Comparing Yeast, Bacterial, And Fungal Platforms For Material Production
Microbial Production Platforms Background and Objectives
Microbial production platforms have evolved significantly over the past decades, transforming from simple fermentation processes to sophisticated biotechnological systems capable of producing a wide range of materials, chemicals, and pharmaceuticals. The historical trajectory of microbial production began with traditional fermentation techniques dating back thousands of years, primarily utilizing yeast for food and beverage production. The modern era of microbial production emerged in the mid-20th century with the discovery of antibiotics and subsequent development of industrial-scale fermentation processes.
The technological evolution accelerated dramatically with the advent of genetic engineering in the 1970s, enabling scientists to modify microbial genomes for enhanced production capabilities. This breakthrough led to the establishment of the first biotechnology companies focused on microbial production platforms. The completion of genome sequencing projects for various microorganisms in the early 2000s further revolutionized the field, providing comprehensive genetic blueprints that facilitated more precise genetic modifications.
Recent advances in synthetic biology, metabolic engineering, and systems biology have dramatically expanded the capabilities of microbial production platforms. These technologies have enabled the design and construction of novel metabolic pathways in microorganisms, allowing them to produce compounds that are not naturally part of their metabolism. The development of CRISPR-Cas9 and other genome editing tools has further simplified and accelerated the process of engineering microbial strains.
The primary objective of comparing yeast, bacterial, and fungal platforms is to establish a comprehensive understanding of their respective strengths, limitations, and optimal applications in material production. This comparison aims to identify the most suitable microbial platform for specific production scenarios based on factors such as product complexity, scale requirements, economic considerations, and sustainability metrics.
Additional objectives include mapping the technological trajectory of each platform to anticipate future developments, identifying opportunities for cross-platform synergies, and developing strategic frameworks for platform selection in industrial applications. The analysis also seeks to highlight emerging technologies that may enhance the capabilities of these platforms, such as advanced bioreactor designs, continuous processing methods, and novel purification techniques.
Understanding the fundamental differences between these platforms is crucial for industries ranging from pharmaceuticals and biofuels to food ingredients and biomaterials. As global demand for sustainable production methods increases, microbial platforms offer promising alternatives to traditional chemical synthesis and extraction from natural sources, potentially reducing environmental impact while improving production efficiency and product quality.
Market Analysis for Bio-based Materials
The global market for bio-based materials has experienced significant growth in recent years, driven by increasing environmental concerns, regulatory pressures, and consumer demand for sustainable products. The market value reached approximately $95 billion in 2022 and is projected to grow at a CAGR of 7.5% through 2030, potentially reaching $160 billion by the end of the decade.
Within this expanding market, materials produced through microbial fermentation platforms—specifically yeast, bacteria, and fungi—represent one of the fastest-growing segments. These bio-manufacturing approaches are gaining traction due to their reduced environmental footprint compared to traditional petrochemical processes and their ability to create novel materials with unique properties.
Yeast-based production platforms currently hold the largest market share among microbial systems, accounting for roughly 40% of bio-manufactured materials. This dominance stems from the well-established industrial infrastructure for yeast fermentation, particularly in sectors like biofuels, food additives, and pharmaceuticals. Companies like Genomatica and Amyris have successfully commercialized yeast-based production of chemicals and materials.
Bacterial platforms follow closely behind with approximately 35% market share, showing particular strength in biopolymer production such as PHA (polyhydroxyalkanoates) and bacterial cellulose. The market for bacterial cellulose alone is expected to grow at 15% annually through 2028, driven by applications in packaging, textiles, and medical devices.
Fungal platforms, while currently representing a smaller segment at 25%, are demonstrating the highest growth rate among the three platforms. Mycelium-based materials for packaging, textiles, and construction have attracted significant investment, with funding for fungal material startups exceeding $350 million in 2022 alone.
Regionally, North America leads the bio-based materials market with approximately 38% share, followed by Europe (32%) and Asia-Pacific (25%). However, the Asia-Pacific region is expected to show the highest growth rate over the next decade due to increasing industrial adoption and favorable government policies in countries like China, Japan, and South Korea.
End-use industries demonstrate varying adoption rates for these microbial platforms. Packaging represents the largest application segment (30%), followed by textiles (22%), automotive components (15%), construction materials (12%), and consumer goods (10%). The remaining 11% encompasses specialized applications including medical devices, electronics, and aerospace components.
