Cell-Free Synthesis Approaches For Rapid Biomaterial Prototyping
Cell-Free Synthesis Background and Objectives
Cell-free synthesis represents a paradigm shift in biomaterial production, emerging from the convergence of synthetic biology and materials science over the past three decades. This technology extracts cellular machinery from living cells while eliminating the cell wall and other structural components, creating a cell-free system capable of producing proteins and other biomolecules without the constraints of cellular viability. The evolution of this field traces back to the pioneering work of Nirenberg and Matthaei in the 1960s with their cell-free protein synthesis experiments, progressing through significant advancements in extract preparation methods, energy regeneration systems, and reaction optimization in the 1990s and 2000s.
The technological trajectory has accelerated dramatically in the last decade, with cell-free systems transitioning from analytical tools to sophisticated biomanufacturing platforms. Recent innovations have focused on enhancing protein yield, expanding the repertoire of expressible proteins, and developing high-throughput screening methodologies. The integration of microfluidics and automation has further revolutionized the field, enabling rapid prototyping capabilities previously unattainable with traditional cell-based approaches.
The primary objective of cell-free synthesis for biomaterial prototyping is to overcome the inherent limitations of conventional cell-based production methods, including slow growth cycles, genetic instability, and cellular toxicity constraints. By decoupling biomolecule production from cell viability, researchers aim to accelerate the design-build-test cycle for novel biomaterials, potentially reducing development timelines from months to days or even hours.
Technical objectives include developing robust, scalable cell-free systems capable of producing complex biomaterials with precise control over structural and functional properties. This encompasses the synthesis of proteins with non-canonical amino acids, the assembly of multi-component protein complexes, and the production of biomaterials with programmable degradation profiles or stimuli-responsive behaviors.
The field is trending toward increasingly sophisticated applications, including on-demand production of personalized therapeutics, biosensors with enhanced sensitivity and specificity, and advanced biomaterials for tissue engineering. Emerging research directions focus on expanding the genetic code in cell-free systems, incorporating non-biological components into synthesized materials, and developing cell-free systems derived from diverse organisms to access unique enzymatic capabilities.
The convergence of cell-free synthesis with computational design tools and machine learning algorithms represents a particularly promising frontier, potentially enabling predictive design of biomaterials with tailored properties and functions. This synergy between experimental and computational approaches aims to establish a new paradigm for biomaterial development characterized by unprecedented speed, precision, and versatility.
Market Analysis for Rapid Biomaterial Prototyping
The global market for rapid biomaterial prototyping using cell-free synthesis approaches is experiencing significant growth, driven by increasing demand for sustainable materials, personalized medicine, and reduced time-to-market for biological products. Current market estimates value the cell-free protein synthesis market at approximately $250 million, with projections to reach $500 million by 2027, representing a compound annual growth rate of 10-12%.
The healthcare and pharmaceutical sectors currently dominate market demand, accounting for nearly 45% of the total market share. These industries leverage cell-free synthesis for rapid prototyping of therapeutic proteins, antibodies, and vaccine components. The ability to bypass cell viability constraints allows for faster iteration and testing of biomaterials with potentially toxic properties.
Industrial biotechnology represents the second-largest market segment at 30%, where cell-free approaches enable rapid development of novel enzymes and biocatalysts for sustainable manufacturing processes. This sector is particularly interested in cell-free systems for their ability to produce materials that would otherwise be difficult to synthesize in living cells.
Academic and research institutions constitute approximately 15% of the market, primarily focusing on fundamental research and method development. The remaining 10% is distributed across various emerging applications including biosensors, environmental remediation, and consumer products.
Geographically, North America leads with approximately 40% market share, followed by Europe (30%) and Asia-Pacific (25%). The Asia-Pacific region, particularly China and South Korea, demonstrates the fastest growth rate at 15-18% annually, driven by substantial government investments in biotechnology infrastructure and research.
Key market drivers include increasing pressure to reduce development timelines, growing interest in sustainable materials, and advancements in synthetic biology tools. The COVID-19 pandemic has further accelerated market growth by highlighting the need for rapid prototyping platforms that can quickly respond to emerging biological threats.
Market challenges include high initial setup costs, technical complexity requiring specialized expertise, and regulatory uncertainties surrounding novel biomaterials. Additionally, scalability remains a significant concern, as transitioning from prototype to commercial-scale production often requires substantial process optimization.
