacturing reduces facility footprint requirements and associated environmental impacts.
Circular economy principles are increasingly applied to bioreactor system design. Modular components that can be easily disassembled, repaired, and upgraded extend equipment lifespan. For single-use systems, biodegradable polymers and recycling programs are being developed to address end-of-life concerns, though significant challenges remain in creating truly sustainable disposable technologies.
Regulatory frameworks worldwide are evolving to incorporate sustainability metrics into biomanufacturing approval processes. Companies implementing sustainable bioreactor technologies not only reduce environmental impact but often realize significant cost savings through reduced resource consumption and waste management expenses, creating alignment between economic and environmental objectives in modern bioprocessing.
Energy efficiency represents one of the most significant sustainability aspects of modern bioreactor systems. Traditional bioreactors often require substantial energy inputs for mixing, aeration, temperature control, and downstream processing. Newer designs incorporate energy recovery systems, improved insulation, and more efficient mixing technologies that can reduce energy consumption by up to 40% compared to conventional systems.
Water usage optimization has emerged as another key sustainability factor. Modern bioreactor formats implement closed-loop water recycling systems that can recover and purify process water, reducing freshwater requirements by 60-80%. Single-use bioreactors, while generating plastic waste, actually demonstrate superior water efficiency profiles compared to traditional stainless steel systems that require extensive cleaning and sterilization between batches.
Raw material efficiency extends beyond water to include culture media components and supplements. Advanced formulations utilizing plant-based hydrolysates and chemically defined media reduce reliance on animal-derived components while improving batch-to-batch consistency. Waste valorization strategies are increasingly integrated into bioreactor operations, converting spent biomass into valuable byproducts or bioenergy feedstocks.
Carbon footprint reduction strategies have become standard in next-generation bioreactor design. Life cycle assessment (LCA) studies indicate that implementing renewable energy sources for bioreactor operations can reduce greenhouse gas emissions by 30-70% compared to fossil fuel-powered systems. Additionally, process intensification through continuous manufacturing reduces facility footprint requirements and associated environmental impacts.
Circular economy principles are increasingly applied to bioreactor system design. Modular components that can be easily disassembled, repaired, and upgraded extend equipment lifespan. For single-use systems, biodegradable polymers and recycling programs are being developed to address end-of-life concerns, though significant challenges remain in creating truly sustainable disposable technologies.
Regulatory frameworks worldwide are evolving to incorporate sustainability metrics into biomanufacturing approval processes. Companies implementing sustainable bioreactor technologies not only reduce environmental impact but often realize significant cost savings through reduced resource consumption and waste management expenses, creating alignment between economic and environmental objectives in modern bioprocessing.
Regulatory Considerations for Novel Bioreactor Formats
The regulatory landscape for novel bioreactor formats presents significant challenges for manufacturers seeking to implement emerging low-cost, high-volume production systems. Regulatory bodies such as the FDA, EMA, and NMPA have established comprehensive frameworks that govern biopharmaceutical manufacturing processes, with particular emphasis on quality control, process validation, and product consistency. These frameworks were primarily designed for traditional bioreactor systems, creating potential regulatory gaps when evaluating novel formats.
For emerging bioreactor technologies, regulatory considerations must address both process and product aspects. Process-related regulations focus on validation protocols, which must demonstrate that the novel bioreactor format can consistently produce the intended product with predefined quality attributes. This includes establishing critical process parameters (CPPs) and their acceptable ranges, which may differ significantly from those of conventional bioreactors.
Product-related regulations center on comparability studies, which are essential when transitioning from traditional to novel bioreactor formats. Manufacturers must provide substantial evidence that products from new bioreactor systems maintain equivalent safety, efficacy, and quality profiles. This often requires extensive analytical characterization and potentially additional clinical studies, depending on the degree of process change.
Regulatory pathways for novel bioreactor formats vary globally, creating additional complexity for manufacturers targeting international markets. The FDA's Quality by Design (QbD) approach offers some flexibility by focusing on understanding process-product relationships rather than rigid process parameters. Similarly, the EMA has implemented the Process Analytical Technology (PAT) framework, which supports continuous manufacturing innovations when properly validated.
Environmental regulations also impact novel bioreactor implementation, particularly for single-use systems that generate significant plastic waste. Manufacturers must develop comprehensive waste management strategies that comply with local environmental regulations, which vary considerably across different regions.
Early engagement with regulatory authorities through programs like the FDA's INTERACT or EMA's Scientific Advice is crucial for novel bioreactor formats. These consultations can provide valuable guidance on regulatory expectations and potential challenges before significant resources are committed to development and validation activities.
Standardization efforts are gradually emerging to address regulatory uncertainties. Organizations such as ASTM International and ISO are developing standards specific to novel bioreactor technologies, which may eventually facilitate regulatory approval processes. However, these standards are still evolving, and manufacturers must stay informed about developments in this rapidly changing regulatory landscape.
For emerging bioreactor technologies, regulatory considerations must address both process and product aspects. Process-related regulations focus on validation protocols, which must demonstrate that the novel bioreactor format can consistently produce the intended product with predefined quality attributes. This includes establishing critical process parameters (CPPs) and their acceptable ranges, which may differ significantly from those of conventional bioreactors.
Product-related regulations center on comparability studies, which are essential when transitioning from traditional to novel bioreactor formats. Manufacturers must provide substantial evidence that products from new bioreactor systems maintain equivalent safety, efficacy, and quality profiles. This often requires extensive analytical characterization and potentially additional clinical studies, depending on the degree of process change.
Regulatory pathways for novel bioreactor formats vary globally, creating additional complexity for manufacturers targeting international markets. The FDA's Quality by Design (QbD) approach offers some flexibility by focusing on understanding process-product relationships rather than rigid process parameters. Similarly, the EMA has implemented the Process Analytical Technology (PAT) framework, which supports continuous manufacturing innovations when properly validated.
Environmental regulations also impact novel bioreactor implementation, particularly for single-use systems that generate significant plastic waste. Manufacturers must develop comprehensive waste management strategies that comply with local environmental regulations, which vary considerably across different regions.
Early engagement with regulatory authorities through programs like the FDA's INTERACT or EMA's Scientific Advice is crucial for novel bioreactor formats. These consultations can provide valuable guidance on regulatory expectations and potential challenges before significant resources are committed to development and validation activities.
Standardization efforts are gradually emerging to address regulatory uncertainties. Organizations such as ASTM International and ISO are developing standards specific to novel bioreactor technologies, which may eventually facilitate regulatory approval processes. However, these standards are still evolving, and manufacturers must stay informed about developments in this rapidly changing regulatory landscape.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!