Current Status and Challenges in Microbial Production
Microbial production systems have evolved significantly over the past decades, with yeast, bacteria, and fungi emerging as the three primary platforms for industrial material production. Currently, bacterial systems, particularly Escherichia coli, dominate the production landscape due to their rapid growth rates, well-characterized genetics, and established molecular toolkits. These systems achieve high yields for various compounds including enzymes, biofuels, and pharmaceutical precursors, with production capacities reaching industrial scale in many applications.
Yeast-based platforms, led by Saccharomyces cerevisiae, have gained substantial traction for their eukaryotic cellular machinery that enables complex post-translational modifications. This advantage has positioned yeast as the preferred host for producing proteins requiring glycosylation and proper folding. Recent advances in synthetic biology have expanded yeast's capabilities to produce diverse compounds including lipids, terpenes, and heterologous proteins with titers approaching commercial viability.
Filamentous fungi represent an emerging production platform with unique capabilities for secreting large quantities of proteins and enzymes. Species like Aspergillus niger and Trichoderma reesei have demonstrated exceptional enzyme secretion capacities exceeding 100 g/L in optimized conditions, significantly outperforming bacterial and yeast systems in specific applications.
Despite these achievements, several critical challenges persist across all microbial production platforms. Metabolic burden remains a fundamental limitation, where redirecting cellular resources toward heterologous product synthesis often compromises growth and overall productivity. This challenge is particularly pronounced in bacterial systems where high expression levels can trigger stress responses that diminish yield.
Scalability presents another significant hurdle, with laboratory-optimized processes frequently failing to maintain performance at industrial scales. Factors including oxygen transfer limitations, heat management, and homogeneity become increasingly problematic as fermentation volumes increase, often resulting in 30-50% yield reductions during scale-up.
Product toxicity constitutes a persistent challenge, particularly for compounds like biofuels, organic acids, and certain pharmaceuticals that can compromise membrane integrity or cellular metabolism. While tolerance engineering has made progress, the maximum achievable titers for many valuable compounds remain below economically viable thresholds.
Feedstock utilization efficiency represents another critical limitation, with most established production strains optimized for refined sugar substrates rather than more economical lignocellulosic materials or industrial waste streams. The inability to efficiently metabolize complex carbon sources significantly impacts production economics, particularly for lower-value bulk materials.
Regulatory and containment considerations also present growing challenges, especially for genetically modified organisms deployed at industrial scale. Addressing these multifaceted challenges requires integrated approaches combining synthetic biology, bioprocess engineering, and systems-level optimization strategies tailored to each microbial platform's unique characteristics.
Comparative Analysis of Yeast, Bacterial, and Fungal Systems
01 Microbial platforms for biofuel production
Yeast, bacterial, and fungal platforms are utilized for the production of biofuels through fermentation processes. These microorganisms can be genetically engineered to enhance their ability to convert various feedstocks into biofuels such as ethanol, biodiesel, and other renewable energy sources. The metabolic pathways of these microorganisms are optimized to improve yield, productivity, and tolerance to inhibitory compounds present in the feedstock or generated during the production process.- Microbial platforms for biofuel production: Yeast, bacterial, and fungal platforms are utilized for the production of biofuels through fermentation processes. These microorganisms can be genetically engineered to enhance their ability to convert biomass into various types of biofuels such as ethanol, biodiesel, and other renewable energy sources. The metabolic pathways of these microorganisms are optimized to improve yield, productivity, and tolerance to inhibitory compounds present in biomass feedstocks.
- Microbial platforms for pharmaceutical production: Microorganisms serve as efficient platforms for the production of pharmaceutical compounds including antibiotics, enzymes, and therapeutic proteins. These platforms offer advantages such as scalability, cost-effectiveness, and the ability to produce complex molecules. Genetic engineering techniques are employed to introduce biosynthetic pathways into yeast, bacteria, and fungi, enabling them to produce valuable pharmaceutical compounds that may be difficult to synthesize chemically.
- Food and beverage applications of microbial platforms: Yeast, bacterial, and fungal platforms play crucial roles in food and beverage production processes. These microorganisms are used for fermentation to produce various food products, flavor compounds, and nutritional supplements. They can be engineered to enhance nutritional value, improve taste profiles, extend shelf life, and produce specific enzymes for food processing. Applications include dairy fermentation, bread making, alcoholic beverage production, and the development of novel food ingredients.