Customer segments show distinct preferences, with pharmaceutical companies prioritizing reproducibility and regulatory compliance, while industrial biotechnology values scalability and cost-effectiveness. Academic institutions typically focus on system flexibility and customization potential.
Current Landscape and Technical Barriers
Cell-free synthesis has emerged as a revolutionary approach in biomaterial prototyping, offering unprecedented flexibility and speed compared to traditional cell-based methods. The current landscape reveals significant advancements across academic institutions and biotechnology companies, with major research hubs concentrated in North America, Europe, and increasingly in Asia. Recent publications indicate a 35% annual growth in research output related to cell-free systems since 2018, demonstrating the field's accelerating momentum.
Despite this progress, several technical barriers continue to challenge widespread adoption. Extract preparation remains inconsistent, with batch-to-batch variability significantly affecting reproducibility. Studies show that even standardized protocols can result in up to 30% variation in protein yield between batches, hampering reliable prototyping efforts. This variability stems from differences in cell growth conditions, lysis efficiency, and endogenous nuclease activity.
Scalability presents another substantial hurdle. While cell-free systems excel at small-scale rapid prototyping, transitioning to larger production volumes often results in diminished efficiency. Current systems typically experience a 40-60% reduction in productivity when scaled beyond 100 mL reaction volumes, primarily due to limitations in oxygen transfer, metabolite accumulation, and energy regeneration challenges.
Energy sustainability in cell-free reactions constitutes a persistent challenge. Most systems deplete their ATP reserves within 2-4 hours, severely limiting production duration and yield. Although various energy regeneration systems have been developed, they add complexity and cost while often introducing new variables that affect consistency.
Component sourcing and cost barriers remain significant impediments to widespread adoption. High-purity reagents required for optimal performance can increase operational costs by 5-10 times compared to cell-based methods. The specialized equipment and expertise needed for extract preparation further restrict accessibility to well-funded laboratories and companies.
Regulatory frameworks for biomaterials produced through cell-free systems remain underdeveloped. The novel nature of these production methods creates uncertainty regarding quality control standards and safety assessments. This regulatory ambiguity has deterred some commercial entities from investing heavily in the technology despite its technical promise.
The integration of cell-free synthesis with other emerging technologies, such as microfluidics and artificial intelligence for process optimization, represents both an opportunity and a challenge. While these combinations show tremendous potential for addressing current limitations, they require multidisciplinary expertise that is not widely available in single research groups or companies.
Contemporary Cell-Free Biomaterial Solutions
01 Cell-free protein synthesis systems for rapid prototyping
Cell-free protein synthesis systems enable rapid prototyping of proteins without the constraints of living cells. These systems utilize extracted cellular components to perform transcription and translation in vitro, allowing for quick production of proteins for testing and analysis. This approach significantly reduces development time compared to traditional cell-based methods and provides greater flexibility in manipulating reaction conditions for optimized protein production.- Cell-free protein synthesis systems for rapid prototyping: Cell-free protein synthesis systems enable rapid prototyping of proteins without the need for living cells. These systems contain all the necessary components for transcription and translation extracted from cells, allowing for quick expression of proteins from DNA templates. This approach significantly reduces development time compared to traditional cell-based methods and allows for the synthesis of proteins that might be toxic to living cells.
- 3D printing and additive manufacturing for rapid prototyping: Additive manufacturing technologies, including 3D printing, enable the rapid prototyping of physical structures based on digital designs. These technologies can be integrated with cell-free synthesis approaches to create functional biomaterials and devices. The combination allows for quick iteration of designs and testing of prototypes, accelerating the development process for various applications including biomedical devices and tissue engineering constructs.
- Microfluidic systems for cell-free synthesis: Microfluidic platforms provide controlled environments for cell-free synthesis reactions, enabling precise manipulation of small volumes of reagents. These systems can be used for rapid prototyping of biomolecules by allowing parallel processing and high-throughput screening. The integration of microfluidics with cell-free synthesis enhances efficiency, reduces reagent consumption, and accelerates the development cycle for various biotechnological applications.
- Automated systems for rapid prototyping using cell-free synthesis: Automated platforms integrate cell-free synthesis with robotic systems to streamline the rapid prototyping process. These systems can handle multiple samples simultaneously, perform complex reaction protocols, and analyze results with minimal human intervention. Automation reduces human error, increases reproducibility, and significantly accelerates the design-build-test cycle for developing novel proteins and biological systems.