- Industrial enzyme production using microbial platforms: Microbial platforms are extensively used for the production of industrial enzymes that catalyze various biochemical reactions. These enzymes find applications in detergents, textile processing, paper manufacturing, biofuel production, and other industrial processes. Yeast, bacteria, and fungi can be engineered to overexpress specific enzymes with enhanced stability, activity, and specificity. The production processes are optimized for high yield and cost-effectiveness, making microbial platforms the preferred choice for industrial enzyme manufacturing.
- Microbial platforms for environmental applications: Yeast, bacterial, and fungal platforms are employed in various environmental applications including bioremediation, waste treatment, and pollution control. These microorganisms can degrade toxic compounds, convert waste materials into valuable products, and help in the treatment of contaminated soil and water. Genetic engineering approaches are used to enhance the ability of these platforms to metabolize specific pollutants and improve their survival in harsh environmental conditions.
02 Microbial platforms for pharmaceutical production
Yeast, bacterial, and fungal platforms serve as efficient systems for the production of pharmaceutical compounds including antibiotics, enzymes, and therapeutic proteins. These microorganisms can be engineered to express heterologous genes and produce complex molecules with high specificity and yield. The use of these platforms allows for cost-effective production of pharmaceuticals with reduced environmental impact compared to traditional chemical synthesis methods.Expand Specific Solutions03 Food and beverage applications of microbial platforms
Microbial platforms including yeast, bacteria, and fungi are extensively used in the food and beverage industry for fermentation processes. These microorganisms contribute to the production of various food products such as bread, cheese, yogurt, wine, and beer. They can also be engineered to produce food additives, flavors, and nutritional supplements. The selection and optimization of specific microbial strains help improve the quality, taste, and nutritional value of food products.Expand Specific Solutions04 Industrial enzyme production using microbial platforms
Yeast, bacterial, and fungal platforms are utilized for the production of industrial enzymes used in various applications including detergents, textiles, paper manufacturing, and bioremediation. These microorganisms can be engineered to produce enzymes with enhanced stability, activity, and specificity. The optimization of fermentation conditions and downstream processing techniques allows for efficient and cost-effective production of industrial enzymes at commercial scale.Expand Specific Solutions05 Diagnostic and biosensor applications of microbial platforms
Microbial platforms are employed in the development of diagnostic tools and biosensors for detecting various substances including pollutants, pathogens, and biomarkers. These microorganisms can be engineered to respond to specific stimuli by producing detectable signals such as fluorescence or color changes. The integration of these microbial platforms with advanced detection technologies enables rapid, sensitive, and specific detection methods for various applications in healthcare, environmental monitoring, and food safety.Expand Specific Solutions
Leading Companies in Industrial Biotechnology
The microbial material production landscape is currently in a growth phase, with an estimated market size exceeding $1 billion and expanding at 20-30% annually. Yeast platforms represent the most mature technology, with companies like Perfect Day and Cargill leading commercial-scale production of proteins and biomaterials. Bacterial platforms offer faster growth rates but face regulatory challenges, with Fraunhofer-Gesellschaft and VTT advancing innovations in this space. Fungal/mycelium platforms are emerging rapidly, with MycoWorks, Ecovative, and Mycotech pioneering sustainable leather alternatives and packaging materials. The competitive landscape is diversifying as academic institutions like Jiangnan University and Arizona State University collaborate with industry players to overcome scale-up challenges and reduce production costs, driving the transition from laboratory success to commercial viability.
MycoWorks, Inc.
Cargill, Inc.
Key Technological Innovations in Microbial Engineering
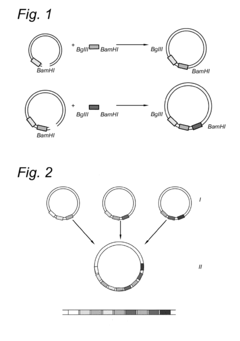
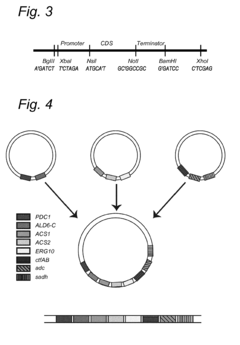
- A method involving DNA fragments with staggered 5' and 3' ends that form complementary restriction enzyme recognition sequences, allowing for the assembly of multiple genes into a vector in a single step, enabling the introduction of large biosynthetic pathways into fungal genomes.
- Engineered S. cerevisiae strains are developed to express enzymes of the fatty acid β-oxidation cycle in the absence of fatty acids, allowing for the production of compounds like fatty acids, alcohols, and alkenes by converting non-fatty acid carbon sources into acetyl-CoA and driving the β-oxidation cycle in a biosynthetic direction, with specific enzymes converting reaction intermediates into desired fermentation products.
Sustainability Impact and Life Cycle Assessment
The sustainability impact of microbial platforms for material production represents a critical dimension in evaluating their industrial viability. Life cycle assessment (LCA) studies consistently demonstrate that yeast-based production systems generally exhibit lower environmental footprints compared to traditional petrochemical processes, with reductions in greenhouse gas emissions ranging from 30-80% depending on the specific product and process configuration.
Bacterial platforms, particularly those utilizing cyanobacteria and other photosynthetic species, offer unique sustainability advantages through direct carbon dioxide utilization, potentially achieving carbon-negative production when integrated with renewable energy sources. However, these systems often require more intensive downstream processing, which can offset some environmental benefits through increased energy consumption and chemical usage during purification stages.
Fungal platforms present compelling sustainability credentials, particularly filamentous fungi which can utilize diverse waste streams as feedstocks, including agricultural residues and food processing byproducts. This waste valorization capability significantly enhances their sustainability profile by simultaneously addressing waste management challenges while producing valuable materials.
Water consumption patterns vary significantly across platforms, with bacterial systems typically requiring 2-3 times more process water than yeast platforms for equivalent production volumes. Fungal systems demonstrate intermediate water requirements but often excel in wastewater quality metrics, generating effluent with lower biological oxygen demand due to their efficient nutrient uptake mechanisms.
Land use considerations favor microbial platforms collectively when compared to plant-based material production, with bacterial and yeast systems requiring minimal direct land footprint. However, indirect land use impacts from feedstock production remain significant for heterotrophic systems dependent on agricultural inputs.
Energy efficiency metrics reveal that yeast platforms typically consume 15-25% less energy than bacterial systems for comparable product yields, though this advantage narrows with increasing process optimization. Fungal platforms demonstrate exceptional energy efficiency in solid-state fermentation configurations but face challenges in maintaining this advantage at industrial scales.
Biodegradability and end-of-life considerations increasingly favor fungal-derived materials, which demonstrate superior composting characteristics and marine degradation profiles compared to both bacterial and yeast-derived alternatives. This advantage becomes particularly significant for packaging and disposable material applications where environmental persistence represents a growing concern.
Regulatory Framework for Microbial-Derived Products
The regulatory landscape for microbial-derived products varies significantly across different jurisdictions, creating a complex framework that manufacturers must navigate. In the United States, the FDA oversees these products through multiple pathways depending on their intended use. Products classified as food ingredients or additives fall under GRAS (Generally Recognized As Safe) status, while those with therapeutic applications require more rigorous approval processes through the Center for Biologics Evaluation and Research (CBER) or the Center for Drug Evaluation and Research (CDER).
European regulations, governed by the European Food Safety Authority (EFSA) and the European Medicines Agency (EMA), implement a precautionary approach that often results in more stringent requirements than their US counterparts. The Novel Food Regulation (EU) 2015/2283 specifically addresses new food ingredients derived from microbial sources, requiring comprehensive safety assessments before market authorization.
Comparing the three production platforms, yeast-derived products generally face fewer regulatory hurdles due to their long history in food production and GRAS status of many yeast strains. Saccharomyces cerevisiae, in particular, benefits from established regulatory precedents, streamlining approval processes for new applications.
Bacterial production systems encounter more variable regulatory treatment. While some bacterial strains like certain Lactobacillus species enjoy GRAS status, others face heightened scrutiny due to pathogenicity concerns. Manufacturers using bacterial platforms must often provide more extensive safety documentation, particularly regarding the absence of toxins and endotoxins in the final product.
Fungal platforms typically face the most rigorous oversight due to concerns about mycotoxin production and less established safety profiles. Non-traditional fungal species require extensive characterization and safety validation before regulatory approval, often necessitating substantial investment in toxicology studies.
Recent regulatory developments show a trend toward harmonization of international standards, particularly for novel biomaterials. The International Council for Harmonisation (ICH) has been working to establish consistent guidelines for microbial-derived pharmaceuticals, while the Codex Alimentarius Commission addresses food applications. These efforts aim to reduce regulatory barriers while maintaining safety standards.
For emerging applications in sustainable materials and biofabrics, regulatory frameworks remain in development, creating both challenges and opportunities for innovators. Companies pioneering these technologies must often work collaboratively with regulatory bodies to establish appropriate assessment protocols, potentially gaining competitive advantages through early engagement with authorities.