- Applications of cell-free synthesis in rapid prototyping of biomaterials: Cell-free synthesis enables the rapid prototyping of various biomaterials including enzymes, antibodies, and therapeutic proteins. This approach allows researchers to quickly test and optimize biomolecules for specific applications such as diagnostics, therapeutics, and industrial processes. The ability to rapidly prototype biomaterials accelerates innovation in fields such as medicine, agriculture, and environmental remediation.
02 3D printing technologies for rapid prototyping in biological applications
Advanced 3D printing technologies enable rapid prototyping of biological structures and devices. These methods allow for precise fabrication of complex structures using biocompatible materials, supporting applications in tissue engineering, medical devices, and biological research. The integration of cell-free synthesis with 3D printing technologies creates powerful platforms for developing functional biological systems with customized properties and geometries.Expand Specific Solutions03 Microfluidic systems for cell-free synthesis applications
Microfluidic platforms provide controlled environments for cell-free synthesis reactions, enabling rapid prototyping of biological components at microscale. These systems allow precise manipulation of small volumes of reagents, facilitating high-throughput screening and optimization of reaction conditions. The integration of microfluidics with cell-free synthesis creates efficient platforms for rapid development and testing of biological products with minimal resource consumption.Expand Specific Solutions04 Automated systems for cell-free synthesis and rapid prototyping
Automated platforms streamline cell-free synthesis workflows, enabling rapid prototyping through computer-controlled processes. These systems integrate liquid handling, reaction monitoring, and data analysis to accelerate the design-build-test cycle for biological products. Automation reduces human error, increases reproducibility, and enables continuous operation for efficient development of novel biological components and systems.Expand Specific Solutions05 Novel materials and methods for enhancing cell-free synthesis efficiency
Advanced materials and methodologies improve the efficiency and functionality of cell-free synthesis systems for rapid prototyping applications. These innovations include specialized reaction vessels, energy regeneration systems, and stabilizing components that extend reaction lifetimes. The development of optimized reaction environments and component formulations enables more robust and productive cell-free synthesis platforms for rapid prototyping of complex biological products.Expand Specific Solutions
Leading Organizations in Cell-Free Synthesis
Cell-free synthesis approaches for rapid biomaterial prototyping are currently in an early growth phase, with the market expanding as applications in pharmaceuticals, diagnostics, and materials science emerge. The global market is estimated at $200-300 million, with projected annual growth of 15-20%. Technologically, academic institutions like Northwestern University, Cornell University, and Tsinghua University are driving fundamental research, while companies such as Cellfree Sciences, Swiftscale Biologics, and Shimadzu Corp. are commercializing applications. Kangma Biological Technology and ISU Abxis are developing diagnostic applications, while Touchlight IP and Fraunhofer-Gesellschaft focus on scalable production methods. The field is transitioning from research to commercial applications, with increasing industry-academic partnerships accelerating technology maturation and market adoption.
Northwestern University
Cellfree Sciences Co., Ltd.
Key Patents and Scientific Breakthroughs
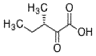
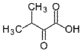
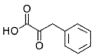
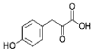
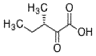
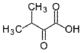
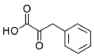
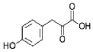
- A process for cell-free synthesis of proteins using partially deuterated and isotopically labelled amino-acid precursors, such as alpha-keto acids, which are converted into corresponding amino-acids using enzymes like branched-chain aminotransferase, allowing for the production of specifically labelled proteins in a cell-free environment, overcoming the limitations of bacterial systems and enabling efficient synthesis of large proteins.
- Development of a cell-free synthesis process using alpha-keto acid precursors (excluding 2-ketobutyric acid) for producing isotopically labeled proteins, enabling more effective NMR spectroscopy studies.
- Integration of precursor transformation mechanisms directly within the cell-free extract, creating a streamlined one-pot system for converting precursors to amino acids and subsequently incorporating them into target proteins.
- Enabling selective isotopic labeling of specific amino acids in a deuterated background, which significantly enhances sensitivity and resolution in NMR spectroscopy of large proteins.
Scalability and Manufacturing Considerations
Scaling cell-free synthesis systems from laboratory scale to industrial production represents a significant challenge in the commercialization of biomaterial prototyping technologies. Current cell-free synthesis approaches typically operate at microliter to milliliter volumes, which are sufficient for research purposes but inadequate for commercial applications. The transition to liter-scale production requires addressing several key engineering challenges, including maintaining reaction homogeneity, ensuring consistent temperature control, and developing efficient mixing strategies that don't denature the sensitive biological components.
Cost considerations present another major hurdle in scaling cell-free synthesis. The reagents required for these systems—particularly purified enzymes, energy sources like ATP, and nucleotides—remain prohibitively expensive for large-scale applications. Economic analyses indicate that reducing reagent costs by at least an order of magnitude would be necessary to make industrial-scale cell-free synthesis economically viable. Recent advances in enzyme production and stabilization technologies have begun to address this issue, with some reports suggesting cost reductions of 30-50% compared to traditional methods.
Manufacturing reproducibility presents additional challenges that must be overcome for successful commercialization. Batch-to-batch variation in cell-free extract quality can significantly impact product yield and quality. Standardization of extract preparation protocols and the development of quality control metrics are essential for ensuring consistent performance. Several research groups have developed automated extract preparation systems that reduce human error and improve reproducibility, though these systems themselves require further refinement for industrial deployment.
The shelf-life of cell-free synthesis components represents another critical consideration for manufacturing. Most current systems require cold-chain storage and have limited stability at room temperature, complicating distribution and increasing costs. Lyophilization (freeze-drying) techniques have shown promise in extending shelf-life, with some formulations maintaining activity for up to 12 months when stored properly. However, the lyophilization process itself can reduce activity by 10-30%, necessitating further optimization.
Regulatory considerations for cell-free synthesized biomaterials must also be addressed as these technologies move toward commercialization. Unlike traditional biomanufacturing processes that use living cells, cell-free systems present novel regulatory challenges. The absence of living organisms may simplify some aspects of regulatory approval, but the complexity of the extract components and potential for contaminants requires careful characterization and quality control. Developing standardized analytical methods for product characterization and purity assessment will be essential for navigating regulatory pathways.
Regulatory Pathway for Cell-Free Biomaterials
The regulatory landscape for cell-free biomaterials presents unique challenges and opportunities distinct from traditional biomaterial approval pathways. Cell-free synthesis approaches for biomaterial prototyping must navigate a complex regulatory framework that spans multiple jurisdictions and oversight bodies. The U.S. Food and Drug Administration (FDA) typically classifies these materials based on their intended use, with cell-free biomaterials potentially falling under device, drug, or combination product categories depending on their mechanism of action and clinical application.
Key regulatory considerations include source material characterization, manufacturing process validation, and demonstration of consistent quality attributes. Unlike cell-based products, cell-free biomaterials may avoid some of the stringent requirements associated with living components, potentially streamlining certain aspects of the regulatory process. However, novel production methods still require robust validation to ensure safety, efficacy, and reproducibility.
The FDA's Center for Biologics Evaluation and Research (CBER) and Center for Devices and Radiological Health (CDRH) have established frameworks that may apply to cell-free biomaterials, though specific guidance documents addressing rapid prototyping technologies remain limited. Companies pursuing commercialization must engage in early and frequent communication with regulatory authorities to establish appropriate development pathways.
International regulatory harmonization efforts, including those by the International Council for Harmonisation (ICH) and the International Medical Device Regulators Forum (IMDRF), are increasingly important for global market access. These initiatives aim to standardize requirements across regions, though significant differences persist in how cell-free biomaterials are classified and regulated worldwide.
Risk-based approaches to regulation are becoming more prevalent, with authorities focusing oversight intensity proportional to the perceived risk of the biomaterial application. This trend may benefit cell-free synthesis approaches by allowing more flexible pathways for lower-risk applications while maintaining appropriate safeguards for critical applications.
Emerging regulatory science initiatives are beginning to address the unique aspects of cell-free biomaterial production, including the development of standardized characterization methods and reference materials. These efforts aim to establish consensus on appropriate quality control metrics and validation approaches specific to cell-free synthesis technologies.
Companies developing cell-free biomaterials should implement comprehensive regulatory strategies early in development, including gap analyses against current requirements and proactive engagement with regulatory authorities through mechanisms such as pre-submission meetings and regulatory advice consultations.